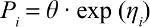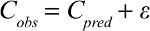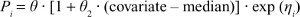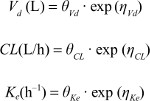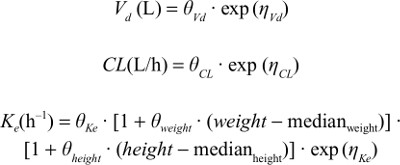Back to Journals » Journal of Pain Research » Volume 11
Population pharmacokinetic modeling of flurbiprofen, the active metabolite of flurbiprofen axetil, in Chinese patients with postoperative pain
Authors Zhang J, Zhang H , Zhao L, Gu J , Feng Y, An H
Received 6 June 2018
Accepted for publication 5 November 2018
Published 30 November 2018 Volume 2018:11 Pages 3061—3070
DOI https://doi.org/10.2147/JPR.S176475
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Jingru Zhang, 1,2,* Hong Zhang, 1,* Libo Zhao, 3 Jian Gu, 4 Yi Feng, 1 Haiyan An 1
1Department of Anesthesiology, Peking University People’s Hospital, Beijing 100044, China; 2Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Sciences, Peking University, Beijing 100191, China; 3Beijing Children’s Hospital, Capital Medical University, Beijing 100045, China; 4Department of Pharmacy, Peking University People’s Hospital, Beijing 100044, China
*These authors contributed equally to this work
Background: Flurbiprofen axetil, a lipid-microsphere-carrier targeting preparation, is a nonsteroidal anti-inflammatory drug indicated for the treatment of postoperative pain.
Aim: The aim of the study was to develop a population pharmacokinetic (PPK) model of flurbiprofen, the active metabolite of flurbiprofen axetil, and optimize the treatment of flurbiprofen axetil in Chinese patients.
Methods: A total of 144 therapeutic drug-monitoring samples of flurbiprofen axetil from 72 patients were included in this study. The pharmacologically active metabolite flurbiprofen was used as the analytical target and determined 5– 45 minutes after intravenous administration. The PPK model for flurbiprofen was developed using Phoenix NLME 1.3 with a nonlinear mixed-effect model. Bootstrap and visual predictive checks were used simultaneously to validate the final PPK model. Potential covariates of age, sex, body weight, height, and body-mass index were tested for PK parameters.
Results: The PPK model of flurbiprofen was explained by a one-compartment model with first-order elimination, in which a hypothetical-effect compartment was linked to a PK compartment. Population mean values of PK parameters estimated in the final model were θKe=0.0015/h, θVd=7.91 L, and θCL=1.55 L/h. Analysis of covariates showed that height and weight influenced the Ke of flurbiprofen. The final model was proved to be robust.
Conclusion: The final PPK model was demonstrated to be appropriate and effective, and can be used to assess the PK parameters of flurbiprofen in Chinese patients with postoperative pain.
