Back to Journals » Hepatic Medicine: Evidence and Research » Volume 11
Portosystemic shunts and refractory hepatic encephalopathy: patient selection and current options
Authors Philips CA , Rajesh S , Augustine P, Padsalgi G, Ahamed R
Received 18 October 2018
Accepted for publication 27 December 2018
Published 25 January 2019 Volume 2019:11 Pages 23—34
DOI https://doi.org/10.2147/HMER.S169024
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Gerry Lake-Bakaar
Video abstract presented by Cyriac Abby Philips and Sasidharan Rajesh.
Views: 2167
Cyriac Abby Philips, Sasidharan Rajesh, Philip Augustine, Guruprasad Padsalgi, Rizwan Ahamed
The Liver Unit, Cochin Gastroenterology Group, Ernakulam Medical Centre, Cochin, Kerala, India
Abstract: Portosystemic shunt (PS) syndrome encompasses a spectrum of disease manifestations ranging from asymptomatic portal hypertension to recurrent and refractory hepatic encephalopathy, ultimately culminating in progressive hepatic failure in patients of cirrhosis and associated large PSs. PSs commonly seen in cirrhosis include splenorenal, gastrorenal, and dilated paraumbilical veins, all of which can present with recurrent or refractory hepatic encephalopathy. In this exhaustive review, we describe the anatomy of PSs, elucidate new theories on their pathophysiology, discuss the clinical implications of PSs in cirrhosis, provide details on different techniques (classical and novel) of shunt embolization, and explore all the pertinent current literature on shunt embolization for refractory and recurrent hepatic encephalopathy, all of which are enumerated with extensive images and illustrations.
Keywords: portosystemic shunts, hepatic encephalopathy, cirrhosis, portal hypertension, embolization, shunt occlusion
Introduction
Chronic liver injury leads to replacement or encapsulation of injured tissue by collagenous scar, leading to the formation of regenerative nodules. This causes an increase in liver stiffness and increases the intrahepatic resistance to portal blood flow, leading to diversion of blood away from the liver (hepatofugal flow) toward low-resistance portosystemic vessels (embryonic channels that recanalize to decompress the portal system). Portal circulatory changes occur when the portal pressure is at least 5 mmHg above the inferior vena cava pressure – a condition called portal hypertension, coined by Gilbert and Villaret in 1906.1 Portal hypertensive collateral formation leads to “varices” that are dilated end-organ veins with a high risk of rupture, as well as “shunts” that are distended vascular collaterals that connect the portal and systemic vasculature. The common sites of end-organ collateral-variceal formation include esophageal, paraesophageal, gastroesophageal, cardiophrenic, and coronary regions, and other areas such as a paraumbilical, perisplenic, anterior abdominal wall, omental, mesenteric, peritoneal, pleural, and anal canal. Portosystemic shunts (PSs) grow by the degree of portal hypertension, and large shunts (defined as those with a diameter ≥8 mm) can clinically lead to “PS syndrome”. The spontaneous large PSs (SPS) include gastrorenal, spleno (or lieno) renal, mesocaval, mesogonadal, mesenterorenal, and splenogastrorenal varieties, of which splenorenal shunts are the most commonly noted on imaging and in patients with PS syndrome. SPS can be left and right (or central) sided. Left-sided shunts are seen on the left of midline or left of the confluence of the splenic and mesenteric veins, of which the gastrorenal shunt is the most common, which is seen in 80% of patients with gastric varices, but in only 10% of patients with portal hypertension. The spontaneous splenorenal shunt is a true anatomical shunt which is the direct communication between the splenic vein and the left renal vein without involving the gastrointestinal system with variceal formation. Such a shunt is, in the true sense, a prototype that can lead to PS syndrome.2
PSs in cirrhosis
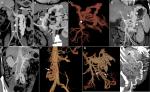
