Back to Journals » International Journal of Women's Health » Volume 15
Placenta Accreta Spectrum Diagnosis Challenges and Controversies in Current Obstetrics: A Review
Authors Arakaza A, Zou L, Zhu J
Received 8 November 2022
Accepted for publication 30 March 2023
Published 20 April 2023 Volume 2023:15 Pages 635—654
DOI https://doi.org/10.2147/IJWH.S395271
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
Arcade Arakaza, Li Zou, Jianwen Zhu
Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, China
Correspondence: Li Zou, Email [email protected]
Background: Placenta accreta spectrum (PAS) is the most common obstetric complication in current obstetrics in which the placenta is fully or partially attached to the uterine myometrial layer at delivery. This is commonly due to the deficiency of the uterine interface between the uterine endometrial and myometrial layers leading to abnormal decidualization at the uterine scar area, which permits the abnormally placental anchoring villous and trophoblasts, deeply invade the myometrium. The prevalence of PAS is globally at rising trends every day in modern obstetrics originally due to the high increasing rate of cesarean sections, placenta previa, and assisted reproductive technology (ART). Thus, the early and precise diagnosis of PAS is imperative to prevent maternal intrapartum or postpartum bleeding complications.
Objective: The main aim of this review is to debate the current challenges and controversies in the routine diagnosis of PAS diseases in obstetrics.
Data Source: We retrospectively reviewed the recent articles on different methods of diagnosing PAS in PubMed, Google Scholar, Web of Science, Medline, Embase, and other website databases.
Results: Despite that, the standard ultrasound is a reliable and key tool for the diagnosis of PAS, the lack of ultrasound features does not exclude the diagnosis of PAS. Therefore, clinical assessment of risk factors, MRI tests, serological markers, and placental histopathological tests are also indispensable for the prediction of PAS. Previously, limited studies reached a high sensitivity rate of diagnosis PAS in appropriate cases, while many studies recommended the inclusion of different diagnosis methods to improve the diagnosis accuracy.
Conclusion: A multidisciplinary squad with well-experienced obstetricians, radiologists, and histopathologists should be involved in the establishment of the early and conclusive diagnosis of PAS.
Keywords: placenta accreta spectrum, PAS, diagnosis, challenges and controversies, obstetrics, review
Introduction
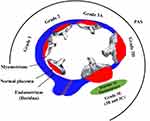
Placenta accreta refers to a variety of aberrant placentation disorders resulting in complete or partial retention of the placenta at delivery. Traditionally, it was divided into three pathologic categories according to the invasiveness of the placenta: placenta accreta when anchoring villi closely adhere to the superficial myometrium without interrupting decidua, placenta increta when the placental villi invade the myometrium, and placenta percreta when placental villi invade the myometrium and through the whole uterine wall reach the serosa, even the surrounding tissues, and organs.1–4 In fact, the profundity degree of placental villous tissues invading the myometrium varies greatly. Therefore, current pathologists have avoided the traditional term and defined this spectrum disorder as placenta accreta spectrum (PAS) disorders, including both morbidly adherent placenta (MAP) covering placenta accreta, and abnormally invasive placenta (AIP) covering placenta increta and placenta percreta.5 The standardized clinical classification and new terminology of PAS including all grades of abnormal placentation descriptively substituted the traditional categorical nomenclature (placenta accreta, increta, percreta) with a pathologic grading classification recently proposed by FIGO (International Federation of Gynaecology and Obstetrics) (Figure 1).4,6,7
PAS was first reported in 1927 by Irving et al with an incidence of only 0.12 in 1000 women.1 In recent years, its incidence in the United States has raised about 13-fold due to a rapidly ascending rate of cesarean deliveries from 5.8% to 32.9%.8 The incidence of PAS increased from 1 in 2510 women before 1994 to 1 in 533 women in 2002, and it counted as 4 in 1000 women in 2003 and 1 in 272 women in 2016.2,9 Previously published data in 2021 have reported that PAS is increasing by about 18-fold in maternal morbidity and up to 30% maternal mortality rate especially when there is no prenatal diagnosis.10 PAS is a serious obstetric complication with numerous challenges and controversies in diagnosis and in managing.3,11,12 Its main harm is the potential maternal life-threatening intrapartum and postpartum hemorrhage,3,5,13 which is the main cause of emergency hysterectomy.10,14 During PAS, it is difficult to separate the placenta from the uterus. If the placenta is not stripped properly, it will affect uterine contraction, while blunt stripping can lead to huge bleeding on the stripping surface, which is difficult to control. The quantity of bleeding in the operation of implantable placenta increta and penetrating placenta percreta is expressively higher than that of adhesive placenta accreta. Penetrating placenta is easy to lead to acute intraperitoneal malignant bleeding, which often leads to hemorrhagic shock. Therefore, the deeper the penetration of the placenta, the more bleeding occurred during the operation, and the more blood transfusion is required.2,15
Kayem et al16 followed up 520 114 deliveries in 176 French hospitals of the 8 districts and studied 242 cases diagnosed with PAS divided into a group of women with placenta previa with a prior cesarean delivery and a group of women without the combination of placenta previa and prior history of cesarean delivery. Among 115 women with the combination of placenta previa and prior history of cesarean delivery, 77% were suspected to be PAS after imaging test, and 53% had a hysterectomy; while among 127 women without the combination of placenta previa and prior history of cesarean delivery, 17% were suspected to be PAS after imaging test, and 21% had a hysterectomy. Crocetto et al17 also pointed out that PAS is one of the primary causes of hysterectomy after cesarean section. Kong et al18 retrospectively counted the reasons for emergency hysterectomies from 2011 to 2014, of which uncontrollable bleeding due to PAS caused about 45% to 73.3%. Atallah et al19 pointed out that as PAS can lead to uncontrollable massive bleeding during labor or postpartum, and even amniotic fluid embolism or disseminated intravascular coagulation in severe cases, it is recommended to retain the placenta in situ and perform an emergency hysterectomy after fetal delivery to ensure the life of patients.3,10 At the same time, the bleeding due to PAS also can bring great harm to the patient’s heart and physiology leading to maternal cardiac arrest and death.
Regarding the management of PAS, most pregnant women with PAS have planned delivery, cesarean section is recommended at 34–36 weeks to improve the outcome for pregnant women and the fetus.5,20 With the continuous improvement of surgical skills level in recent years, many scholars have explored new surgical methods during cesarean section to improve pregnancy outcomes, such as vascular interventional surgery, bilateral uterine artery ligation or embolization, iliac arteries balloon embolization or abdominal aortic balloon occlusion, intrauterine balloon placement, uterine breakwater-like surgery to reduce the amount of bleeding and strive for opportunities to preserve the uterus.3,10,20–22 If all the surgical procedures fail, cesarean hysterectomy along with leaving the placenta in situ remains the safest and most suitable method for managing PAS since both can prevent massive blood loss concomitant with manual removal of the placenta.3,10,23 However, some of the methods such as interventional radiology or placental bed suturing and uterine compression sutures or cesarean hysterectomy and so on require the availability of multidisciplinary team care with well-experienced interventional radiologists, obstetricians, anesthesiologists, neonatologists, urologists, nursing experts, and blood bank specialists to establish the accurate management of PAS and to improve the fetomaternal outcomes.5,10,14,24
Concerning the goal of this manuscript, at present, the diagnosis of PAS lacks specific manifestations and characteristic serological indicators. Therefore, prenatal diagnosis is difficult, and imaging examinations are often needed. In addition, not all cases are suitable for auxiliary hysterectomy surgery, the reasonable implementation of hysterectomy depends on the accurate diagnosis before the operation, and because the clinical manifestations of PAS are not specific, it puts forward higher requirements for various techniques of antenatal diagnosis of PAS. Hence, we would like to debate the current controversies and challenges in diagnosing PAS.
Pathogenesis of Placenta Accreta Spectrum
The precise pathogenesis of PAS is still mysterious, only some risk factors can provide us the clues. Concerning the trigger of PAS diseases, the most ideal hypothesis is the deficiency of the uterine interface between the endometrium and myometrium leading to an abnormal decidualization at the uterine scar area, which permits the placental anchoring villous and trophoblasts deeply penetrate the uterine myometrial layer.3,5,25,26 Normal endometrial decidualization plays a vital role in early placental formation and development; the decidual tissues can not only provide a channel that provides nutrients for fetal development, but also resist the excessive invasion of villous trophoblast cells to the mother.27,28 Endometrial decidualization occurs between the placenta and the mother, in which decidual stromal cells (DSCs) are the main cell components.29 DSCs and Extravillous trophoblast cells (EVTs) in early pregnancy are related to the process of early blastocyst implantation through autocrine and/or paracrine regulation mechanism,30,31 and all of the two can secrete a variety of growth factors, cytokines, and cell adhesion factors playing the double function of fetal immunity and nutrition.28,32
The balance of the maternal–fetal immune interface is broken by various factors from time to time and regains the balance under the regulation of various factors secreted by EVTs and DSCs. Therefore, the existence of decidual tissues is only of great significance for the balance of the maternal–fetal immune interface, so that the villous trunk can be fixed without excessive invasion.29,33 If the local endometrium is missing or the scarring is formed due to surgical trauma and so on, resulting in local failure of decidualization or poor decidualization, and secondly, the regulation between DSCs and EVTs is continuously unbalanced, the invasive behavior of villous cells is uncontrolled and the EVTs intensely infiltrate the uterine myometrium to form PAS.27
However, some cases with PAS without any history of previous cesarean sections or other uterine surgeries were reported5, which needs further investigations for their pathogenesis.