Keywords: population pharmacokinetics, flurbiprofen, postoperative pain, weight, height
Expression of Concern for this paper has been published
Corrigendum for this paper has been published
Introduction
Approximately 80% of patients undergoing surgical procedures suffer acute postoperative pain, of which 86% report moderate–severe pain.1 Flurbiprofen axetil, an ester prodrug of flurbiprofen, is one of the most commonly used nonsteroidal anti-inflammatory drugs worldwide indicated for the treatment of postoperative pain.2 It is a lipid-microsphere-carrier targeting preparation to reduce venous irritation caused by flurbiprofen.3 Flurbiprofen axetil is rapidly metabolized to flurbiprofen through the hydrolysis of esterase, which is the pharmacologically active moiety. Flurbiprofen reaches peak plasma concentrations at 5–10 minutes after the intravenous injection of flurbiprofen axetil, with a terminal half-life of 5.8 hours.4 Urinary excretion in 0–48 hours accounts for about 85% of dose. The main urinary metabolite is a conjugate.5
Adverse effects of flurbiprofen include injection-site pain, headache, and elevation of liver enzymes, among others. The lowest effective dose can be used in the shortest treatment time to reduce pain and minimize the adverse reactions. The dosage for patients with hepatic or renal insufficiency should be carefully considered. Moreover, as we reported previously, lipid microspheres loaded with flurbiprofen can penetrate the blood–brain barrier into cerebrospinal fluid (CSF) after intravenous injection,6 which may lead to central toxicity in the circumstance of overdose. Indeed, flurbiprofen is highly bound to plasma proteins and has a low volume of distribution, of which the binding of flurbiprofen to human serum albumin and normal plasma is >99%.5 Published data suggest that the drugs most likely to exhibit interindividual differences in their pharmacokinetics (PK) are those that are highly bound to plasma proteins or undergo significant hepatic metabolism.7 Therefore, it is important to optimize therapy with flurbiprofen axetil by therapeutic drug monitoring. There have been studies on the traditional PK of flurbiprofen in healthy volunteers or highly selected patients.8–12 However, population PK (PPK) modeling is a scientific and highly efficient approach to describe the PK behavior of the investigated drug and to evaluate potential covariates that may contribute to PK intra- and intersubject variability,13 wherein participants can be more representative of the target population treated with the drug.14 It has been assumed that patient characteristics may contribute to interindividual differences in drug responses, and it is essential to take into account the influence of these covariates on the PPK of flurbiprofen.15
To date, there is limited PPK information available on flurbiprofen axetil and its active metabolite flurbiprofen. In the present study, we used nonlinear mixed-effect modeling by Phoenix NLME to analyze data of flurbiprofen axetil in plasma and CSF simultaneously and to consider the influence of covariates on PK parameters. The aim of our study was to develop a PPK model of flurbiprofen intravenously in Chinese patients with postoperative pain and identify the influences of patient characteristics on flurbiprofen PK variability to provide a basis for clinical individualized dosage regimens.
Methods
As further exploration of our earlier research, subjects and their study data were obtained from our previous report.6 Briefly, a total of 72 subjects aged 18–72 years and receiving flurbiprofen axetil injections for treatment in Peking University People’s Hospital were recruited in this study. The study was approved by the Medical Ethics Committee of Peking University People’s Hospital (ChiCTR-TRC-11001791) and conformed to the ethical principles of the Declaration of Helsinki. All participants provided written informed consent before enrollment.
The study design followed was random and sparse sampling. Every subject was given an intravenous injection of 1 mg/kg flurbiprofen axetil (5050E; Tide Pharmaceutical, Beijing, China) under subarachnoid anesthesia. All patients were randomly assigned into nine groups (eight subjects in each group), and every group had blood- and CSF-sample collection simultaneously at 5, 10, 15, 20, 25, 30, 35, 40, or 45 minutes after the flurbiprofen axetil intravenous administration. The collected samples were immediately centrifuged at 2,500 rpm for 10 minutes and kept at –20°C until further analysis. Patients’ demographic data were collected including age, sex, body weight, height, and body-mass index (BMI).
Flurbiprofen concentrations in blood and CSF were determined using reverse-phase HPLC.6 In brief, flurbiprofen was extracted with acetonitrile. The mobile phase was phosphate buffer (pH 7.0):acetonitrile (75:25) at a flow rate of 1 mL/min. Chromatographic peaks were detected at a wavelength of 247 nm. Concentrations of flurbiprofen were calculated by peak area. The lower limit of detection was 0.5 µg/mL for plasma and 2.5 ng/mL for CSF, respectively.
