Back to Journals » OncoTargets and Therapy » Volume 8
Positive feedback between oncogenic KRAS and HIF-1α confers drug resistance in colorectal cancer
Authors Wang Y, Lei F, Rong W, Zeng Q, Sun W
Received 30 December 2014
Accepted for publication 1 April 2015
Published 26 May 2015 Volume 2015:8 Pages 1229—1237
DOI https://doi.org/10.2147/OTT.S80017
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 10
Editor who approved publication: Dr Faris Farassati
Yanzhao Wang,1 Fuming Lei,2 Wanshui Rong,2 Qingmin Zeng,2 Wenbing Sun1
1Department of Hepatobiliary Surgery, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of General Surgery, Peking University Shougang Hospital, Beijing, People’s Republic of China
Abstract: Approximately 30%–50% of colorectal cancers (CRCs) harbor the somatic mutated KRAS gene. KRAS G12V, one of the most common KRAS mutations in CRCs, is linked to increased tumor aggressiveness, less response to anti-epidermal growth factor receptor (EGFR) therapy, and poor survival rate. In this study, we sought to determine whether resistance to EGFR inhibitors in colorectal cancer cells harboring KRAS G12V mutation is associated with hypoxia. Our data indicated that HIF-1α was induced by KRAS G12V signaling at transcription level. Hypoxia or HIF-1α overexpression could increase KRAS G12V activity. Therefore, a positive feedback between hypoxia and KRAS G12V activation was formed. Cetuximab, an EGFR inhibitor, which has a minor effect on KRAS-mutant CRCs, could effectively inhibit the proliferation of CRC cells harboring KRAS G12V mutation when combined with HIF-1α inhibitor PX-478. Our data indicated that hypoxia was involved in resistance to anti-EGFR therapy, and a combination therapy might be necessary for CRC patients with KRAS mutation.
Keywords: combination therapy, EGFR inhibitor resistance, KRAS G12V
Introduction
Colorectal cancer (CRC) is one of the most common cancers in developed countries. Mortality rates of CRC declined by ~3% during the past decade due to improvement in treatments.1,2 The epidermal growth factor receptor (EGFR) signaling pathway, which regulates cell differentiation, proliferation, migration, angiogenesis, and apoptosis, becomes deregulated frequently in colorectal-cancer cells.3 Upregulation of the EGFR gene is associated with poor survival. Thus, the application of the EGFR-targeted monoclonal antibodies, such as cetuximab and panitumumab, has been indicated for individualized treatment of metastatic CRCs.4–6 Several studies have demonstrated that oncogenic mutations of KRAS, a downstream signaling effector of EGFR, are associated with the effectiveness of these drugs.7,8 KRAS mutations are present in approximately 30%–50% of CRC patients. About 80% of KRAS mutations are found in codon 12 and 15% in codon 13, with the most common mutations being G12D, G12A, G12R, G12C, G12S, G12V, and G13D.9,10 These mutations result in accumulation of GTP-bound KRAS, a constitutive active form, due to impairment of intrinsic GTPase of KRAS. Cells harboring KRAS G12V are reported to have high oncogenic potential and are more aggressive than those harboring other KRAS mutations.11 Patients with KRAS-mutant tumors rarely benefit from anti-EGFR therapy. Recent studies have shown that cells carrying different KRAS mutations responded differently to cetuximab treatment.12 The proliferation of cancer cells harboring KRAS G13D mutation could be inhibited by cetuximab while little inhibitory effect was observed on cells with KRAS G12V mutation.12
KRAS G12V mutant functions predominately through RAS–RAF–MAPK signaling and, to a lesser extent, through the PI3K/AKT pathway.13 PI3K/AKT pathway activation has been associated with resistance to anti-EGFR therapy in some studies, but not in others.14,15 There are no defined mechanisms for EGFR inhibitor resistance that apply to all KRAS mutant cancers.