Ruysch and Schmiedeal first observed portosystemic connections in the years 1738 and 1744, respectively. They demonstrated vascular connections between inferior vena cava and the mesenteric system through injection venography. The portosystemic channels are fine vessels that existed before the development of portal hypertension, and their extent and growth are significantly proportional to the severity of portal hypertension.3,4 Portosystemic circulation was found to develop through two major processes: one, through a previously patent vasculature with end-organ veins appearing dilated and tortuous leading to variceal formation and two, in the event of repermeabilization of the embryonic vascular channels.5 Anatomically, common shunts seen in cirrhosis patients include direct splenorenal and indirect splenorenal (also called gastrosplenorenal or gastrorenal) types. The direct splenorenal shunts are seen in the left subphrenic compartment, has a portal radicular origin, and terminate within the left renal vein. The gastrorenal shunts or indirect splenorenal shunt is formed by either the superior polar veins of Pigache and Worms that form the uppermost of the short vessels of spleen, which drains the posterior and left gastric fundus anastomosing with the inferior phrenic vein, or the posterior gastric vein of Rio Branco that transverses the posterior aspect of the stomach draining into the splenic vein.6 The different variations of the direct splenorenal shunt are shown in Figure 1A–C and those of the indirect type or gastrorenal shunt in Figure 1D. The commonest portosystemic collateral noted in patients with cirrhosis and portal hypertension is the left gastric vein (or the coronary vein) which has anterior and posterior branches that supply esophageal or paraesophageal varices: the left gastric vein in itself or the posterior branch, when prominent, can function as a shunt leading to hepatic encephalopathy (HE) other than its role in variceal bleeding. Rarely localized PSs in cirrhosis include mesocaval (Figure 1E, F), mesorenal (Figure 1G), recanalized paraumbilical vein (PUV; Figure 1H), mesoadrenogonadal, and splenoadrenorenal shunts.
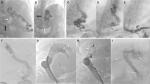
Kim and Lee suggested the “electric circuit” theory based on Ohm’s law as the pathophysiology of PS formation and deduced treatment options and outcomes based on the same. The authors proposed classifying PS according to the two distinct underlying mechanisms – the increase in portal venous pressure (PVP) and the decrease in shunt resistance (SR). In the normal state, SR is sufficiently high, due to which shunt flow is negligible. Since the portal pressure is directly proportional to portal venous resistance, in liver cirrhosis, when the portal pressure increases, the pressure difference across the shunt increases. By Ohm’s law, the shunt flow is defined as PVP/SR. When the PVP becomes sufficiently high, the shunt flow can become greater than zero, resulting in SPS formation. The other way for shunt flow to increase is for SR to decrease at a fixed PVP, as seen with aneurysmal dilatation of the collateral channel. When the SR decreases, the PVP also decreases because of the resistance of intrahepatic portal channels and of the shunt as in a “parallel” circuit. The portal venous flow decreases consequently, resulting in portal flow bypassing the liver. The authors reviewed the management results of shunt occlusion in 49 cases in the literature and found that in only ten cases, the morphologic evolution of the PS was identified. PS disappeared or collapsed in seven cases, while in three, it persisted or thrombosed post-procedure. They also found that, there was no case in which PSS persisted after inflow occlusion, while there were two cases in which PS had only “collapsed” after outflow occlusion (Figure 2A, B). This latter detail is important when it comes to recanalization of large SPS years after occlusion in patients with refractory encephalopathy. As per the electric circuit model, it was suggested that the PS be classified into portal hypertensive (increase in PVP) and spontaneous (decrease in SR) types. The PS circuit model (Figure 3) also suggests that when blocking a portal hypertensive type PS, the outflow should not be occluded because further portal pressure increments can worsen portal hypertension and related events, as seen in patients with higher grades of liver disease severity.7
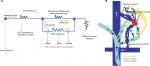  | Figure 2 Circuit theory and hemodynamics of portosystemic shunts. Notes: (A) The circuit theory of portosystemic shunt formation. (B) Demonstration of the shunt hemodynamics and the potential sites of occlusion. Adapted from Kim M, Lee K-Y. Understanding the pathophysiology of portosystemic shunt by simulation using an electric circuit. Biomed Res Int. 2016;2016(81):ID 2097363.7 Copyright © 2016 Moonhwan Kim and Keon-Young Lee. Creative Commons license available at: https://creativecommons.org/licenses/by/4.0/legalcode. Abbreviations: IVC, inferior vena cava; LGV, left gastric (or coronary) vein; LRV, left renal vein; PGV, posterior gastric vein; PV, portal vein; SGV, short gastric veins; SMV, superior mesenteric vein; SV, splenic vein. |