Population pharmacokinetic model
PPK analysis was performed using a nonlinear mixed-effect model with Phoenix NLME 1.3. The first-order conditional estimation–extended least squares method with the η–ε interaction option was used during the PPK model-development process. Initially, the basic model was examined. One- and two-compartment structural kinetic models with first-order absorption were evaluated. The best structural model was chosen based on assessment of the objective function value (equal to the twice the negative log likelihood [–2LL] value) and visual inspection of standard goodness-of-fit plots, including individual fit.16
The exponential model was used to describe the interindividual variability of the PK parameters:
|
|
where Pi is the OK parameter for the ith individual, including the clearance (CL), the apparent volume of distribution (Vd), and the elimination rate constant (Ke), θ is the typical population value of the corresponding parameter in this population, and ηi is a random variable for the ith individual following normal distribution with a mean of 0 and a variance of ω.2
Intraindividual variability (residual error) was evaluated using an addition model:
|
|
where Cobs is the observed serum flurbiprofen concentration, Cpred is the corresponding model predicted concentration, and ε is assumed to follow a normal distribution with a mean of 0 and a variance of σ.2 Additionally, given that the flurbiprofen concentration in CSF was far below that in plasma, we added CSF drug concentrations as an effect-compartment model linked to the PK compartment.17
Population covariate analysis
Potential covariates of age, sex, body weight, height, and BMI were tested for PK parameters. The categorical covariate (sex) was incorporated using indicator variables with an exponential function. Influences of continuous covariates, such as age, weight, height, and BMI, were included in the model using a power function after normalization to the median value. A visual covariate-screening procedure was performed before modeling. For visual screening, scatterplots for continuous variables and box plots for discrete variables were used. Variables showing a potential relationship with a certain PK parameter in the screening procedure were included in the model to be selected as important covariates. Then, important covariates were selected and chosen using stepwise forward selection–backward elimination with a likelihood-ratio test. Because the objective function value follows a χ2 distribution, a covariate was considered significant when inclusion of the covariate resulted in a decrease in –2LL >3.84 (P<0.05) and elimination of the covariate resulted in an increase in –2LL >6.63 (P<0.01).18
A linear model was used for the continuous covariate candidates:
|
|
where θ2 is a coefficient representing the relationship between the covariate and the typical population value of the parameter and median the median of the covariate in the population.
Model validation
Adequacy of the final model was simultaneously evaluated using bootstrapping and visual predictive checks (VPCs). A bootstrap (n=1,000) was performed by resampling the subjects from the original data set. The final-model parameter estimates obtained from the data set were compared with medians and 95% CIs of the bootstrap estimates. A VPC was performed using 1,000 data-set simulations. The 5th, 50th, and 95th percentiles of the simulated concentrations were plotted against time with the observed flurbiprofen concentrations.
Results
As previously reported, a total of 72 patients (27 males and 45 females) treated with flurbiprofen intravenously were involved in the dataset.6 There were no statistically significant differences in age (P=0.64), gender (P=0.95), body weight (P=0.95), height (P=0.98), or BMI (P=0.86) among all nine time-point groups. The demographics of the subjects are summarized in Table 1. A total of 72 plasma flurbiprofen concentrations and 72 CSF flurbiprofen concentrations were available in our previous study.6
  | Table 1 Demographics of the study population (n=72) Note: Data from Zhang et al.6 Abbreviation: CSF, cerebrospinal fluid. |
Population pharmacokinetic modeling
On the basis of goodness-of-fit criteria, flurbiprofen concentrations as a function of time were best described by a one-compartment PK/pharmacodynamic model with first-order kinetics. According to the reported study6 and lower level of flurbiprofen in CSF, we considered flurbiprofen concentrations as the index of pharmacodynamics using the effect–compartment link model. The basic model was:
|
In this study, age, sex, body weight, height, and BMI were appropriate to study as covariates for flurbiprofen metabolism. After visual screening through scatterplots and box plots and stepwise forward selection–backward elimination, the covariate analysis indicated strong influence of weight and height on Ka. The final model was:
|
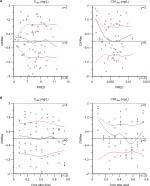
Parameter estimates and 95% CIs of the basic and final models are shown in Table 2. Observed flurbiprofen concentrations vs individual predicted value (IPred) and predicted value (Pred) for the final model are shown in Figure 1, which revealed predicted vs observed flurbiprofen-concentration data points were aligned on the identity line. Figure 2 plots the conditional weighted residuals (CWRes) vs predicted flurbiprofen concentrations, showing random distribution around zero, and does not reflect any systemic deviations.