Hypoxic microenvironment of cancer cells has been suggested to result in drug resistance.16–18 Hypoxia has been shown to associate with many signaling pathways, including PI3K/AKT, MAPK, and NOTCH signaling.19 Since KRAS is linked to all these pathways, in this study, we determine whether resistance to anti-EGFR therapy in KRAS-mutant CRCs is related to the condition of hypoxia. We found a positive feedback regulation between hypoxia and KRAS G12V activity in which hypoxia inducible factor 1-alpha (HIF-1α) was induced by KRAS G12V expression and, in turn, KRAS activity was upregulated by hypoxia. Therefore, hypoxia was involved in anti-EGFR therapy resistance. Our data provided a rationale for combination therapy of EGFR inhibitor and HIF-1α inhibitor for CRC patients carrying KRAS G12V mutation.
Materials and methods
Reagents
pBabe-puro plasmid was purchased from Addgene (Cambridge, MA, USA); antibodies for phospho-AKT, AKT, phosphor-ERK, ERK, and RAS-GTP were from Abcam plc. (Cambridge, MA, USA); and rabbit anti-actin, anti-HIF-1α, and anti-KRAS were from Cell Signaling Technology (Beverly, MA, USA). Real-time polymerase chain reaction (PCR) primers for HIF-1α, KRAS, and actin were purchased from Gene Copoeia Inc. (Beijing, People’s Republic of China). Cetuximab and PX-478 were from Merck Serono (Darmstadt, Germany). siRNA against KRAS 3′-UTR was designed using an online tool on the Invitrogen website. The sequences are as follows: si-1: CGAGTGGTTGTACGATGCATTGGTT; si-2: GGGTGGTGGTGTGCCAAGACATTAA.
Cell culture and transfection
Colon cancer cell lines HCT116, Colo205, SW480, HT29, and Caco-2 were obtained from the Cell center in Peking Union Medical College. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum, supplemented with penicillin/streptomycin and 2 mM glutamine. All cells were kept in a humidified 5% CO2 incubator at 37°C. For hypoxic conditions, cells were cultured in 1% O2, 5% CO2, and 94% N2 at 37°C.
Lentiviruses for pBabe-puro, pBabe-puro–wild-type KRAS, and pBabe-puro–KRAS G12V were prepared as described.33 Briefly, 1.5 μg of plasmids of interest and 1.5 μg of packing plasmids (pCMV delta 89 and VSVG) were transfected into 293 T-cells in a six-well plate. Viruses were collected at 48 hours and 72 hours after transfection. CaCo2 or HT29 cells were seeded in a six-well plate until 80% confluent and then were infected with pBabe-puro, pBabe-puro–wild-type KRAS, and pBabe-puro-KRAS G12V lentiviruses, followed by 5 μg/mL puromycin selection. Stable cell lines were confirmed by examining KRAS expression by Western blot.
Western blot
Cell lysates were harvested at indicated conditions using Laemmli lysis buffer. Samples were boiled and separated by SDS gel electrophoresis and then were transferred to PVDF membranes. Membranes were blocked with 5% nonfat milk in TBS-T (0.1% Tween 20) at room temperature for 1 hour and incubated with primary antibodies (1:1,000 dilution) at 4°C overnight. After incubation with secondary antibody (1:3,000 dilution) at room temperature for 1 hour, the membranes were then subjected to development with chemiluminescence ECL reagent (LumiGold™; SignaGen Laboratories, Rockville, MD, USA).
Cell proliferation assay (MTT)
Cell-proliferation assays were performed using Thiazolyl Blue Tetrazolium Bromide (MTT) methods. Cells were seeded in 96-well plates at a density of 5×103 cells per well. Cetuximab or PX-478 was added to cells 12 hours after being seeded. Absorbance values were measured at 12, 24, 48, and 72 hours in a microplate reader.