Epidemiology and Risk Factors for Placenta Accreta Spectrum
PAS is actually increasing in the entire world due to the increasing rate of cesarean deliveries, placenta previa, and ART (assisted reproductive technology).5,34 However, there are many studies on the risk factors of PAS. Wu et al35 used the logistic regression (LR) model to analyze 64,359 cases of delivery from 1982 to 2002 according to the statistics of the University of Chicago, and it was early found that placenta previa and multiple cesarean sections are the main risk factors of PAS. Recently, it was also found that placenta previa, multiple cesarean sections, uterine surgeries (endometrial curettage, etc.), maternal advanced age, smoking multiple births, multiple abortions, and hypertensive disorders of pregnancy, and other unknown obstetric complications were the risk factors for PAS.5,10,36 Jauniaux et al20 reported that the occurrence of PAS raised from 1.7 per 10.000 women to 577 to 10.000 women with both cesarean delivery and placenta previa. They believed that the history of cesarean delivery and different types of placenta previa, ART, and smoking are autonomous risk factors for PAS.
Solheim et al37 established a prediction model, suggesting that PAS increases with the increase of the incidence of cesarean section and placenta previa. Wu et al35 concluded that the odds ratio (OR) of placenta previa patients with PAS was 51.4%, indicating that the risk of placenta previa accreta elevated to 51 times, while the risk of PAS in pregnant women with two or more cesarean sections raised to 8 times. They also found out that the possibility of placenta previa in pregnant women with a history of more than three cesarean sections and placenta previa elevated to 4000 times. In the same direction in 2018, Cahill et al2 also pointed out that the probability of PAS in women with one cesarean section and five or more cesarean sections were 0.3% and 6.74%, respectively, showing a significant upward advantage, and then a certain correlation between cesarean sections and PAS.
The main reason may be that the lower segment of the uterus is relatively weak, and cesarean section can cause local endometrial loss or damage, resulting in local decidual dysplasia or no formation of the decidua. When the decidua is missing or stunted, the maternal–fetal immune interface is continuously unbalanced, which leads to the uncontrolled invasion of placental villi to the deep myometrial layer and the formation of PAS.
Recently in 2021, Matsuzaki et al38 studied the trends, features and outcomes of PAS women who delivered with cesarean section in the United States from October 2015 to December 2017. The results found that 8030 cases (0.29%) out of 2,727,477 cases had PAS diseases (placenta accreta (n = 6205, 0.23%), placenta percreta (n = 1060, 0.04%), and placenta increta (n = 765, 0.03%)). The number of PAS cases raised to 2.1% every three months. Moreover, PAS diseases due to cesarean sections were associated with surgical harms (78.3% vs 10.6%), maternal diseases (60.3% vs 3.1%), hemorrhage (54.1% vs 3.9%), coagulation disorders (5.3% vs 0.3%), shock (5.0% vs 0.1%), urinary tract harm (8.3% vs 0.2%), and maternal demise (0.25% vs 0.01%) compared to those with cesarean delivery without PAS diseases. Moreover, the placenta increta’s ratio was 19.9, and the placenta percreta’s odds ratio was 32.1, indicating that women with invasive placenta increta and penetrating placenta percreta are highly associated with risks of postoperative complications compared with those with abnormally adherent placenta accreta. Although some results are different, it can be confirmed that placenta previa and multiple cesarean sections are currently recognized as autonomous risk factors for PAS.2,5
Besides placenta previa and multiple cesarean sections, it has also been recently found in some cases of PAS due to assisted reproductive technology (ART) in women with a history of previous uterine surgeries.5,13,20 Lin et al39 also pointed out that women who underwent ART to conceive had an elevated risk for PAS and other placental implantation abnormalities in women with a history of cesarean section. Recently, Ogawa et al40 involved 472,301 singleton pregnancies, including 426 women having PAS with placenta previa and 1827 women having PAS without placenta previa. In PAS with placenta previa group, the number of prior cesarean sections considerably predicted PAS [(aRR) for one previous cesarean section was 5.34, 95% CI 3.70–7.71, while aRR for two or more prior cesarean sections was 16.5, 95% CI 11.5–23.6]. In PAS without placenta previa group, the prior cesarean section did not considerably predict PAS, whereas conception through ART (aRR 5.05, 95% CI 4.50–5.66) powerfully predicted PAS.
Therefore, for pregnant women with a history of placenta previa, cesarean sections, and ART, we must be vigilant, select appropriate examination methods, and carefully evaluate whether they have PAS. For patients with PAS, we should further clarify the degree of placental implantation and fully communicate with patients to inform them of the risk.15 If some pregnant women insist on pregnancy, we should closely observe and timely terminate the pregnancy to minimize maternal harm.
Diagnosis of Placenta Accreta Spectrum
At present, the definite diagnosis of PAS depends on intraoperative (or) postoperative pathology. Those who meet at least one of the following three items can be diagnosed as PAS:
- After actively handling the third stage of labor of vaginal delivery and observing for at least 20 minutes, the placenta is not stripped itself and needs to be stripped by hand or in the process of stripping, the placenta adheres to the uterus, and even the stripping operation is difficult, and the placenta is broken,
- After the placenta is adhered to or implanted during manual stripping in a cesarean section if there is serious bleeding at the placental site and local hemostasis is required, or part of the uterine wall needs to be removed to preserve the uterus, or a total hysterectomy is required,
- Placental tissues implanted in the myometrium confirmed by histopathologic study.31,35
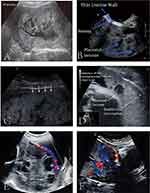
Due to the serious harm of PAS to pregnant women, although the prenatal diagnosis is still not clear, some effective information can be provided to help clinicians make diagnoses and treatment plans, and then prevent the peripartum bleeding complications due to PAS. The antenatal diagnosis is mainly based on the clinical history of the patients, imaging examinations such as ultrasound and MRI, serological test, and finally confirmed by placental histopathology at delivery (Figure 2).5,8,18,31
 |
Figure 2 Placenta accreta spectrum diagnosis diagram. Abbreviations: MRI, magnetic resonance imaging; PAS, placenta accreta spectrum. |
The antenatal diagnosis of PAS by ultrasound (transvaginal, transabdominal, and color Doppler) is made during the second trimester of pregnancy when assessing the fetal anatomy and it may be revised again at 32 weeks of gestation.10,23,24 Recent researches have recommended that PAS diagnosis and exclusion should be conducted between 18 and 24 weeks of gestation.5 However, PAS can be suspected early in the first trimester of pregnancy if an ultrasound scan reveals a gestational sac located in the lower uterine segment and close or lower than the caesarean scar, or if a caesarean scar pregnancy (CSP) is early found on the ultrasound.5 The serological examination is also feasible when necessary, but its specificity is relatively low. However, ultrasound has always been the first choice for antenatal diagnosis of PAS because of its simple, noninvasive and economic advantages.5,15,41 MRI has been specifically used for ambiguous features on ultrasound or when the placenta is posterior and in doubted placenta percreta or placenta invading the parametrium. Nevertheless, in some clinics, it is done consistently in all cases with doubted PAS.10,20,42 Hence, the diagnosis of PAS requires the availability of multidisciplinary team care with well-experienced radiologists in ultrasound and MRI, histopathologists, obstetricians, nursing staff experts, blood bank, and laboratory specialists to establish the accurate diagnosis and management of PAS.5,24
Serological Examination
In recent years, many scholars have focused on the study of serum markers in maternal peripheral blood for PAS because of some misdiagnosed or undiagnosed cases with ultrasound and MRI.43 It mainly includes maternal serum alpha-fetoprotein (AFP), serum creatine kinase (CK), serum pro-BNP, Troponin, pregnancy-related protein (PAPP-A), fetal free DNA (cffDNA), placental free mRNA (β-hCG mRNA and hPL mRNA), vascular endothelial growth factor (VEGF), placental growth factor (PlGF), human soluble-FMS hemolytic vascular endothelial growth factor receptor 1(sFlt-1), insulin-like growth factor (IGF), and several other maternal serological indexes.1,2,44–46
Alpha-Fetoprotein (AFP)
AFP is a common glycoprotein in serum during pregnancy, produced in the fetal liver through the yolk sac during early pregnancy.47 The level of alpha-fetoprotein in serum reaches the peak at 12–15 weeks of pregnancy and decreases to the normal level within 2 weeks after delivery (<20 ng/mL). When the placenta is implanted, alpha-fetoprotein in fetal blood will directly enter maternal blood circulation, so AFP in maternal serum increases, up to 2–5 times that of normal pregnant women.48 Morlando et al49 found that the serum AFP and β-hCG at 14–22 weeks were higher in cases of the PAS, while Fyala et al50 found that elevated third-trimester maternal serum AFP levels could predict PAS in placenta previa patients.