Clinical relevance of PSs in cirrhosis
Lam et al were the first to initially examine the effect of large PS on the incidence of variceal bleeding and HE. The authors concluded that large PSs did not protect against variceal bleeding and were associated with an increased risk for spontaneous HE.8 It was demonstrated that the occurrence of hepatocellular carcinoma was higher in patients with large splenorenal shunts than in those without them, higher body mass index predicted the development of large PS, and large esophageal varices were detected less frequently in patients with large PS. The authors of the study also found that the prevalence of ascites was similar in patients with and without large splenorenal shunts.9 It was shown that the prevalence of HE was higher and that of acute variceal bleeding lower in patients with large PS. However, patients with large PS had significantly higher Child–Pugh and model for end-stage liver disease (MELD) scores with similar in-hospital mortality.10 Riggio et al showed that presence of large PS in patients with cirrhosis was associated with chronic HE (continuous cognitive defects) as well as bouts of overt HE (recurrent HE, at least two episodes in 1 year requiring hospital admission) leading to poor quality of life.11 The opacification of large spontaneous PS before the creation of transjugular intrahepatic PS (TIPS) was shown to be associated with an increased risk of early and severe complications. It was proposed that the embolization of opacified large PS during procedure could reduce post-TIPS complications.12 The presence of large spontaneous PS was associated significantly with moderate and severe portopulmonary hypertension and a lack of response to medical treatment in portopulmonary hypertension.13 In a recent study by Simón-Talero et al, patients with large PS developed episodic HE more frequently than patients with small PSs. Patients with large PS had a higher prevalence of persistent and chronic HE. The most common shunts noted in their study were splenorenal and paraumbilical types. The presence and size of PS increased with higher grades of liver disease and with the severity of portal hypertension. The presence of PS had a significantly higher risk of ascites and variceal bleeding with lower transplant-free survival. Interestingly, this was found to be significant in the subset of patients with MELD score 6–9 or Child–Pugh class A. The authors also found that in patients with clinically significant portal hypertension with MELD score of <10, the presence of large PS greatly increased decompensations compared to that in those without large PS (68% vs 44%). Additionally, the authors also noted that overt as well as chronic HE occurred among patients with low MELD scores (≤15) without precipitating factors on optimal ammonia-lowering therapies.14 Multiple studies have shown that gastric varices were more commonly associated with gastrorenal shunts. Saks et al, in their retrospective study, showed that patients with lienorenal PS were more likely to have gastroesophageal varices on imaging without significant differences in the incidence of variceal bleeding. However, portal hypertension-related hemorrhage occurred in 46% of patients with any shunt (large or small) vs no shunt. These data may point toward an important clinical relevance that favors PS as a surrogate for severe and worsening portal hypertension.15 The presence of ascites was less likely to occur in patients with cirrhosis and large PS and HE in a retrospective study.11 However, the recent study demonstrated that ascites was more common in patients with large or small PS.14 It is important to note that in the spectrum of PS syndrome, ascites heralds end-stage liver disease in the presence of progressive hepatic parenchymal decline and portal vein thrombosis. The short- and long-term benefits of shunt embolization of large PS in patients other than those with refractory or recurrent HE are lacking. Current literature, however, supports the utility of shunt embolization of large PS in patients with HE associated with PS syndrome and provides clinical insights into adequate patient selection and associated outcomes post-embolization.