Real-time PCR assay
RNA was prepared using Trizol reagent (Thermo Fisher Scientific) according to instructions and quantitative reverse transcription-PCR was performed using the QuantiTect SYBR Green PCR (Qiagen NV, Venlo, the Netherlands). HIF-1α and KRAS mRNA level were determined using corresponding primers with β-actin served as a loading control.
Colony formation assay
SW480 cells were treated respectively with control, cetuximab, PX-478, and cetuximab plus PX-478 for 8 hours. Cells were counted and seeded to six-well plates, with a density of 200 cells per well. After 15 days when there were visible colonies, cells were fixed with ice-cold methanol for 10 minutes. Methanol was aspirated from the plates and enough 0.5% crystal violet solution (made in 25% methanol and stored at room temperature) was added to cover bottom of the plates. Incubation occurred at room temperature for 10 minutes. Crystal violet solution was poured off and the plates were carefully rinsed in double-distilled H2O until color no longer came off during rinsing. Plates were allowed to dry at room temperature (overnight) and then colonies were counted.
Immunohistochemistry
Clinical CRC samples were obtained from Beijing Chao-yang Hospital, Beijing, People’s Republic of China. KRAS genotype was determined by Capital Medical University, Beijing, People’s Republic of China. Samples with wild-type KRAS and KRAS G12V mutation were fixed with 4% paraformaldehyde, embedded in paraffin, and then affixed onto the slides. The slides were stained with HIF-1α antibody according to online protocol from the Abcam company website (http://www.abcam.com/ps/pdf/protocols/ihc_p.pdf).
Results
Induction of HIF-1α by KRAS G12V signaling in colon cancer cells
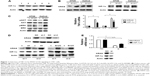
HIF-1α, the most well established form of hypoxia inducible factor, is expressed in all human cells and plays an essential role in mediating hypoxia response. HIF-1α can be regulated at both protein and transcriptional levels.20 To investigate whether hypoxia is involved in drug resistance in CRCs harboring KRAS G12V, we first compared HIF-1α protein level among five CRC cell lines, including three KRAS wild-type cell lines (HT29, CaCo2, and Colo205), one KRAS G13D mutant cell line (HCT116), and one KRAS G12V mutant cell line (SW480). As shown in Figure 1A, a higher level of HIF-1α was detected in KRAS G12V mutant cells (SW480), indicating that KRAS G12V signaling might be able to induce HIF-1α expression. To confirm this result, we overexpressed KRAS G12V in HT29 and CaCo2 cells and found HIF-1α protein increased (Figure 1B). We then examined mRNA level by real-time PCR and found that HIF-1α was upregulated by KRAS G12V signaling at transcriptional level (Figure 1B). We also analyzed the most important two signaling pathways downstream of RAS, the PI3K/AKT and MAPK pathways. Both pAKT and pERK MAPK signaling were activated moderately by KRAS G12V overexpression as indicated by increased pAKT and pERK levels (Figure 1C).
Since both HT29 and CaCo2 cells harbor wild-type KRAS, to exclude the effect of this endogenous KRAS, two siRNAs targeting 3’-UTR of KRAS were introduced into HT29 cells. Then we overexpressed wild-type KRAS and KRAS G12V individually in these KRAS-knockdown cells. While there was a slight increase of HIF-1α level in wild-type-KRAS-overexpressed cells, a significant increase was found in KRAS G12V-overexpressed cells (Figure 1D). Furthermore, we utilized the SW480 cell, which is homozygous KRAS G12V-mutated, to validate our results. When KRAS G12V was knocked down in SW480 cells, HIF-1α was downregulated at both the protein and mRNA level (Figure 1E). These data suggested hypoxia played an important role in the pathophysiology of KRAS G12V-expressing cancers.