It was found that the abnormal increase in AFP is also related to preeclampsia, preterm birth, FGR, neonatal asphyxia, and placental dysfunction.47,48 Therefore, McDuffie et al51 believed that PAs should be considered in case of elevated serum AFP of pregnant women if fetal malformation and intra-placental hemorrhage and other conditions could be excluded.
Although prenatal maternal serum AFP detection is convenient, according to some domestic scholars, the sensitivity and specificity of prenatal maternal serum AFP in the diagnosis of placenta previa complicated with PAS are only 71% and 46%, respectively.46 This high detection rate might be accidental and can be explained by the fact that elevated serum level of AFP is also found in multiple pregnancies, fetal growth defects, neural tube diseases, ventral wall defects, fetal aneuploidies, fetal kidney defects, fetal demise, and so on.52,53 In order to avoid false-positive results, the detection of maternal AFP in screening PAS must be combined with the detection of high-risk women to screen for PAS with serological tests, and then the use of higher resolution imaging tools like 3-dimensional ultrasound or MRI to exclude these fetal abnormalities.25 Invasive amniocentesis can be performed after 15 weeks of gestation if ultrasound and MRI cannot rule out those fetal abnormalities.54 Hence, the pickup of high-risk women to screen for PAS with the serological test is fundamental and can be done through the clinical assessment of PAS risk factors or the use of other highly specific diagnostic tools such as using color Doppler ultrasound and MRI or invasive amniocentesis to exclude any fetal abnormalities that can falsify the results. However, antenatal maternal serum AFP is an auxiliary, simple and objective method in combination with the clinical assessment of high-risk factors of PAS and the use of highly specific imaging tools and invasive amniocentesis for antenatal diagnosis of PAS.53
Creatinine Kinase (CK)
The determination of serum CK can be used as a serological test index for the diagnosis.55 CK usually exists in the cytoplasm and mitochondria of animal hearts, muscles and brains. It is a fundamental enzyme playing a role in intracellular energy-carrying, muscle contraction, and ATP metabolism.56
Ersoy et al12 conducted a prospective study on 100 pregnant women including 54 women with complete placenta previa and 46 normal pregnant women. Among the 54 pregnant women with complete placenta previa, 14 pregnant women were diagnosed with PAS by placental histopathology. The results showed that the prenatal serum indexes, including N-terminal pro-hormone B-type brain natriuretic peptide (NT-proBNP), CK, CK-MB, and Troponin I, showed the statistical significance in Troponin I, NT-Pro BNP between the two groups (P < 0.05), while the values of CK and CK-MB between the two groups did not show any statistical significance (P > 0.05). Therefore, it is considered that the increase in maternal serum troponin I and NT-proBNP may be due to the increase in neovascularization in patients with placenta previa accreta. Fayed et al57 found that the serum CK value of pregnant women having abnormal placentation was higher than that of pregnant women having normal placentation, 1/19 patients (5.26%) with normal placenta and 10/31 patients (32.26%) with abnormal placentation had elevated serum CK (>160 U/l), which was used as the diagnostic threshold. The accuracy, affectability, specificity, positive prognostic value, and negative prognostic value of the corresponding diagnosis of abnormal placentation were 55%, 30%, 95%, 90%, and 45%, respectively.
It is speculated that the reason for the increase of CK may be that during PAS, due to the developmental defects of uterine decidua, the trophoblasts invade the myometrium, which can lead to muscle cell damage, and then CK is released into the blood, which raises the CK level in serum’s pregnant women. However, the level of CK in uterine smooth muscle cells is not specific, so it is easy to be affected by many factors, such as mechanical skeletal or muscle injury, myocardial infarction, and other diseases, which will also lead to an increase in serum CK. Therefore, when the serum CK of pregnant women increases, it is necessary to fully eliminate other possible diseases before diagnosing PAS.58 There are also reports that the combination of serum AFP, CK, and ultrasonography can improve the diagnostic rate of PAS.44,53,57
Pregnancy-Associated Serum Protein (PAPP-A)
PAPP-A is a pregnancy-related glycoprotein molecule produced by the placental syncytiotrophoblast and the decidua.59 During pregnancy, a large number of decidua are produced and released into the maternal blood circulation, increasing maternal serum PAPP-A until delivery. PAPP-A is an insulin-like growth factor-binding protein playing a fundamental role in early trophoblast intrusion, placental development and vascularization, and invasion. As a biological marker of placental trophoblast, PAPP-A can predict PAS, preeclampsia, fetal growth restriction, spontaneous abortion, preterm birth, and so on.59–61
Desai et al62 measured the serum PAPP-A concentration of 16 pregnant women with placenta previa combined with PAS (accreta group) and 66 pregnant women without placenta previa and PAS (non-accreta group). The results showed that the serum PAPP-A concentration of the accreta group was 1.68 MoM, which was considerably higher than 0.98 MoM of the non-accreta group and 1.00 MoM for normal pregnant women at the same gestational age. Therefore, this study found that the concentration of serum PAPP-A has diagnostic value for judging the high-risk cases of placenta previa accreta. Wang et al61 conducted a retrospective cohort study of 177 pregnant women with placenta previa accreta detecting their serum PAPP-A in the first trimester. The results revealed that PAPP-A MoM of the placenta previa accreta group was considerably higher than those of the placenta previa group and control group, serum PAPP-A was associated with the occurrence of PAS.
However, the sensitivity of serum PAPP-A concentration detection combined with transabdominal color Doppler ultrasonography in predicting placenta previa complicated with PAS is higher than that of the two alone and needs further research. PAPP-A has also some limitations during pregnancy, and it has been found positive in non-pregnant females and males.63
Placental-Free mRNA
Maternal plasma placental-free mRNA can be stably separated from maternal plasma, its concentration can be quantitatively detected, and the detection results can reflect abnormal placental formation.
Kawashima et al64 measured the concentration of free hPL mRNA (human placental lactogen mRNA) in maternal plasma in 5 pregnant women with PAS, 13 pregnant women with placenta previa without PAS, and 92 healthy pregnant women at the gestational age of 28–32 weeks. The results showed that by quantitatively detecting the concentration of hPL mRNA, the diagnostic specificity and sensitivity of PAS were 61.5% and 100%, respectively. Zhou et al44 included 12 pregnant women with placenta previa complicated with PAS, 21 pregnant women with placenta previa without PAS, and 35 healthy pregnant women at the gestational age of 28–30 weeks a, and the concentrations of free β-hCG mRNA and hPL mRNA in the maternal plasma samples were measured. The results showed that the concentrations of free β-hCG mRNA and hPL mRNA in maternal plasma of patients with PAS were considerably higher than those of healthy pregnant women. In particular, it is worth noting that PAS patients who underwent hysterectomy during cesarean section had higher plasma-free β-hCG mRNA and hPL mRNA concentrations than PAS patients who did not undergo a hysterectomy.
Therefore, the quantitative determination of plasma-free mRNA concentration may be used as a biomarker to predict the clinical diagnosis of PAS and can be combined with ultrasound and MRI to improve the accuracy of prenatal diagnosis of PAS.65 Compared with maternal serum cffDNA, placental-free mRNA has gender-independent and detection advantages, and its clinical application prospect is wider.
Cell-Free Fetal DNA (cffDNA)
Maternal serum cffDNA was shown to originate from direct maternal metastasis of villous trophoblast apoptosis. In the process of PAS, the maternal immune response to the invasion of uterine myometrium can lead to the destruction of villous trophoblast and the increase of cffDNA in maternal peripheral blood; PAS is not an abnormality itself. During PAS, placental villous trophoblast cells are destroyed by the maternal immune system, resulting in the increase of cffDNA concentration in maternal blood.
Some literature has reported that the concentration of cffDNA in maternal peripheral blood is related to the functional state of placental tissues, that is, the degree of destruction of placental trophoblast.66 Samuel et al67 included 20 pregnant women in the study on the correlation between the increase of maternal serum cffDNA concentration and PAS. Among them, six were placental adhesions, seven were placenta previa, and seven had a history of a previous cesarean section but normal placenta. The results showed that there was no significant difference (P > 0.05) in the detection of serum cffDNA concentration between pregnant women with placenta previa accreta and pregnant women with normal placenta. The reason behind this is that the sample size included in the study may be too small, the maternal DNA is highly similar to fetal DNA, and the content of fetal DNA is relatively small. The level of cffDNA can only be compared by detecting non-maternal DNA sequences.
Therefore, the clinical application of maternal serum cffDNA concentration detection in the prediction of placenta previa accreta is limited.
Vascular Endothelial Growth Factors (VEGF, PLGF, and sFlt-1)
Vascular Endothelial Growth Factors are various and useful cytokines acting on vascular endothelial cells. They are of great significance in stimulating angiogenesis and growth and maintaining the integrity and normal permeability of the vascular wall. During normal pregnancy, there is an abundant expression of VEGF and its receptors in the placenta and decidua, which is the key factor for the construction of placental and decidual vascular network growth and development.