PS syndrome
Portal hypertension forces portal blood toward portosystemic collaterals of low resistance. When this flow occurs through very low resistance but large-caliber PSs, effective liver detoxification coupled with hepatic impairment in the wake of decreased blood flow to the liver leads to systemic accumulation of inflammatory and neurotoxic agents that cause cognitive and psychomotor disturbances in the patient, which further progress to complications of liver failure and end-stage liver disease. This spectrum of asymptomatic severe portal hypertension at one end that progresses to end-stage liver disease (reduction in hepatic reserve over time) in the presence of large PS is termed the PS syndrome, a term coined by Kumamoto et al in 2010.16 This observation was later modified to characterize asymptomatic patients with large SPS who present with recurrent HE progressing to persistent HE punctuated with bouts of overt HE and associated diminishing liver function and reduction in portal blood flow (portal vein thrombosis/hepatic parenchymal decline). HE can be broadly classified into two forms with regards to the mechanism of HE: the encephalopathy related to the hepatic failure, due to synthetic dysfunction as in late stages of cirrhosis or in a critically ill patient with cirrhosis, or in a noncirrhotic patient as seen in acute liver failure, and encephalopathy related to PSs (iatrogenic such as TIPS or SPS). The incidence of SPS in patients with HE varies from 46% to 71% in published series, and most patients with recurrent or persistent HE become refractory to medical therapy. HE due to SPS, early in its course, is associated with a preserved liver function with relatively low MELD score, thereby excluding priority listing for timely liver transplantation. The Saad staging system of PSS clearly defines this progression in three stages. In the first stage, (Saad-A), the patient remains asymptomatic from cirrhosis and PS point of view. In the next stage (Saad-B), overt episodes of HE manifest. In the final stage (Saad-C), as the liver disease rogresses, both hepatic failure and shunting lead to persistent or recurrent HE with evidence of portal vein thrombosis or jaundice. It is in the Saad-B stage that shunt embolization proves beneficial, whereas in Saad-C, liver transplantation becomes the ideal treatment of choice.17
Shunt embolization for HE: techniques and considerations
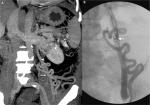
HE in the absence of precipitating factors or bouts of HE in patients with cirrhosis with persistent cognitive deficits (after exclusion of other neurological diseases) and a low MELD score should warrant evaluation for large spontaneous PS. The diagnosis is made by performing contrast-enhanced computed tomography imaging or magnetic resonance imaging/portography. Large shunts, defined as those of diameter ≥8 mm, are amenable to shunt embolization through a variety of techniques.14 Initial embolization procedures were done by balloon-assisted retrograde transvenous occlusion (BRTO). BRTO (Figure 4A–C) is a well-established procedure for treatment of gastric variceal bleeding that is at present frequently utilized for the management of shunt-related HE. BRTO (via the transfemoral or transjugular approach) embolizes the shunt outflow by occluding the shunt per se with an occlusion balloon, followed by injection of a sclerosing agent (most commonly used, sodium tetradecyl sulfate foam with or without lipiodol and air or carbon dioxide mixture; earlier utilized ethanolamine oleate not favored due to hemolysis, hemoglobinuria, and renal failure) directly into the variceal complex by an endovascular approach. The indwelling balloon acts as the hemostatic unit within the shunt and prevents the leak of sclerosant into the systemic circulation. BRTO is technically most suitable for splenorenal or gastrosplenorenal shunt (Figure 3A, B), wherein the shunt is catheterized through the left renal vein. It is less useful and more technically demanding for other types of PSs. In BRTO, the balloon occlusion inflation is maintained from 6 hours to sometimes up to 20 hours and is removed only after the sclerosant stagnation is confirmed on abdominal radiography. The long procedural timing and monitoring, balloon rupture, and sclerosant embolism are some of the major concerns associated with the BRTO technique, which have led to several modifications of the BRTO for improving the technical concerns and patient safety.18,19 Coil-assisted retrograde transvenous obliteration (CARTO) procedure was introduced by Lee et al, in which coils and gelfoam slurry replace indwelling balloons and vascular plugs (plug-assisted retrograde transvenous occlusion [PARTO]). This technique is useful in conditions where the shunt size, angulation, and tortuosity exclude BRTO and PARTO utilization. The approximate average time to procedure completion in BRTO, PARTO, and CARTO would be 8–10 hours, 2–3 hours, and from 30 minutes to 1 hour, respectively, as per multiple studies in the literature. However, CARTO can prove to be more expensive with the use of a larger number of coils and detachable coils. There are two technical types of CARTO. In one type, multiple coils are deployed into the narrowest portion (improper deployment can lead to displacement of the coil into the pulmonary vasculature or the heart) of the shunt followed by gelfoam and sclerosant mix injection (Figure 3C–E) and