Positive feedback regulation between HIF-1α and KRAS activation
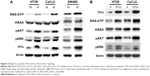
As a small GTPase, KRAS exists in two forms, the active GTP-bound form and the inactive GDP-bound form. Transition between these two activation states can be regulated by several upstream signals. Here, we intended to see whether hypoxia, which often accompanies cancer progression, would affect KRAS activity. HT29 and CaCo2 cells with KRAS G12V expression were cultured in hypoxic conditions (1% O2) or normoxic conditions. Compared with normal culture conditions, hypoxia induced elevation of GTP-bound RAS as detected by Western blot using a RAS-GTP specific antibody (Figure 2A). To provide further proof that KRAS signaling was activated by hypoxia, we checked pAKT and pERK in these cells. As indicated in Figure 2A, both pAKT and pERK level were upregulated in cells that underwent hypoxia. We then overexpressed HIF-1α in cells harboring KRAS G12V to see if HIF-1α mediated this signal activation. GTP-bound RAS, as well as the levels of its corresponding signal effectors pAKT and pERK, were all upregulated by HIF-1α expression (Figure 2B). Together, these results indicated that KRAS G12V signaling in CRC cells induced HIF-1α expression and, in turn, hypoxia or HIF-1α overexpression activated KRAS signaling to favor cancer development.
HIF-1α protein was reduced by cetuximab treatment in KRAS wild-type cells but not in KRAS mutant cells
Multiple studies have demonstrated that the use of the EGFR inhibitor cetuximab is limited to CRCs with wild-type KRAS gene since cetuximab has little effect on cancers harboring KRAS-activating mutations.21,22 The mechanism of resistance to cetuximab is currently not clear. Based on our results that hypoxia is involved in KRAS signaling regulation, we sought to determine whether hypoxia would affect drug resistance in KRAS G12V mutant cancer cells. Control and KRAS G12V-overexpressed CaCo2 cells were treated with cetuximab for 24 hours, then cell lysates were prepared for Western blot analysis. HIF-1α protein level was reduced by cetuximab treatment in KRAS wild-type cells but remained the same in cells harboring KRAS G12V mutation (Figure 3A). Consistent with this, in SW480 cells, cetuximab treatment did not decrease HIF-1α protein level (Figure 3A). The different presentation of HIF-1α after cetuximab treatment between KRAS wild-type cells and cells harboring KRAS G12V mutation strongly suggested that hypoxia might be involved in resistance to anti-EGFR therapy.
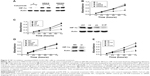
HIF-1α inhibitor sensitized KRAS G12V CRC cells to cetuximab treatment
Proliferation rates of control and KRAS G12V-overexpressed CaCo2 cells, with and without cetuximab treatment, were analyzed by MTT. Consistent with other studies, cetuximab could inhibit proliferation of cells with wild-type KRAS while having no obvious effect on cells harboring KRAS G12V mutation (Figure 3B). PX-478 (S-2-amino-3-[4′-N, N,-bis (2-chloroethyl)amino]-phenyl propionic acid N-oxide dihydrochloride), a HIF-1α inhibitor, has been shown to induce tumor cell apoptosis and decrease cell proliferation.23 One study suggested that PX-478 could enhance radiosensitivity of prostate carcinoma cells.24 Here, we examined the inhibition of PX-478 on the proliferation of CRC cells harboring KRAS G12V mutation. We found that PX-478 alone had a slight inhibitory effect while a significant inhibitory effect was found when using in combination with cetuximab (Figure 3C). We then examined HIF-1α expression by Western blot and found that combination treatment with cetuximab and PX-478 reduced HIF-1α level dramatically (Figure 3C). To further confirm these results, we knocked down HIF-1α in SW480 cells and then examined the effect of cetuximab on SW480 cell proliferation. As expected, resistance to cetuximab was observed in control SW480 cells while HIF-1α knockdown sensitized the cells to cetuximab treatment, as indicated in Figure 3D. We then tried to see if hypoxia could render cetuximab-sensitive cells resistant to cetuximab. CaCo2 cells were cultured under normoxia or hypoxia conditions for 24 hours before being treated with cetuximab. Proliferation rate was examined and we found that hypoxia promoted CaCo2 cell proliferation, and cells under hypoxic conditions were less responsive to cetuximab compared with those under normoxia (Figure 3E). All of these data illustrated that hypoxia played an important role in resistance to Cetuximab in KRAS G12V cells.