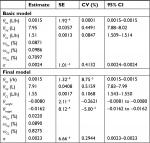  | Table 2 Population pharmacokinetic parameters of flurbiprofen for basic and final models Abbreviations: SE, standard error; CV, coefficient of variation. |
The population Eta QQ plot of the final model is displayed in Figure 3, which shows that flurbiprofen concentrations were adequately described between most of the observed and predicted values.
  | Figure 3 Final-model population Eta QQ plot. Notes: (A) nCL; (B) nKe; (C) nVd. |
Model validation
The results of the bootstrap validation are shown in Table 3. Parameter estimates of the final model were consistent to those of the bootstrap, indicating robustness and stability of the final model. The VPC for the final model showed that the majority of observed flurbiprofen data fell within the boundaries of the 5th and 95th percentiles of the simulated flurbiprofen-concentration data, suggesting that the final model adequately explained the observed data (Figure 4).
  | Table 3 Results of bootstrap analysis Abbreviation: CV, coefficient of variation. |
Discussion
To our knowledge, this is the first PPK model of flurbiprofen, the active metabolite of its prodrug, flurbiprofen axetil, using both plasma and CSF therapeutic drug-monitoring data in a Chinese population with postoperative pain. Additionally, it provides the first description of the effects of covariates on the CL, Vd, and Ke of flurbiprofen in a large number of patients. The results of this study indicated that the Ke of flurbiprofen was influenced by weight and height. The stability and predictive performance of the final PPK model were established by bootstrapping and VPC evaluation, the results of which revealed the robustness and stability of the final model.
The PK data of flurbiprofen intravenously in patients with postoperative pain was best described by a one-compartment linear model with first-order elimination. Allowing that the concentration of flurbiprofen in CSF was much lower than that in plasma and that flurbiprofen may have central analgesic action, we chose the concentration in CSF as the pharmacodynamic effect–compartment link model, which might be more appropriate if it took the time lag into consideration. For an accurate description of flurbiprofen PK/pharmacodynamics, a one-compartment model was considered most suitable to describe the concentration–time profile of flurbiprofen in our previous report.6
The typical population values of the PK parameters estimated in the final model were θKe=0.0015/h, θVd=7.91 L, θCL=1.55 L/h, and corresponding time to maximum concentration of 10 minutes. After a single oral dose of 100 mg flurbiprofen, Suri et al9 reported that the CL and Vd of S-flurbiprofen were 1.52 L/h and 6.00 L in US subjects, and Qayyum et al11 found that these were 1.89 L/h and 12.07 L in Pakistani subjects, whereas Galasko et al8 showed after multidose administration of R-flurbiprofen that CL was 1.465 L/h. All studies were performed in healthy volunteers. In the current study, the population value of flurbiprofen clearance in Chinese patients was approximately the same as in Suri et al and Galasko et al, but a little lower than that in Qayyum et al. We suppose that differences in physiological status between healthy volunteers and patients or different ethnic populations might be possible reasons for the disparity.
PPK analysis showed that PK between-subject variability for flurbiprofen was related to weight and height. It has been reported that in children aged 3 months to 13 years, estimated CL was 0.96 L/h/70 kg and volume of distribution at steady state 8.1 L/70 kg,12 which was much lower compared with studies in adults, indicating PK differences related to body size (such as weight or body-surface area). In our study, the drug was given by weight, and weight was included in the final model as a covariate finally. It suggested the current clinical application of flurbiprofen axetil by weight is reasonable. Moreover, because body-surface area increases with height, it is logical to include a height covariate into the final model. Our analysis did not describe the impact of sex on the PK of flurbiprofen. A large number of epidemiological studies have shown that prevalence in women is higher than in men in many chronic pain diseases; therefore, women also use analgesics more frequently than men.19 Some experimental analyses and clinical studies have also found sex differences in type, sensitivity, degree, and threshold of pain.20 However, current research illustrates that sex difference is small in postoperative pain, while more significant in painful diseases,21 which not only was consistent with our findings but also provided an explanation for the results.