in the other type, standard BRTO procedure is performed, followed by coil embolization (performed through the occlusion balloon) of the large shunt.20 Using a permanent vascular plug, Gwon et al described a modified BRTO approach, typically through the transfemoral route (only in acute angulations of the gastrorenal shunt, the internal jugular route is used) that is performed, at most times, under short moderate sedation to reduce the procedure time and post-procedure monitoring during shunt embolization. The PARTO technique eliminates the complications associated with the indwelling balloon catheters and excludes the use of sclerosing agents. Using a 10F sheath at all times makes it possible to leave the guidewire and the microcatheter in the varices before plug deployment. A smaller size sheath would warrant removal of the guidewire and catheter before plug insertion and deployment, making the process tedious. The vascular plug size is decided upon based on the diameter of the narrowest part of the shunt measured on contrast imaging, and a plug ~30% larger than the size of shunt needs to be used to prevent migration and sclerosant reflux. Shunts that lack a narrowed “neck” area and in which such areas cannot be mapped on imaging cannot be embolized using this technique. Very large shunts, ≥25 mm in size, cannot be embolized with PARTO due to restricted availability of larger diameter plugs. Additionally, large collaterals can be embolized using coils or hand cut gelatin sponge and contrast media mix (gelfoam slurry). A follow-up abdominal radiograph is pertinent the following day to ensure complete filling and sclerosant retention within the shunt and collaterals.21–23 Balloon-occluded antegrade transvenous obliteration (BATO) uses a portal venous approach to embolize a variety of shunts other than gastrorenal and splenorenal shunts. In literature, this technique is most commonly used to obliterate gastric varices in the absence of a gastrorenal shunt. In the presence of multiple afferent veins, the largest afferent may be tackled using a balloon occlusion catheter, while the smaller ones are embolized using coils or a vascular plug. Sometimes, combining BRTO with BATO increases technical success and improves shunt and collateral obliteration in select patients. At our center, BATO is used for the obliteration of recanalized PUV and prominent large coronary gastric veins in patients with recurrent HE.
We have also performed two novel techniques of shunt embolization, specifically for large dilated recanalized PUV. In the first approach, when transhepatic access to PUV through an attenuated portal vein is difficult, direct puncture of the PUV (lying within the anterior abdominal wall) through the anterior abdominal wall (provides scaffolding for hemostasis/retrograde approach) and occluding it using a vascular plug (direct-plug-assisted retrograde transvenous obliteration; Figure 3F–H) has been performed with 100% technical success. Alternatively, in the second approach for embolization, the PUV (when present at the posterior-most aspect of the abdominal wall with extension into the abdominal cavity) is accessed by a percutaneous transhepatic approach through the right portal vein; antegrade occlusion of the dilated collateral is performed using coils (coil-assisted antegrade transvenous obliteration; Figure 3I) and stasis of contrast within the PUV is confirmed with reflux of contrast into the left portal vein.
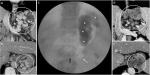
Initial studies on PARTO treatment of gastric varices showed a higher recurrence rate of collaterals when compared to BRTO due to recanalization through gelfoam (permanent endothelial destruction and thrombosis is lower with gelfoam compared to sclerosing agents). In such a scenario, a double embolization technique (Figure 5A, B) using gelfoam slurry as well as sclerosant injection (the latter done cautiously so as not to displace the recently introduced gelfoam) is more effective. Patil et al performed a systematic review and meta-analysis of six studies that utilized embolization of large PS for medically refractory HE in patients with cirrhosis with Child–Pugh A class and MELD score <15. In their study, splenorenal shunts were predominant and 90% of the procedures were technically successful. Amelioration of HE was seen in pooled percentage of 76.2%. New onset variceal disease was noted in 6% and new onset or worsening ascites in 14%. The authors stated that PS embolization was safe, associated with minimal complications in those with good functional liver reserve.24 Modified BRTO procedures have a slightly higher recurrence rate of shunt recanalization and collateral reformation than standard BRTO (Figure 6A–E). Incomplete obliteration of all collateral vessels in CARTO/PARTO can lead to worsening of missed or existing collateral vessels, especially the esophageal varices, leading to bleeding events in the intermediate and long term. Selection of the technique is completely dependent on the expertise of the interventional radiologist, availability of technical staff, patient affordability, hepatic reserve, and more importantly, an apt collateral vascular anatomy. At times, a combination approach would be more efficacious than a single standard approach and can only be decided on intraprocedure. In literature, the most common adverse event was worsening esophageal varices, which ranged from 19% to 46%. Studies on comparisons between BRTO, CARTO, and PARTO or BATO for PS-related HE are lacking in the current literature.25,26