Wehrum et al45 believed that in the process of placenta formation, if VEGF, PLGF, and sFlt-1 are unbalanced, it may lead to placenta previa complicated with PAS. The results of maternal serum VEGF, PLGF, and sFlt-1 in 90 pregnant women with complete placenta previa and 45 pregnant women without high-risk factors of placenta previa showed that the level of serum VEGF in pregnant women with complete placenta previa was significantly lower than that in pregnant women with single pregnancy without high-risk factors of placenta previa (P = 0.020), while there were no significant differences in PlGF levels (P = 0.137) and sFlt-1 levels (P = 0.758).
The results of Biberoglu et al68 are inconsistent with Wehrum. He included 68 pregnant women with placenta previa in the study group (33 without PAS, 17 with placenta accreta/increta, and 18 with placenta percreta). Healthy pregnant women who finally delivered at 37 weeks of gestation were included in the control group (n = 30). The results of maternal serum VEGF, PLGF, and sFlt-1 concentrations and the ratio of PLGF and sFlt-1 (sFlt-1/PLGF) were compared between the two groups, and there was no statistical significance (P > 0.05 for all). To investigate the reason, he believes that the circulating biological indicators of angiogenesis and vascularization should be comparable regardless of the degree of placental invasion because the concentration of maternal serum VEGF, PLGF, and sFlt-1 cannot reflect the local level of decidual and placental interface.
Uyanıkoğlu et al69 measured the maternal serum levels of VEGF, PLGF, and sFlt-1 before and after surgery in 44 pregnant women who delivered by cesarean section, 22 women due to placenta percreta, and 22 due to other diseases. The maternal serum levels of VEGF, PLGF, and sFlt-1 before surgery were found lower in women with placenta percreta than that in women with other diseases in the controls (p < 0.001, p < 0.001, and p < 0.05 respectively), while the maternal serum levels of VEGF and sFlt-1 after surgery were found higher with the placenta percreta than that in the controls (p = 0.001 and p < 0.001 respectively), PLGF levels were the same in both groups (p = 0.72).
Therefore, measuring the concentration of those serum biomarkers of angiogenesis was defective for the diagnosis of placenta previa combined with PAS while it appears more significant in the diagnosis of the placenta percreta. Perhaps, the current detection technology could not show the difference, which needs to be confirmed by further research.
Insulin-Like Growth Factor-1 (IGF-1)
IGF-1 is a cytokine with various functions in the fetal growth and development pathways in the regulation of cell multiplication, necrosis, and cell death.70
Insulin-like growth factors belong to a family of peptides essential for fetal growth and development, and may also play a role as a modulator of cell growth in fetal membranes and placenta via autocrine/paracrine mutual actions and the uterine decidua has been considered as its location of action.71 Umana-Perez et al72 believed that IGF-1 and TGF-β (Transforming growth factor-β secreted by the maternal decidua and by the placenta and highly expressed only during the first trimester of pregnancy) play oppositely key roles in the regulation of human placenta growth. IGF-1 role is to promote the multiplication and differentiation of trophoblastic cells, while the TGF-β role is to limit the invasion of trophoblastic cells. Their study results showed that serum concentration abnormalities of IGF-1 and TGF-β have been associated with pregnancies complicated by PAS, preeclampsia, FGR, infertility, miscarriage, and gestational trophoblastic diseases. Therefore, whether IGF-1 and TGF-β can be used as an early diagnostic intervention tools in high-risk pregnant women with probable PAS needs to be confirmed by further research.
Overall, the characteristics of the main studies included in the serological analysis of multiple markers that may predict PAS were comparatively listed below (Table 1).
 |
Table 1 Characteristics of Main Studies Included in the Serological Analysis |
Ultrasonic Examination
Ultrasound, as the most preferred method in diagnosing PAS, has received the attention of many scholars.31 Comstock et al41 pointed out that if ultrasound found the low position of the gestational sac before 10 weeks, and in the regular interface between villi and myometrium, we should be particularly vigilant against the occurrence of PAS.
At present, there are few domestic studies on the diagnosis of PAS by ultrasound in early pregnancy in clinical practice, but many scholars have turned their attention to the study of early ultrasound images of PAS. If the diagnosis of the degree of PAS can be determined in the early pregnancy, the possible risks of uterine rupture in the middle and late pregnancy can be evaluated to decide whether to continue the pregnancy or terminate the pregnancy and to improve the outcome of pregnant women.
There are many studies on the ultrasonic image characteristics of PAS in the middle and late pregnancy, such as partial or complete disappearance of the posterior low echo of the placenta, placental lacunae, thinning of the posterior muscular layer of the placenta, the irregular boundary between uterus and bladder, abundant blood flow signals of the placenta and so on.31 However, there are different reports on the sensitivity and specificity of ultrasound in the diagnosis of PAS.
Bowman et al36 studied the ultrasonic images of 55 pregnant women with simple placenta previa and 56 pregnant women with placenta previa and accreta, and obtained that the sensitivity and specificity of ultrasonic diagnosis of placenta previa were 53.5% and 88.0%, respectively. In the same year, Riteau et al73 retrospectively analyzed the ultrasound images of 42 patients with PAS and concluded that the sensitivity and specificity of ultrasound diagnosis of PAS were 100% and 37.5%, respectively.
The reasons for the statistical differences in various kinds of literature may be as follows: the ultrasound images of PAS are complex, the operators are highly subjective, there are great differences in experience, and there is no objective and standardized diagnostic standard. Therefore, we strive to find more objective indicators to diagnose PAS.
Ultrasonic Doppler Blood Flow Vessels Indexes Assessment
Uterine artery and umbilical artery Doppler velocimetry are becoming the key tools for the prediction of placenta previa and PAS. Cho et al74 assessed the significance of PI of uterine artery Doppler velocimetry in diagnosing PAS. Their results found that the mean uterine artery PI was significantly decreased in the PAS group compared to the previa group alone (0.51 versus 0.57). The area under the receiver operating characteristic curve was 0.72 for the mean prior cesarean section alone, and 0.77 for the mean PI and prior cesarean section together. This implies that the mean uterine artery PI is reduced in patients with PAS compared to those without PAS, which concludes that the assessment of the uterine artery Doppler together with the history of prior cesarean sections can improve the diagnostic accuracy of PAS. Zamanskaya et al75 analyzed the uterine, fetal, and placental characteristics of 50 patients with normal placenta, 50 patients with placenta previa, and 28 patients with PAS on 20–22nd, 30–32nd, and 35–36th weeks of gestation. The results found that the blood flow indexes of the uterine arteries of patients with placenta previa were considerably high compared to those with normal placentation. Moreover, the pulsatility index (PI) in the right uterine artery was considerably low in pregnant women with PAS compared to those with normal placentation and placenta previa. Moreover, fetoplacental hemodynamic changes in the umbilical artery blood flow in women with placenta previa, PAS, and normal placentation were found, while the middle cerebral artery blood flow indices did not change considerably in the three groups of patients at any period of gestation. Bostan et al76 prospectively examined the uterine artery Doppler velocimetry changes in 15 pregnant women with anterior placenta previa and 15 pregnant women with anterior placenta previa accreta in the third trimester of pregnancy. The data obtained showed that the mean uterine artery Doppler velocimetry indices as well as mean resistance index (RI) and mean pulsatility index (PI) were significantly decreased in women with PAS as compared with women with placenta previa (0.45 versus 0.451 for mean RI and 0.56 versus 0.50 for mean PI). Similarly, Fahmy et al77 pointed out the contribution of PI of uterine artery Doppler in the diagnosis of PAS in 32 patients with PAS and 22 patients with placenta previa. The results revealed that PI was importantly decreased in the accreta group (0.62 ± 0.20) than in the non-accreta control group (0.89 ± 0.23). The above two studies concluded that the reduction of RI and PI indices of uterine artery Doppler in combination with the maternal history of previous cesarean sections could improve the diagnosis accuracy of patients with PAS compared to those without PAS. Therefore, uterine artery Doppler low PI can become a useful prediction of placenta previa and PAS. Lu et al78 conducted a single-center retrospective cohort study of 504 patients at 22–24 weeks of gestation, divided into three groups: 64 cases in the placenta previa group, 89 cases in the resolving group and 351 cases in the control group, and uterine artery S/D ratio, RI, and PI were calculated. The results showed that the means of S/D ratio, PI, and RI of uterine arteries in the placenta previa group were much lower than that in the other groups. This study also found out that the elevated mean values of S/D ratio, PI, and RI designated the good prognosis of placenta previa, while the low mean values of S/D ratio, PI, and RI designated the bad prognosis of placenta previa at 36 weeks of gestation. This implies the probable predictive value of S/D ratio, PI, and RI of uterine artery Doppler velocimetry modifications at mid-term of gestation on persistent placenta previa or resolved placenta previa at the end of the third trimester.