Combination of HIF-1α inhibitor and anti-EGFR therapy effectively inhibits the reproductive ability of CRC cells
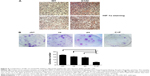
To see if the regulation between KRAS G12V and HIF-1α was true in vivo, we collected colon-cancer patient samples for immunohistochemistry assay. Compared with patients carrying wild-type KRAS, the samples from patients with KRAS G12V had a much higher level of HIF-1α staining (Figure 4A). This result strongly indicated that CRC patients carrying KRAS G12V mutation might benefit from combination treatment with cetuximab and HIF-1α inhibitors. To see a long-term effect of combination cetuximab and PX-478, we performed colony formation assay. SW480 cells were treated with cetuximab, PX-478, or cetuximab together with PX-478. After 8 hours of treatment, cells were seeded in a six-well plate for colony formation assay. The colonies formed were counted and pictured 15 days later. Although the cells were resistant to cetuximab or PX-478 treatment individually, they were sensitive to the combination of cetuximab and PX-478 (Figure 4B). These data suggest that combination of HIF-1α inhibitor and EGFR inhibitor might be necessary for patients carrying KRAS G12V mutation.
Discussion
KRAS, a member of the RAS superfamily, is a plasma membrane-anchored GTP-/GDP-binding protein and is expressed in most human cells. Normally, KRAS switches between the active, GTP-bound state and the inactive, GDP-bound state in response to upstream signals.25 KRAS mutations are usually point mutations that result in activation of downstream signaling pathways.26 EGFR is found to be an important player in CRC initiation and progression and has therefore become a key therapeutic target to treat CRC patients. KRAS is involved in several intracellular signal transductions but is mainly responsible for EGFR signaling.27 Thus, activating KRAS mutations have been recognized as a strong predictor of resistance to anti-EGFR therapy. However, the mechanisms involved remain largely unknown. Finding out the biomarkers for prediction of EGFR-targeted therapy in CRC would have great significance for treatment decisions and clinical outcome. In this study, we designed experiments to see whether cancer hypoxia microenvironment affected resistance to EGFR inhibitors in KRAS G12V-mutant cells. We found that KRAS G12V signaling in colon cancer cell lines increases HIF-1α at transcription level. Moreover, HIF-1α or hypoxic condition could in turn upregulate KRAS activity and activation of its downstream signaling. Thus, a positive feedback regulation between KRAS activation and hypoxia is formed. We speculated that these two signals worked together to promote cell proliferation and enable cancer cells to escape from EGFR-inhibitor treatment. One study showed that hypoxic conditions could increase the activity of wild-type KRAS but not that of mutant KRAS.28 In our case, both HIF-1α expression and hypoxic conditions led to increased activation of KRAS G12V mutant as indicated by more presence of GTP-KRAS. The different conclusions that we obtained are probably due to the cells being treated with hypoxia for different times. In our study, we incubated cells under hypoxic conditions for at least 24 hours while in the other study incubation was for 4 hours.28 The increase of KRAS G12V activity by hypoxia might be an adapted process in which a certain time period was required.
Activation of EGFR downstream signals, such as MAPK, PI3K/AKT pathway, and the Jak2/STAT3 pathway,29 lead to induction of HIF-1α. Expressions of HIF-1α target genes lead to cell proliferation and resistance to apoptosis. Indeed, we found HIF-1α level was dramatically induced by KRAS G12V expression while slightly induced by wild-type KRAS expression. HIF-1α level was reduced by cetuximab treatment in cells harboring wild-type KRAS cells but not in cells with KRAS G12V mutation. Consistent with this, proliferation of cells harboring KRAS G12V could be inhibited by combination treatment with cetuximab and PX-478 (an inhibitor of HIF-1α) effectively, but not by either of them when used individually.