Shunt embolization for HE: patient selection and outcomes
The various technical success rates of shunt embolization are reportedly as high as 78.7%–100%, while complete obliteration of gastric varices and improvement in HE have been reported in 79.6%–100% and 100%, respectively. It was demonstrated that the portal venous blood flow increased significantly at 1 week and 3 months after shunt occlusion in cirrhosis patients with Child–Pugh A and B status. The hepatic arterial blood flow became significantly lower than the baseline 1 month after the procedure, while no difference was noted in total liver blood flow at all study times after shunt embolization. Improvement in the synthetic function of the liver evidenced by increase in the serum albumin levels was also an interesting finding.27 Multiple studies on shunt occlusion for refractory HE have been published in literature. The key studies and their salient features are shown in Table 1. In 1997, Sakurabayashi et al demonstrated for the first time that successful shunt embolization abolished overt HE in cirrhosis.28 The largest series in this regard was a retrospective multicenter study in 37 patients. Approximately 60% of the patients had overt HE-free survival at 3 months, while 50% did not suffer from overt HE at 2 years of follow-up. In patients with MELD >11 at baseline, the recurrence of HE was found to be higher. A single-center series in 15 patients suggested that a MELD of 15 be used as the cut-off for identification of potential candidates for shunt emobilization, in contrast to the previous study. Both these studies identified patients who would have a recurrence of HE after the procedure, but not high-risk patients who would worsen after the procedure.29,30 A study in 14 patients showed that 93% of patients had long-term absence of overt HE at 27 months of follow-up.31 It was reported from Korea that post-shunt embolization, overt HE was absent in 60% of patients at 2 years of follow-up. The authors also found that among patients with MELD <15 without hepatocellular carcinoma, the overall survival rate was better in patients undergoing shunt embolization.32 A single-center study in 20 patients from the USA showed that benefit was achieved in 100% (18/18) and 92% (11/12) at 1–4 and 6–12 months, respectively. The majority of patients (67%) were free from HE-related hospitalizations over 1 year. Ten percent of patients developed procedure-related complications that were self-limiting. Thirty percent of patients developed new or worsening ascites that was controlled with diuretics.33 The largest single-center study on shunt embolization in patients with recurrent and persistent HE was published by our group from India. Twenty-one patients (Child–Pugh score, 6–13) with mean MELD-sodium score 19.3, with medically refractory recurrent or persistent HE, including three patients with cirrhosis-related Parkinsonism (CP), underwent occlusion of a total of 29 shunts (1 surgical, 20 nonsurgical). Some patients with cirrhosis with HE have extrapyramidal and cerebellar symptoms that progress to develop into a progressive bradykinetic-rigidity syndrome referred to as “cirrhosis-related Parkinsonism”. However, atypical features such as progressive ataxia, dystonia, choreoathetosis, or spastic paraparesis may also be noted with a slow progressive decline in cognitive dysfunction. This rare but difficult to treat chronic progressive form of HE is frequently associated with the presence of large PS.34 In our study, recurrent and persistent HE and CP markedly improved in the short (3 months), intermediate (6 months), and long (9 months) periods of follow-up. None of the patients developed spontaneous or persistent HE at a median follow-up of 105 (30–329) days (P<0.05). Our group was the first to demonstrate the benefit of shunt embolization in patients with CP. All patients survived on a median follow-up of 193 (42–329) days. Post-procedure, the Hoehn and Yahr grade (for Parkinson disease) improved significantly in 71.4% and the parkinsonian features resolved completely in 28.5% of patients. Interestingly, we also found that Child–Pugh score >11 predicted mortality post-shunt occlusion, and hence, such patients need to be excluded from shunt embolization for recurrent or persistent HE and be listed for liver transplantation as the treatment of choice.35 Even though there is strong evidence of the benefits of post-shunt embolization for recurrent or persistent HE in patients with cirrhosis, related procedural complications do occur and worsening of portal hypertension is seen in a small subset of patients. Treatable complications include new onset or worsening ascites, while life-threatening uncontrolled acute esophageal variceal bleeding, hemoperitoneum, capsular bleeding, and multiple organ failures do occur in some patients. As per our protocol, post-shunt embolization, all patients undergo upper gastrointestinal endoscopy to look for worsening esophageal varices at 6 months and after that annually and undergo primary endoscopic band ligation for high-risk varices thereafter until eradication. The hepatocellular carcinoma surveillance is performed every 6 months, while a repeat contrast or magnetic resonance imaging of the abdomen for development of new shunts is performed at 12 months post-embolization.