These studies all suggest that uterine arcuate artery blood flow Doppler confirmed the significant value in the diagnosis of placenta previa accreta in different studies. However, further studies are needed to reevaluate the efficacy of umbilical artery blood flow parameters in the diagnosis of placenta previa accreta.
Common Ultrasonic Image Features
Placental Lacunae
During PAS, anechoic lacunae are common in the placental parenchyma, which is irregular in shape as insect erosion (Figure 3A). On the ultrasonic image, they are usually called Swiss cheese syndrome or placental lacunae. Jauniaux et al26 pointed out that Swiss cheese is a word used to describe chorionic edema during hydatidiform mole, and it is distributed in any part of the placenta without blood flow, so it is not recommended to use Swiss cheese to describe PAS. During PAS, trophoblasts further invade the vascular wall of the proximal uterine spiral artery, radial artery, arcuate artery, and even uterine artery, so that the arterioles with high pulse pressure directly open in the placental parenchyma and affect the placental parenchyma to form different placental lacunae. Therefore, the placental lacunae of different sizes are often located on the deep side of the placenta (Figure 3B–D).
Riteau et al73 believed that the sensitivity of using placental lacunae to diagnose PAS is the highest in various two-dimensional ultrasound features, which is 88%. Many kinds of literature can be consulted to score the possibility of PAS by using placental lacunae. For example, Finberg et al79 proposed that the absence of placental lacunae is recorded as 0; 1–3 small lacunae are recorded as 1 +; 4–6 large lacunae are recorded as 2 +; When multiple lacunae are penetrating the placenta, it is recorded as 3 +; Normal placenta or adhesions are mostly graded 0–1, implantation or penetration is mostly graded 2–3.
Partial or Complete Loss of the Retroplacental Hypoechoic Zone
On the 2D (two-dimensional) gray-scale ultrasound image, a clear solitary hypoechoic zone can be seen behind the placenta, which is formed by the trophoblast and decidua of fixed villi. During pregnancy, the branches of the uterine spiral artery and uterine vein expand and interlace, so it shows low echo. If the loss of endometrium leads to poor local decidua formation, the villi can directly invade the myometrium and cause PAS. On the ultrasonic image, it shows that the retroplacental hypoechogenicity disappears partially or completely (Figure 3C and D).
Finberg et al79 pointed out that the loss of the retroplacental hypoechoic zone is the primary cause of false-positive ultrasound diagnosis of PAS. Therefore, if a retroplacental hypoechoic zone exists, PAS can be excluded, but it should not be used as a single diagnostic criterion. Cali et al80 studied the ultrasound images of 187 patients with PAS, which also confirmed this view.
Thinning of Retroplacental Myometrial Thickness
In the case of PAS, due to the invasion of the placental villi, the myometrium behind the placenta may be thinner than its surrounding area, which can be displayed on two-dimensional gray-scale ultrasound images. In their study, Riteau et al73 selected the thickness of the myometrial layer after the placenta was less than 1mm as the standard, and obtained that its sensitivity was 73%. They used the thickness of the uterine myometrium at the placental attachment of less than 1mm as the standard in diagnosing PAS. However, because the myometrium of the lower segment of the uterus and the scar of cesarean section is thinner than that of the uterine body and fundus, the myometrium will become thinner and thinner as pregnancy progresses.
Thinning or Loss of Uterine -Bladder Boundary
On the two-dimensional gray-scale ultrasound image, the uterus-bladder boundary shows a smooth and continuous line like a strong echo, which is the serosa echo of the uterus and bladder. It is more clearly displayed when the bladder is filled. Jauniaux et al81 believed that it may also be the ultrasonic artifact caused by the folded peritoneum rich in neovascularization between the anterior myometrium and the posterior bladder wall, which should be distinguished.
If the placental villi invade the uterine serosa or penetrate the uterus to reach the bladder and invade the serosa of the bladder, the linear strong echo at the junction of the uterus and bladder becomes thinner, irregular, or disappears (Figure 3D). Pilloni et al82 retrospectively analyzed the ultrasonic images of 314 pregnant women with placenta previa, and concluded that the uterine-bladder boundary became thinner, the irregular sensitivity was 40.5%, and the specificity was 97.8%. This sign often appears when the placental is heavily implanted or penetrated, and generally does not appear when there is adhesion or mild implantation, so its specificity is high.
Abundant Blood Flow Signal Behind the Placenta
During PAS, the blood vessels of the uterus and placenta abundantly expand. Doppler ultrasound can show abundant blood flow signals behind the placenta (Figure 3E and F), which is better when the bladder is filled. Studies found that the diagnostic sensitivity of transabdominal Doppler ultrasound with abundant blood flow signals behind the placenta was 94%.73 Likewise, Daney et al83 believed that the sensitivity and the specificity of transabdominal Doppler ultrasound to the diagnosis of PA were 85% and 78%, respectively. However, Oyelese et al84 believed that transvaginal ultrasound does not increase the rate of vaginal bleeding.
Nevertheless, transvaginal ultrasound is rarely used to evaluate PAS during pregnancy. Although the accuracy of this feature is high, the ultrasonic definition of abundant blood flow signals behind the placenta is not clear, and the adjustment of the velocity scale is not clearly defined, which is greatly affected by the experience of ultrasonic diagnostic doctors.
MRI Examination
MRI has recently become an effective auxiliary examination method besides ultrasound, especially when ultrasound cannot provide conclusive diagnostic information and in the case of the posterior placenta or placenta percreta. However, in some clinics, it is required for all patients with doubted PAS.42 MRI is also used to assist the ultrasound in the evaluation of the placental deepness invasion, the lateral outspreading degree of myometrial and parametrial invasion in doubted features found on ultrasound.20 MRI has the advantages of sensitivity to blood flow, large imaging range, high soft tissue separation rate, and is not affected by maternal obesity, less amniotic fluid, posterior placenta, and so on. However, it has the disadvantages of high examination cost, long inspection time, and easy to be affected by fetal movement.
MRI findings of the normal placenta:85
- In early pregnancy, the outer contour of the uterine wall is continuous, smooth, and without a bulge.
- On T2 weighted imaging (T2WI), there are clear three-layer signal bands: the uterine myometrium with medium signal in the middle, the uterine junction band and uterine serosa with low signal inside and outside.
- In late pregnancy, the uterus is in an “inverted pear shape”, and the uterine wall becomes thinner, but the boundary of each signal band is still clear on T2WI.
Common MRI signs of PAS:42
Direct signs: on T2WI (T2-weighted images), the placenta invaded the myometrium or other surrounding organs forming dark intra-placental bands (Figure 4A and B), and the uterine layer becomes thinner and interrupted, the junction zone between the uterus and placenta becomes thinner or disappears. The low signal of uterine serosa also disappears, and the bladder wall is not smooth due to the uterus bulging inside (Figure 4C).
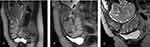 |
Figure 4 MRI images in placenta accreta spectrum. (A and B) White arrows show dark intra-placental bands on T2-weighted images. (C) White arrows indicate the uterus bulging into the bladder. |
Indirect signs: an uneven thickening of the placenta, an uneven signal of the placenta, multiple migrations and expansion of blood vessels in the placenta, localized swelling of the uterus, thinning, and interruption of the myometrium, swelling of the lower segment of the uterus, abnormal changes in the surrounding or tissues of the uterus like the bladder (Figure 4C).
Riteau et al73 retrospectively analyzed MRI images of 42 patients with PAS and concluded that the sensitivity and specificity of ultrasound diagnosis of PAS were 76.9% and 90%, respectively. After a meta-analysis of 20 studies, Familiar et al86 concluded that the diagnostic sensitivity of MRI for placental adhesion, implantation and penetration were 94.4%, 100%, and 86.5%, respectively, this indicates that MRI has high accuracy in the diagnosis of PAS, and has certain significance in the differentiation of PAS degree. Mahalingam et al42 retrospectively analyzed the MRI and histopathological tests of 60 pregnant women with probable PAS. It was concluded that the sensitivity, specificity, and accuracy of MRI in the diagnosis of PAS were 85.71%, 94.87%, and 92.5%, respectively.
Recently in 2023, Bai et al87 conducted a multicenter retrospective and it was revealed that MRI test has better reliability in predicting PAS than ultrasonography test. The sensitivity and specificity of MRI in predicting PAS were 79.2% and 97.8%, respectively, while the sensitivity and specificity of ultrasonography in predicting PAS were 47.5% and 88.4%, respectively.
Histopathological Diagnosis of Placenta Accreta
Histologically, PAS is currently defined by a partial or complete loss of decidual basalis, leading to placental villi attaching directly to the surface of the myometrium or invading the scarred myometrium81 or aberrant implantation with a layer of fibrinoids and intermediate trophoblast cells between chorionic villi and myometrium on the microscopic test of placenta bed.6
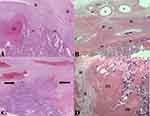
Although the histopathological features in PAS have not yet been studied by many scholars and standardized, the most recognized histopathological findings are the followings:88
- Presence in number and depth of Extravillous trophoblasts cells (EVTs) invasion in the myometrium (Figure 5A–C),
- Absence of the decidual layer between the anchoring villi and the myometrium (Figure 5C),
- Vascular lesions (Intervillous thromboses IVT, excessive fibrinoid deposits at the uteroplacental interface and villous infarction (Figure 5D)),
- Superficial myometrial vessels change (Figure 5B).