It has been suggested that PX-478, as a small molecular drug, has potent antitumor activity against a variety of human solid tumors.30,31 Also, a study by Jacoby et al demonstrated that addition of the HIF-1α inhibitor PX-478 enhanced the therapeutic efficacy of EGFR inhibitors in an orthotopic human lung adenocarcinoma model,32 but no indication of this combination therapy in CRC has been reported so far. Clinical CRC samples from patients carrying wild-type KRAS and KRAS G12V mutant were analyzed by immunohistochemistry in our study. We found a significantly higher level of HIF-1α expression in samples with KRAS G12V mutation than that with wild-type KRAS. This result, together with other data from cell culture, suggests that HIF-1α inhibitors might be effective to treat CRC patients carrying KRAS G12V mutation. To this end, we performed colony formation experiments using SW480 cells (KRAS G12V mutation). We found that although no inhibitory effect was observed when used individually, combined treatment with PX-478 and cetuximab dramatically reduced colony formation.
All of the data here strongly indicate that hypoxia or high level of HIF-1α was responsible for cetuximab resistance in CRC patients carrying KRAS G12V mutation. Combination treatment with HIF inhibitors could be an effective strategy to overcome the relative resistance to EGFR inhibition. Further studies in animal models are still needed to confirm this therapeutic strategy.
Acknowledgments
We thank Dr Zhang from Capital Medical University for providing KRAS genotype information of CRC patients’ samples. This work was supported by National Natural Science Foundation of China (No 30872490; 81172320) and by Dr Jieping Wu Medical Foundation (No 32067501207).
Disclosure
The authors report no conflicts of interest in this study.
References
Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. | ||
Hawley ST, Chang S, Risser D, Zhang Q. Colorectal cancer incidence and mortality in Texas 1990–1992: a comparison of rural classifications. J Rural Health. 2002;18(4):536–546. | ||
Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7(9):493–507. | ||
Maseki S, Ijichi K, Nakanishi H, Hasegawa Y, Ogawa T, Murakami S. Efficacy of gemcitabine and cetuximab combination treatment in head and neck squamous cell carcinoma. Mol Clin Oncol. 2013;1(5): 918–924. | ||
Fensterer H, Schade-Brittinger C, Müller HH, et al. Multicenter phase II trial to investigate safety and efficacy of gemcitabine combined with cetuximab as adjuvant therapy in pancreatic cancer (ATIP). Ann Oncol. 2013;24(10):2576–2581. | ||
Kullmann F, Hartmann A, Stöhr R, et al. KRAS mutation in metastatic pancreatic ductal adenocarcinoma: results of a multicenter phase II study evaluating efficacy of cetuximab plus gemcitabine/oxaliplatin (GEMOXCET) in first-line therapy. Oncology. 2011;81(1):3–8. | ||
Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–3995. | ||
Siddiqui AD, Piperdi B. KRAS mutation in colon cancer: a marker of resistance to EGFR-I therapy. Ann Surg Oncol. 2010;17(4):1168–1176. | ||
Monzon FA, Ogino S, Hammond ME, Halling KC, Bloom KJ, Nikiforova MN. The role of KRAS mutation testing in the management of patients with metastatic colorectal cancer. Arch Pathol Lab Med. 2009;133(10):1600–1606. | ||
Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J Gastroenterol. 2012;18(37):5171–5180. | ||
Céspedes MV, Sancho FJ, Guerrero S, et al. K-ras Asp12 mutant neither interacts with Raf, nor signals through Erk and is less tumorigenic than K-ras Val12. Carcinogenesis. 2006;27(11):2190–2200. | ||