It is well established that the CYP2C9 genotype is a significant predictor of flurbiprofen metabolism and accounts for 59% of the variability in flurbiprofen area under the curve (0–∞) and approximately 50% of the variability in flurbiprofen clearance.11,22 As reported, different genotypes of CYP2C9 could cause differences in PK characteristics of flurbiprofen, where CYP2C9*1/*3 code for an enzyme with decreased flurbiprofen-clearance rate compared with the allele CYP2C9*1/*1, and the clearance rate of flurbiprofen had no significant difference in CYP2C9*1/*2 and CYP2C9*1/*1 populations.23 Wang et al suggested that 33 CYP2C9 allelic variants could change the intrinsic clearance value of flurbiprofen in vitro, in which 31 allelic isoforms significantly decreased the metabolic activities of CYP toward the drug flurbiprofen.24 However, for the Chinese, there are three main genotypes: *1/*1, *1/*3, and *1/*13. The *1/*1 genotype is the most frequent, and the *1/*3 and *1/*13 genotyping populations are both <10%, accounting for 4.3%–7.7% and <1.2%, respectively, while the *1/*2 genotyping is quite rare in China.25 We did not consider CYP2C9 gene polymorphisms as a covariate in the present study due to the small sample, something which should be further investigated.
Our study also has some limitations. Due to medical ethical issues, it is difficult to collect CSF specimens from the same patient at multiple time points in a clinical setting; therefore, in this study, we designated multiple groups of patients for collection of CSF specimens at different time points, which may have been the cause of deviation in the CSF drug concentration. In addition, we did not discuss the influence of drug combinations in consideration of the strict inclusion criteria, unified drug regimen, and small sample in our study. Therefore, large-scale studies will be necessary in future to verify PPK parameters further.
Based on the principle of Bayesian feedback, a patient’s individual PK parameters can be estimated using the established PPK model in our study combined with Bayesian feedback when taking one or two blood samples and CSF samples from the patient. Also, along with pharmacodynamic indices such as visual analog pain scores and prostaglandin E2, we can find optimal dosage and adjust the administration of flurbiprofen axetil to optimize the treatment of postoperative pain.
Conclusion
In this study, we developed a PPK model of flurbiprofen, the active metabolite of flurbiprofen axetil, in Chinese patients with postoperative pain. It was well described by a one-compartment linear model with first-order elimination and an effect–compartment link model, in which the exponential model represented interindividual variability and the addition model interpreted intraindividual variability. Weight and height were included as significant covariates in the final model. The final PPK model was shown to be stable and effective in the prediction of serum flurbiprofen concentrations by bootstrapping and VPC validation. Our findings may help to facilitate individualized dosage schemes and improve the safety and efficacy of drug therapy, while providing a foundation for future PK/pharmacodynamic study of flurbiprofen.
Acknowledgment
This work was supported by the Peking University People’s Hospital Research and Development Funds (RDC2010-04).
Disclosure
The authors report no conflicts of interest in this work.
References
Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. | ||
Zhang L, Zhu J, Xu L, et al. Efficacy and safety of flurbiprofen axetil in the prevention of pain on propofol injection: a systematic review and meta-analysis. Med Sci Monit. 2014;20:995–1002. | ||
Yamazaki Y, Sonoda H, Seki S. [Effects of preoperatively administered flurbiprofen axetil on the action of inhaled anesthesia and postoperative pain]. Masui. 1995;44(9):1238–1241. Japanese. | ||