Parra-Herran et al89 pointed out that the histopathologic degree of PAS relies on the intrusion of placental chorionic villi into myometrial vascular spaces and EVTs proliferation after analyzing the histopathologic and immunohistochemistry features of 61 women who underwent hysterectomies, 44 with PAS, 17 without PAS. The results revealed that the mean invasion of chorionic villi in the myometrial vascular spaces was 70.4% (31/44) in PAS and 5.8% (1/17) in the control group, and the features were found more in women with placenta percreta (87.5%) and placenta increta (84%) than in the women with placenta accreta (27.2%). The mean depth of trophoblast myometrial invasion was 47.9% in the PAS group and 14.5% in the control group. The mean intermediate trophoblast cell count using GATA3 immunohistochemistry was 664 in the PAS group and 288 in the control group.
A panel conducted by FIGO experts in 2020 has consensually proposed the new classification and guidelines criteria for the pathologic diagnosis of PAS diseases separately, including PAS and BPMF:6
Placenta Accreta Spectrum (PAS)
(1) PAS Grades (1–3).
(1) PAS Grade 1: Noninvasive morbidly adherent placenta (placenta accreta): Myometrial sections display a clear and complete placental–myometrial boundary and uniform myometrial thickness without thinning.
(2) PAS Grade 2: Superficial invasion of the placenta (placenta increta): Myometrial sections display an irregular placental–myometrial interface without the outer myometrium (with conservation of at least 25% of the wall thickness relative to the detached myometrium).
(3) PAS Grade 3A: Deep invasion of the placenta: Myometrial sections display an irregular placenta–myometrial interface with involvement of the outer myometrium (with conservation of less than 25% of the wall thickness relative to the detached myometrium). The serosa is completely safe (A = Abnormal invasion).
(4) PAS Grade 3D: Deep invasion of the placenta with disturbance of the serosa (placenta percreta): grossly invasive placenta with disruption of the uterine serosa surface (D = Deep invasion).
(5) PAS Grade 3E: Deep invasion of the placenta with adherent extra uterine tissues and organs: placental invasion into nearby organs (commonly the bladder) or extra-uterine fibro adipose tissues, confirmed by microscopy (E = Extra uterine invasion).
PAS Grades 3B and 3C are pathologically inserted in Grade 3E.
(2) Other elements are described as follows:
(a) Location of invasion in the uterus.
(b) % (average) of the disc that is attached or invading the myometrium.
(c) Location of invasive component:
• Lower uterine segment (nearby to the bladder).
• Anterior wall (above bladder).
• Posterior wall or lateral wall/broad ligament.
(d) Maximum percentage invasion 75%.
(e) Location/extent of serosal disturbance (bladder, colon).
(f) Implantation over cesarean scar dehiscence.
Basal Plate Myometrium Fibers (BPMF)
BPMF refers to the slight attachment of the myometrial smooth muscle fibers to the placental basal plate with or without the involvement of the decidual layer between the anchoring villi and the myometrium (Figure 5A and B).
A diagnosis of BPMF is made based on criteria including stage and size as follows:
(1) Stage 1—Presence of decidua.
(2) Stage 2—Lack of decidua.
(3) Size (mm)—linear length along with the basal plate in the biggest focus.
(4) Number of detached foci.
However, BPMF can be a supplementary feature confirming noninvasive PAS cases in clinical practice. Stage 1 BPMF (with intervening decidua) is a more probable supplementary feature than stage 2 (without intervening decidua), but it is suggested to elucidate the clinical relationship between the two stages. BPMF are sectioned from delivered placentas or curetting specimens while PAS are sectioned from partial or complete placental hysterectomy specimens.
We recommend the usage of this new histologic grading system because it is intended to deal with the clinical and histopathologic divergences in PAS literature by distinguishing adherent and invasive placentation and to standardize the communication between community clinics and specialty hospitals.4,7
Conclusions
PAS is becoming a common emergency disease in current obstetrics due to the growing number of cesarean sections, placenta previa, and assisted reproductive technology (ART). The early and precise diagnosis of PAS is imperative to prevent maternal intrapartum or postpartum bleeding complications. Despite standard ultrasound being a reliable and key tool for the diagnosis of PAS, the lack of ultrasound features does not exclude the diagnosis of PAS. Therefore, the clinical assessment of risk factors, MRI tests, serological markers, and placental histopathological tests are also indispensable all together with the basic ultrasound for the early and accurate diagnosis of PAS as no one of those tests has reached the maximum sensitivity for the diagnosis of PAS. Moreover, a multidisciplinary squad with well-experienced obstetricians, radiologists, and histopathologists should be involved in the establishment of the conclusive diagnosis of PAS.
However, further investigations about the pathogenesis and the diagnosis of PAS in primiparous pregnancies without any history of cesarean sections or other uterine surgeries or ART are necessary for the future.
Ethical Approval
This study was approved by the institutional ethical board of Wuhan Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology.
Acknowledgments
We thank all patients who participated in this study. We also thank National Nature Science Foundation of China that supported this study.
Funding
This work was supported by the National Nature Science Foundation of China (No.81873844 to ZouLi).
Disclosure
No potential conflict of interest relevant to this article was reported.
References
1. Bartels HC, Postle JD, Downey P, Brennan DJ. Placenta accreta spectrum: a review of pathology, molecular biology, and biomarkers. Dis Markers. 2018;2018:1507674. doi:10.1155/2018/1507674
2. Cahill AG, Beigi R, Heine RP, Silver RM, Wax JR. Placenta accreta spectrum. Am J Obstet Gynecol. 2018;219(6):B2–b16. doi:10.1016/j.ajog.2018.09.042
3. Cırpan T, Akdemir A, Okmen F, Hortu I, Ekici H, Imamoglu M. Effectiveness of segmental resection technique in the treatment of placenta accreta spectrum. J Matern Fetal Neonatal Med. 2021;34(19):3227–3233. doi:10.1080/14767058.2019.1702019
4. Jauniaux E, Zheng W, Yan J. Confirming the diagnosis and classifying placenta accreta spectrum (PAS) disorders: minutes of 2020 online international workshop on PAS in Beijing. Maternal Fetal Med. 2021;3(4):229–231.
5. Liu X, Wang Y, Wu Y, et al. What we know about placenta accreta spectrum (PAS). Eur J Obstetr Gynecol Reprod Biol. 2021;259:81–89. doi:10.1016/j.ejogrb.2021.02.001
6. Hecht JL, Baergen R, Ernst LM, et al. Classification and reporting guidelines for the pathology diagnosis of placenta accreta spectrum (PAS) disorders: recommendations from an expert panel. Mod Pathol. 2020;33(12):2382–2396. doi:10.1038/s41379-020-0569-1
7. Salmanian B, Shainker SA, Hecht JL, et al. The Society for Pediatric Pathology Task Force grading system for placenta accreta spectrum and its correlation with clinical outcomes. Am J Obstet Gynecol. 2022;226(5):720.e721–720.e726.
8. Ayati S, Leila L, Pezeshkirad M, et al. Accuracy of color Doppler ultrasonography and magnetic resonance imaging in diagnosis of placenta accreta: a survey of 82 cases. Int J Reprod Biomed. 2017;15(4):225–230.