Conclusion
Embolization of large PSs (diameter ≥8 mm) in carefully selected cirrhosis patients suffering from recurrent or persistent HE with Child–Pugh score <11 improves related liver outcomes and abolishes the risk of overt HE in the long term. Additionally, MELD score >11 at baseline predicts recurrence of HE after shunt embolization. However, MELD score alone is not the true predictor of such recurrences and the shunt anatomy, the functional shunt physiology of inflow and outflow dynamics, and the choice of embolization technique also play an important part in recanalization and recurrence of shunt postembolization. BRTO, CARTO, PARTO, and BATO are well-established techniques initially described for variceal bleeding that are increasingly utilized for shunt embolization in chronic HE, and the choice of technique depends on the expertise of the interventional radiologist and conducive collateral anatomy. Since inflow and outflow hemodynamics in the shunt complex affects the outcomes after shunt embolization, larger studies describing the same with antegrade and retrograde techniques are an unmet need. It is important to classify cirrhosis patients into Saad stages for clinical decision making on shunt embolization to either improve quality of life, increase transplant-free survival, or list for liver transplantation to increase survival.
Disclosure
The authors report no conflicts of interest in this work.
References
Gilbert A, Villaret M. Contribution al’etude Du syndrome d’hypertension portale. Cytologie des liquides d’ascite dans less cirrhoses. Comptes Rendus Soc Biol. 1906;60:820–823. | ||
Philips CA, Arora A, Shetty R, Kasana V. A comprehensive review of portosystemic collaterals in cirrhosis: historical aspects, anatomy, and classifications. Int J Hepatol. 2016;2016(2):1–15. | ||
Ruysch F. Curae posteriores, seu thesaurus anatomicus omnium praecedentium maximus. Jaussonio-Waesbergios, Amsterdam; 1738. Latin. | ||
Schmiedel G. De varietatibus vasorum magni pleurumque momenti. Germany: Vitembergae, Literis Jo. Gothofr. Meyeri; 1744. Latin. | ||
Retzius. Bemerkungen über anastomosen zwischen der perfortader und der unteren hohlader ausserhalb der leber. Physiol Chem. 1835;5:105. | ||
Wind P, Alves A, Chevallier JM, et al. Anatomy of spontaneous splenorenal and gastrorenal venous anastomoses. Review of the literature. Surg Radiol Anat. 1998;20(2):129–134. | ||
Kim M, Lee K-Y. Understanding the pathophysiology of portosystemic shunt by simulation using an electric circuit. Biomed Res Int. 2016;2016(81):ID 2097363. | ||
Lam KC, Juttner HU, Reynolds TB. Spontaneous portosystemic shunt: relationship to spontaneous encephalopathy and gastrointestinal hemorrhage. Dig Dis Sci. 1981;26(4):346–352. | ||
Tarantino G, Citro V, Conca P, et al. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol. 2009;9(1):89. | ||
Qi X, Qi X, Zhang Y, et al. Prevalence and clinical characteristics of spontaneous splenorenal shunt in liver cirrhosis: a retrospective observational study based on contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) scans. Med Sci Monit. 2017;23:2527–2534. | ||
Riggio O, Efrati C, Catalano C, et al. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case–control study. Hepatology. 2005;42(5):1158–1165. | ||
Borentain P, Soussan J, Resseguier N, et al. The presence of spontaneous portosystemic shunts increases the risk of complications after transjugular intrahepatic portosystemic shunt (TIPs) placement. Diagn Interv Imaging. 2016;97(6):643–650. | ||
Talwalkar JA, Swanson KL, Krowka MJ, Andrews JC, Kamath PS. Prevalence of spontaneous portosystemic shunts in patients with portopulmonary hypertension and effect on treatment. Gastroenterology. 2011;141(5):1673–1679. | ||
Simón-Talero M, Roccarina D, Martínez J, et al. Association between portosystemic shunts and increased complications and mortality in patients with cirrhosis. Gastroenterology. 2018;154(6):1694–1705. | ||
Saks K, Jensen KK, Mclouth J, et al. Influence of spontaneous splenorenal shunts on clinical outcomes in decompensated cirrhosis and after liver transplantation. Hepatol Commun. 2018;2(4):437–444. | ||