Kumar SS, Price TJ, Mohyieldin O, Borg M, Townsend A, Hardingham JE. KRAS G13D Mutation and Sensitivity to Cetuximab or Panitumumab in a Colorectal Cancer Cell Line Model. Gastrointest Cancer Res. 2014;7(1):23–26. | ||
Ihle NT, Byers LA, Kim ES, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. 2012;104(3):228–239. | ||
Berg M, Soreide K. EGFR and downstream genetic alterations in KRAS/BRAF and PI3K/AKT pathways in colorectal cancer: implications for targeted therapy. Discov Med. 2012;14(76):207–214. | ||
Wee S, Jagani Z, Xiang KX, et al. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69(10):4286–4293. | ||
Brown JM. Tumor hypoxia, drug resistance, and metastases. J Natl Cancer Inst. 1990;82(5):338–339. | ||
Minard FN, Grant DS. Hypothermia as a mechanism for drug-induced resistance to hypoxia. Biochem Pharmacol. 1982;31(7):1197–1203. | ||
Sakata K, Kwok TT, Murphy BJ, Laderoute KR, Gordon GR, Sutherland RM. Hypoxia-induced drug resistance: comparison to P-glycoprotein-associated drug resistance. Br J Cancer. 1991;64(5):809–814. | ||
Santoni M, Pantano F, Amantini C, et al. Emerging strategies to overcome the resistance to current mTOR inhibitors in renal cell carcinoma. Biochim Biophys Acta. 2014;1845(2):221–231. | ||
Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19(16):4298–4309. | ||
Liu X, Jakubowski M, Hunt JL. KRAS gene mutation in colorectal cancer is correlated with increased proliferation and spontaneous apoptosis. Am J Clin Pathol. 2011;135(2):245–252. | ||
Shaib W, Mahajan R, El-Rayes B. Markers of resistance to anti-EGFR therapy in colorectal cancer. J Gastrointest Oncol. 2013;4(3): 308–318. | ||
Ruzzo A, Graziano F, Canestrari E, Magnani M. Molecular predictors of efficacy to anti-EGFR agents in colorectal cancer patients. Curr Cancer Drug Targets. 2010;10(1):68–79. | ||
Jacoby JJ, Erez B, Korshunova MV, et al. Treatment with HIF-1alpha antagonist PX-478 inhibits progression and spread of orthotopic human small cell lung cancer and lung adenocarcinoma in mice. J Thorac Oncol. 2010;5(7):940–949. | ||
Palayoor ST, Mitchell JB, Cerna D, Degraff W, John-Aryankalayil M, Coleman CN. PX-478, an inhibitor of hypoxia-inducible factor-1alpha, enhances radiosensitivity of prostate carcinoma cells. Int J Cancer. 2008;123(10):2430–2437. | ||
Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–551. | ||
Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;7;101(19):1308–1324. | ||
Zeng M, Kikuchi H, Pino MS, Chung DC. Hypoxia activates the K-ras proto-oncogene to stimulate angiogenesis and inhibit apoptosis in colon cancer cells. PLoS One. 2010;5(6):e10966. | ||
Mills CN, Joshi SS, Niles RM. Expression and function of hypoxia inducible factor-1 alpha in human melanoma under non-hypoxic conditions. Mol Cancer. 2009;8:104. | ||
Koh MY, Spivak-Kroizman T, Venturini S, et al. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2008;7(1):90–100. | ||
Lee K, Kim HM. A novel approach to cancer therapy using PX-478 as a HIF-1α inhibitor. Arch Pharm Res. 2011;34(10):1583–1585. | ||
Jacoby JJ, Erez B, Takahashi O, et al. Abstract 3632: Addition of the HIF-1α inhibitor PX-478 enhances the therapeutic efficacy of EGFR inhibitors in an orthotopic human lung adenocarcinoma model. Cancer Res. 2010;70:3632. | ||
Ma J, Meng Y, Kwiatkowski DJ, et al. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J Clin Invest. 2010;120(1):103–114. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.