Fujii Y, Itakura M. Comparison of lidocaine, metoclopramide, and flurbiprofen axetil for reducing pain on injection of propofol in Japanese adult surgical patients: a prospective, randomized, double-blind, parallel-group, placebo-controlled study. Clin Ther. 2008;30(2):280–286. | ||
Ohmukai O. Lipo-NSAID preparation. Adv Drug Deliv Rev. 1996;20(2–3):203–207. | ||
Zhang H, Gu J, Feng Y, An H. Absorption kinetics of flurbiprofen axetil microspheres in cerebrospinal fluid: A pilot study. Int J Clin Pharmacol Ther. 2017;55(11):875–880. | ||
Johnson JA. Predictability of the effects of race or ethnicity on pharmacokinetics of drugs. Int J Clin Pharmacol Ther. 2000;38(2):53–60. | ||
Galasko DR, Graff-Radford N, May S, et al. Safety, tolerability, pharmacokinetics, and Abeta levels after short-term administration of R-flurbiprofen in healthy elderly individuals. Alzheimer Dis Assoc Disord. 2007;21(4):292–299. | ||
Suri A, Grundy BL, Derendorf H. Pharmacokinetics and pharmacodynamics of enantiomers of ibuprofen and flurbiprofen after oral administration. Int J Clin Pharmacol Ther. 1997;35(1):1–8. | ||
Aarons L. The kinetics of flurbiprofen in synovial fluid. J Pharmacokinet Biopharm. 1991;19(3):265–269. | ||
Qayyum A, Najmi MH, Farooqi ZU. Determination of pharmacokinetics of flurbiprofen in Pakistani population using modified HPLC method. J Chromatogr Sci. 2011;49(2):108–113. | ||
Kumpulainen E, Välitalo P, Kokki M, et al. Plasma and cerebrospinal fluid pharmacokinetics of flurbiprofen in children. Br J Clin Pharmacol. 2010;70(4):557–566. | ||
Hu P, Chen J, Liu D, Zheng X, Zhao Q, Jiang J. Development of population pharmacokinetics model of icotinib with non-linear absorption characters in healthy Chinese volunteers to assess the CYP2C19 polymorphism and food-intake effect. Eur J Clin Pharmacol. 2015;71(7):843–850. | ||
Kiang TK, Sherwin CM, Spigarelli MG, Ensom MH. Fundamentals of population pharmacokinetic modelling: modelling and software. Clin Pharmacokinet. 2012;51:515–525. | ||
Balant LP, Balant-Gorgia EA. Cultural differences: implications on drug therapy and global drug development. Int J Clin Pharmacol Ther. 2000;38(2):47–52. | ||
Yu Y, Zhang Q, Xu W, Lv C, Hao G. Population pharmacokinetic modeling of oxcarbazepine active metabolite in Chinese patients with epilepsy. Eur J Drug Metab Pharmacokinet. 2016;41(4):345–351. | ||
Lin S, Chien YW. Pharmacokinetic-pharmacodynamic modelling of insulin: comparison of indirect pharmacodynamic response with effect-compartment link models. J Pharm Pharmacol. 2002;54(6):791–800. | ||
Yang L, Guo T, Sun LL, et al. Population pharmacokinetic of losartan and its active metabolite E-3174 in five different ethnic populations of China. J Chin Pharm Sci. 2014;23(8):548–557. | ||
Maurer AJ, Lissounov A, Knezevic I, Candido KD, Knezevic NN. Pain and sex hormones: a review of current understanding. Pain Manag. 2016;6(3):285–296. | ||
Hashmi JA, Davis KD. Deconstructing sex differences in pain sensitivity. Pain. 2014;155(1):10–13. | ||
Pereira MP, Pogatzki-Zahn E. Gender aspects in postoperative pain. Curr Opin Anaesthesiol. 2015;28(5):546–558. | ||
Wang B, Wang J, Huang SQ, Su HH, Zhou SF. Genetic polymorphism of the human cytochrome P450 2C9 gene and its clinical significance. Curr Drug Metab. 2009;10(7):781–834. | ||
Lee CR, Pieper JA, Frye RF, Hinderliter AL, Blaisdell JA, Goldstein JA. Differences in flurbiprofen pharmacokinetics between CYP2C9*1/*1, *1/*2, and *1/*3 genotypes. Eur J Clin Pharmacol. 2003;58(12):791–794. | ||
Wang L, Bao SH, Pan PP, et al. Effect of CYP2C9 genetic polymorphism on the metabolism of flurbiprofen in vitro. Drug Dev Ind Pharm. 2015;41(8):1363–1367. | ||
Zhang Y, Si D, Chen X, et al. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on pharmacokinetics of gliclazide MR in Chinese subjects. Br J Clin Pharmacol. 2007;64(1):67–74. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.