Kumamoto M, Toyonaga A, Inoue H, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol. 2010;25(6):1129–1135. | ||
Saad WEA, Lippert A, Saad NE, Caldwell S. Ectopic varices: anatomical classification, hemodynamic classification, and hemodynamic-based management. Tech Vasc Interv Radiol. 2013;16(2):108–125. | ||
Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12(3):327–336. | ||
Saad WE, Sabri SS. Balloon-occluded retrograde transvenous obliteration (BRTO): technical results and outcomes. Semin Intervent Radiol. 2011;28(3):333–338. | ||
Lee EW, Saab S, Gomes AS, et al. Coil-assisted retrograde transvenous obliteration (CARTO) for the treatment of portal hypertensive variceal bleeding: preliminary results. Clin Transl Gastroenterol. 2014;5(10):e61. | ||
Gwon DI, Ko GY, Yoon HK, et al. Gastric varices and hepatic encephalopathy: treatment with vascular plug and gelatin sponge-assisted retrograde transvenous obliteration – a primary report. Radiology. 2013;268(1):281–287. | ||
Kim YH, Kim YH, Kim CS, Kang UR, Kim SH, Kim JH. Comparison of balloon-occluded retrograde transvenous obliteration (BRTO) using ethanolamine oleate (Eo), BRTO using sodium tetradecyl sulfate (STS) foam and vascular plug-assisted retrograde transvenous obliteration (PARTO). Cardiovasc Intervent Radiol. 2016;39(6):840–846. | ||
Kim T, Yang H, Lee CK, Kim GB. Vascular plug assisted retrograde transvenous obliteration (PARTO) for gastric varix bleeding patients in the emergent clinical setting. Yonsei Med J. 2016;57(4):973–979. | ||
Patil R, Rassameehiran S, Patel R, Balakrishnan M, Sood GK. Embolization for closure of spontaneous porto-systemic shunts in patient with cirrhosis and refractory hepatic encephalopathy: a systematic review and meta-analysis. Gastroenterology. 2017;152(5):S1140. | ||
Saad WE, Sze DY. Variations of balloon-occluded retrograde transvenous obliteration (BRTO): balloon-occluded antegrade transvenous obliteration (BATO) and alternative/adjunctive routes for BRTO. Semin Intervent Radiol. 2011;28(3):314–324. | ||
Saad WE, Kitanosono T, Koizumi J. Balloon-occluded antegrade transvenous obliteration with or without balloon-occluded retrograde transvenous obliteration for the management of gastric varices: concept and technical applications. Tech Vasc Interv Radiol. 2012;15(3):203–225. | ||
Kako Y, Yamakado K, Jomoto W, et al. Changes in liver perfusion and function before and after percutaneous occlusion of spontaneous portosystemic shunt. Jpn J Radiol. 2017;35(7):366–372. | ||
Sakurabayashi S, Sezai S, Yamamoto Y, Hirano M, Oka H. Embolization of portal-systemic shunts in cirrhotic patients with chronic recurrent hepatic encephalopathy. Cardiovasc Intervent Radiol. 1997;20(2):120–124. | ||
Laleman W, Simon-Talero M, Maleux G, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57(6):2448–2457. | ||
Singh S, Kamath PS, Andrews JC, Leise MD. Embolization of spontaneous portosystemic shunts for management of severe persistent hepatic encephalopathy. Hepatology. 2014;59(2):735–736. | ||
Naeshiro N, Kakizawa H, Aikata H, et al. Percutaneous transvenous embolization for portosystemic shunts associated with encephalopathy: long-term outcomes in 14 patients. Hepatol Res. 2014;44(7):740–749. | ||
An J, Kim KW, Han S, Lee J, Lim YS. Improvement in survival associated with embolisation of spontaneous portosystemic shunt in patients with recurrent hepatic encephalopathy. Aliment Pharmacol Ther. 2014;39(12):1418–1426. | ||
Lynn AM, Singh S, Congly SE, et al. Embolization of portosystemic shunts for treatment of medically refractory hepatic encephalopathy. Liver Transpl. 2016;22(6):723–731. | ||
Tryc AB, Goldbecker A, Berding G, et al. Cirrhosis-related parkinsonism: prevalence, mechanisms and response to treatments. J Hepatol. 2013;58(4):698–705. | ||
Philips CA, Kumar L, Augustine P. Shunt occlusion for portosystemic shunt syndrome related refractory hepatic encephalopathy – a single-center experience in 21 patients from Kerala. Indian J Gastroenterol. 2017;36(5):411–419. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.