9. Berezowsky A, Pardo J, Ben-Zion M, Wiznitzer A, Aviram A. Second trimester biochemical markers as possible predictors of pathological placentation: a retrospective case-control study. Fetal Diagn Ther. 2019;46(3):187–192. doi:10.1159/000492829
10. Shazly SA, Hortu I, Shih JC, et al. Prediction of success of uterus-preserving management in women with placenta accreta spectrum (CON-PAS score): a multicenter international study. Int J Gynaecol Obstet. 2021;154(2):304–311. doi:10.1002/ijgo.13518
11. Pegu B, Thiagaraju C, Nayak D, Subbaiah M. Placenta accreta spectrum-a catastrophic situation in obstetrics. Obstet Gynecol Sci. 2021;64(3):239–247. doi:10.5468/ogs.20345
12. Ersoy AO, Oztas E, Ozler S, et al. Can venous ProBNP levels predict placenta accreta? J Matern Fetal Neonatal Med. 2016;29(24):4020–4024. doi:10.3109/14767058.2016.1152576
13. Jin T, Kyozuka H, Fujimori M, et al. Unexpected placenta accreta spectrum after the use of assisted reproductive technology in women with adenomyomectomy. Fukushima J Med Sci. 2021;67(1):45–48. doi:10.5387/fms.2021-02
14. Schwickert A, van Beekhuizen HJ, Bertholdt C, et al. Association of peripartum management and high maternal blood loss at cesarean delivery for placenta accreta spectrum (PAS): a multinational database study. Acta Obstet Gynecol Scand. 2021;100(Suppl 1):29–40. doi:10.1111/aogs.14103
15. Yu FNY, Leung KY. Antenatal diagnosis of placenta accreta spectrum (PAS) disorders. Best Pract Res Clin Obstet Gynaecol. 2021;72:13–24. doi:10.1016/j.bpobgyn.2020.06.010
16. Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. Bjog. 2021;128(10):1646–1655. doi:10.1111/1471-0528.16647
17. Crocetto F, Saccone G, Raffone A, et al. Urinary incontinence after planned cesarean hysterectomy for placenta accreta. Urol Int. 2021;105(11–12):1099–1103. doi:10.1159/000518114
18. Kong X, Kong Y, Yan J, Hu JJ, Wang FF, Zhang L. On opportunity for emergency cesarean hysterectomy and pregnancy outcomes of patients with placenta accreta. Medicine. 2017;96(39):e7930. doi:10.1097/MD.0000000000007930
19. Atallah D, Abou Zeid H, Moubarak M, Moussa M, Nassif N, Jebara V. “You only live twice”: multidisciplinary management of catastrophic case in placenta Accreta Spectrum-a case report. BMC Pregnancy Childbirth. 2020;20(1):135. doi:10.1186/s12884-020-2817-2
20. Jauniaux E, Alfirevic Z, Bhide AG, et al. Placenta praevia and placenta accreta: diagnosis and management: green-top guideline no. 27a. Bjog. 2019;126(1):e1–e48. doi:10.1111/1471-0528.15306
21. Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. The management and outcomes of placenta accreta, increta, and percreta in the UK: a population-based descriptive study. Bjog. 2014;121(1):62–70;discussion 70–61. doi:10.1111/1471-0528.12405
22. Murayama Y, Seki H, Takeda S. Intra-arterial balloon occlusion to reduce operative bleeding for placenta previa accreta spectrum. Surg J. 2021;7(Suppl 1):S11–s19. doi:10.1055/s-0040-1721491
23. Shazly SA, Hortu I, Shih JC, et al. Prediction of clinical outcomes in women with placenta accreta spectrum using machine learning models: an international multicenter study. J Matern Fetal Neonatal Med. 2022;35(25):6644–6653. doi:10.1080/14767058.2021.1918670
24. Anderson-Bagga FM, Sze A. Placenta previa. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
25. Aboughalia H, Bastawrous S, Revzin MV, Delaney SS, Katz DS, Moshiri M. Imaging findings in association with altered maternal alpha-fetoprotein levels during pregnancy. Abdominal Radiol. 2020;45(10):3239–3257. doi:10.1007/s00261-020-02499-2
26. Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218(1):75–87. doi:10.1016/j.ajog.2017.05.067
27. James-Allan LB, Whitley GS, Leslie K, Wallace AE, Cartwright JE. Decidual cell regulation of trophoblast is altered in pregnancies at risk of pre-eclampsia. J Mol Endocrinol. 2018;60(3):239–246. doi:10.1530/JME-17-0243
28. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. doi:10.1210/er.2014-1045
29. Ng SW, Norwitz GA, Pavlicev M, Tilburgs T, Simón C, Norwitz ER. Endometrial decidualization: the primary driver of pregnancy health. Int J Mol Sci. 2020;21(11):4092. doi:10.3390/ijms21114092
30. Lacroix MC, Guibourdenche J, Fournier T, et al. Stimulation of human trophoblast invasion by placental growth hormone. Endocrinology. 2005;146(5):2434–2444. doi:10.1210/en.2004-1550
31. Goh WA, Zalud I. Placenta accreta: diagnosis, management and the molecular biology of the morbidly adherent placenta. J Matern Fetal Neonatal Med. 2016;29(11):1795–1800. doi:10.3109/14767058.2015.1064103
32. Pollheimer J, Knöfler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta. 2005;26(Suppl A):S21–30. doi:10.1016/j.placenta.2004.11.013
33. Knöfler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54(2–3):269–280. doi:10.1387/ijdb.082769mk
34. Lin H, Li L, Lin Y, Wang W. Accuracy of magnetic resonance imaging in diagnosing placenta accreta: a systematic review and meta-analysis. Comput Math Methods Med. 2022;2022:2751559. doi:10.1155/2022/2751559
35. Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192(5):1458–1461. doi:10.1016/j.ajog.2004.12.074
36. Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211(2):177.e171–177. doi:10.1016/j.ajog.2014.03.029
37. Solheim KN, Esakoff TF, Little SE, Cheng YW, Sparks TN, Caughey AB. The effect of cesarean delivery rates on the future incidence of placenta previa, placenta accreta, and maternal mortality. J Matern Fetal Neonatal Med. 2011;24(11):1341–1346. doi:10.3109/14767058.2011.553695
38. Matsuzaki S, Mandelbaum RS, Sangara RN, et al. Trends, characteristics, and outcomes of placenta accreta spectrum: a national study in the United States. Am J Obstet Gynecol. 2021;225(5):534.e531–534.e538. doi:10.1016/j.ajog.2021.04.233
39. Lin Y, Chen Q, Huang X, et al. Obstetric and perinatal outcomes after assisted reproductive technology in women with cesarean scar. Front Physiol. 2022;13:808079. doi:10.3389/fphys.2022.808079
40. Ogawa K, Jwa SC, Morisaki N, Sago H. Risk factors and clinical outcomes for placenta accreta spectrum with or without placenta previa. Arch Gynecol Obstet. 2022;305(3):607–615. doi:10.1007/s00404-021-06189-2
41. Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. Bjog. 2014;121(2):171–181;discussion 181–172. doi:10.1111/1471-0528.12557
42. Mahalingam HV, Rangasami R, Premkumar J, Chandrasekar A. Placenta accreta scoring system (PASS)—assessment of a simplified clinico-radiological scoring system for antenatal diagnosis of placenta accreta. Egypt J Radiol Nuclear Med. 2021;52(1):42. doi:10.1186/s43055-021-00427-y
43. Al-Khan A, Youssef YH, Feldman KM, et al. Biomarkers of abnormally invasive placenta. Placenta. 2020;91:37–42. doi:10.1016/j.placenta.2020.01.007
44. Zhou J, Li J, Yan P, et al. Maternal plasma levels of cell-free β-HCG mRNA as a prenatal diagnostic indicator of placenta accrete. Placenta. 2014;35(9):691–695. doi:10.1016/j.placenta.2014.07.007
45. Wehrum MJ, Buhimschi IA, Salafia C, et al. Accreta complicating complete placenta previa is characterized by reduced systemic levels of vascular endothelial growth factor and by epithelial-to-mesenchymal transition of the invasive trophoblast. Am J Obstet Gynecol. 2011;204(5):411.e411–411.e411. doi:10.1016/j.ajog.2010.12.027
46. Zhang T, Wang S. Potential serum biomarkers in prenatal diagnosis of placenta accreta spectrum. Front Med. 2022;9:860186. doi:10.3389/fmed.2022.860186
47. García-Cavazos R, Colín-Valenzuela A, Espino y Sosa S. Alfa fetoproteína como predictor temprano de desenlace perinatal adverso [Alpha-fetoprotein as an early predictor of adverse perinatal outcome]. Ginecol Obstet Mex. 2010;78(5):268–274. Spanish.
48. Butler EL, Dashe JS, Ramus RM. Association between maternal serum alpha-fetoprotein and adverse outcomes in pregnancies with placenta previa. Obstet Gynecol. 2001;97(1):35–38. doi:10.1016/s0029-7844(00)01095-4
49. Morlando M, Collins S. Placenta accreta spectrum disorders: challenges, risks, and management strategies. Int J Womens Health. 2020;12:1033–1045. doi:10.2147/IJWH.S224191
50. Fyala EA. Value of measurement of maternal serum alpha fetoprotien in diagnosis of pathologically adherent placenta in cases of placenta pravia. J Egypt J Fertil Steril. 2018;22(2):25–31. doi:10.21608/egyfs.2018.65838
51. McDuffie RS, Harkness L, McVay RM, Haverkamp AD. Midtrimester hemoperitoneum caused by placenta percreta in association with elevated maternal serum alpha-fetoprotein level. Am J Obstet Gynecol. 1994;171(2):565–566. doi:10.1016/0002-9378(94)90304-2
52. Uldbjerg CS, Lim YH, Glazer CH, Hauser R, Juul A, Bräuner EV. Maternal serum α-fetoprotein levels during pregnancy and testicular cancer in male offspring: a cohort study within a Danish pregnancy screening registry. Int J Environ Res Public Health. 2022;19(21):14112. doi:10.3390/ijerph192114112
53. Lyell DJ, Faucett AM, Baer RJ, et al. Maternal serum markers, characteristics and morbidly adherent placenta in women with previa. J Perinatol. 2015;35(8):570–574. doi:10.1038/jp.2015.40
54. Giovannopoulou E, Tsakiridis I, Mamopoulos A, et al. Invasive prenatal diagnostic testing for aneuploidies in singleton pregnancies: a comparative review of major guidelines. Medicina. 2022;58(10):1472. doi:10.3390/medicina58101472
55. Ophir E, Tendler R, Odeh M, Khouri S, Oettinger M. Creatine kinase as a biochemical marker in diagnosis of placenta increta and percreta. Am J Obstet Gynecol. 1999;180(4):1039–1040. doi:10.1016/S0002-9378(99)70683-6
56. Shoji S. Kureachinkināze (CK) [Creatine kinase (CK)]. Nihon Rinsho. 1995;53(5):1136–1140. Japanese.
57. Fayed MR, Mourad AW, Mahmoud MA, Mohamed AA. Role of Doppler ultrasound and creatine kinase as a biochemical marker in diagnosis of placenta accreta. J Benha J Appl Sci. 2020;5(Issue1 part (2)):191–197.
58. Kumbhare D, Parkinson W, Dunlop B. Validity of serum creatine kinase as a measure of muscle injury produced by lumbar surgery. J Spinal Disord Tech. 2008;21(1):49–54. doi:10.1097/BSD.0b013e31805777fb
59. Nishizawa H, Pryor-Koishi K, Suzuki M, et al. Increased levels of pregnancy-associated plasma protein-A2 in the serum of pre-eclamptic patients. Mol Hum Reprod. 2008;14(10):595–602. doi:10.1093/molehr/gan054
60. Wagner PK, Otomo A, Christians JK. Regulation of pregnancy-associated plasma protein A2 (PAPPA2) in a human placental trophoblast cell line (BeWo). Reprod Biol Endocrinol. 2011;9:48. doi:10.1186/1477-7827-9-48
61. Wang F, Chen S, Wang J, et al. First trimester serum PAPP-A is associated with placenta accreta: a retrospective study. Arch Gynecol Obstet. 2021;303(3):645–652. doi:10.1007/s00404-020-05960-1
62. Desai N, Krantz D, Roman A, Fleischer A, Boulis S, Rochelson B. Elevated first trimester PAPP--a is associated with increased risk of placenta accreta. Prenat Diagn. 2014;34(2):159–162. doi:10.1002/pd.4277
63. Bischof P, Schindler AM, Wyss R, Herrmann WL, Sizonenko PC. Progesterone dependence and extratrophoblastic origin of pregnancy-associated plasma protein-A (PAPP-A) in early pregnancy. Arch Gynecol. 1986;237(3):109–116. doi:10.1007/BF02133854
64. Kawashima A, Sekizawa A, Ventura W, et al. Increased levels of cell-free human placental lactogen mRNA at 28–32 gestational weeks in plasma of pregnant women with placenta previa and invasive placenta. Reprod Sci. 2014;21(2):215–220. doi:10.1177/1933719113492209
65. El Behery MM, Rasha LE, El Alfy Y. Cell-free placental mRNA in maternal plasma to predict placental invasion in patients with placenta accreta. Int J Gynaecol Obstet. 2010;109(1):30–33. doi:10.1016/j.ijgo.2009.11.013
66. Sekizawa A, Jimbo M, Saito H, et al. Increased cell-free fetal DNA in plasma of two women with invasive placenta. Clin Chem. 2002;48(2):353–354. doi:10.1093/clinchem/48.2.353
67. Samuel A, Bonanno C, Oliphant A, Batey A, Wright JD. Fraction of cell-free fetal DNA in the maternal serum as a predictor of abnormal placental invasion-a pilot study. Prenat Diagn. 2013;33(11):1050–1053. doi:10.1002/pd.4195
68. Biberoglu E, Kirbas A, Daglar K, et al. Serum angiogenic profile in abnormal placentation. J Matern Fetal Neonatal Med. 2016;29(19):3193–3197. doi:10.3109/14767058.2015.1118044
69. Uyanıkoğlu H, Incebıyık A, Turp AB, Çakmak G, Sak S, Hilali NG. Serum angiogenic and anti-angiogenic markers in pregnant women with placenta percreta. Balkan Med J. 2018;35(1):55–60. doi:10.4274/balkanmedj.2016.1890
70. Agrogiannis GD, Sifakis S, Patsouris ES, Konstantinidou AE. Insulin-like growth factors in embryonic and fetal growth and skeletal development (Review). Mol Med Rep. 2014;10(2):579–584. doi:10.3892/mmr.2014.2258
71. Goh W, Zalud I. Placenta accreta: a review of the etiology, diagnosis, and management. Donald School J Ultrasound Obstetr Gynecol. 2016;10:352–363. doi:10.5005/jp-journals-10009-1484
72. Umana-Perez A, Novoa Herrán S, Castro Badilla J, et al. Role of the Insulin-like growth factor axis and the Transforming growth factor-β in the regulation of the placenta and the pathogenesis of Gestational Trophoblastic Diseases. Med Res Archiv. 2020;8:2–38. doi:10.18103/mra.v8i10.2247
73. Riteau AS, Tassin M, Chambon G, et al. Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of placenta accreta. PLoS One. 2014;9(4):e94866. doi:10.1371/journal.pone.0094866
74. Cho HY, Hwang HS, Jung I, Park YW, Kwon JY, Kim YH. Diagnosis of placenta accreta by uterine artery Doppler velocimetry in patients with placenta previa. J Ultrasound Med. 2015;34(9):1571–1575. doi:10.7863/ultra.15.14.08039
75. Zamanskaya TA, Bushtyrev AV, Bushtyreva IО, Stashkevich VV, Antimirova VV. The uterine and fetoplacental hemodynamics in pregnant women with placenta previa and placenta accreta. Obstetr Gynecol Reprod. 2017;11(3):5–10. doi:10.17749/2313-7347.2017.11.3.005-010
76. Bostan M. Uterine artery Doppler velocimetry in placenta previa and placenta accreta. Med J Cairo Univ. 2019;87:3009–3013.
77. Fahmy M, Alhalabi A, Hamza H, El Zaher E, El Fattah Khourshid Y, El Sibai M. Role of uterine artery Doppler in the diagnosis of placenta accrete in patients with placenta previa. Menoufia Med J. 2020;33(2):497–500.
78. Lu Y, Wu Y, Yang L, Huang F, Ren M. Uterine artery Doppler velocimetry at mid-term gestation as a potential predictive factor for the resolution of placenta previa at the end of third trimester of pregnancy. J Obstet Gynaecol Res. 2020;46(6):883–889. doi:10.1111/jog.14246
79. Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11(7):333–343. doi:10.7863/jum.1992.11.7.333
80. Calì G, Giambanco L, Puccio G, Forlani F. Morbidly adherent placenta: evaluation of ultrasound diagnostic criteria and differentiation of placenta accreta from percreta. Ultrasound Obstet Gynecol. 2013;41(4):406–412. doi:10.1002/uog.12385
81. Jauniaux E, Bhide A. Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217(1):27–36. doi:10.1016/j.ajog.2017.02.050
82. Pilloni E, Alemanno MG, Gaglioti P, et al. Accuracy of ultrasound in antenatal diagnosis of placental attachment disorders. Ultrasound Obstet Gynecol. 2016;47(3):302–307. doi:10.1002/uog.14893
83. Daney de Marcillac F, Molière S, Pinton A, et al. Diagnostic anténatal des placentas accreta : apport de l’échographie et de l’IRM dans une population à risque [Accuracy of placenta accreta prenatal diagnosis by ultrasound and MRI in a high-risk population]. J Gynecol Obstet Biol Reprod (Paris). 2016;45(2):198–206. French. doi:10.1016/j.jgyn.2015.07.004
84. Oyelese Y. Placenta previa: the evolving role of ultrasound. Ultrasound Obstet Gynecol. 2009;34(2):123–126. doi:10.1002/uog.7312
85. Chen X, Shan R, Zhao L, et al. Invasive placenta previa: placental bulge with distorted uterine outline and uterine serosal hypervascularity at 1.5T MRI - useful features for differentiating placenta percreta from placenta accreta. Eur Radiol. 2018;28(2):708–717. doi:10.1007/s00330-017-4980-z
86. Familiari A, Liberati M, Lim P, et al. Diagnostic accuracy of magnetic resonance imaging in detecting the severity of abnormal invasive placenta: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97(5):507–520. doi:10.1111/aogs.13258
87. Bai GQ, Chen WL, Huang XH, et al. 剖宫产术后再次妊娠合并前置胎盘孕妇胎盘植入的风险评估及不良结局预测全国多中心回顾性研究 [Risk factor assessment and adverse outcome prediction of placenta accreta in pregnant women after cesarean section complicated with placenta previa: a national multicenter retrospective study]. Zhonghua Fu Chan Ke Za Zhi. 2023;58(1):26–36. Chinese. doi:10.3760/cma.j.cn112141-20221009-00615
88. Jauniaux E, Zosmer N, Subramanian D, Shaikh H, Burton GJ. Ultrasound-histopathologic features of the utero-placental interface in placenta accreta spectrum. Placenta. 2020;97:58–64. doi:10.1016/j.placenta.2020.05.011
89. Parra-Herran C, Djordjevic B. Histopathology of placenta creta: chorionic villi intrusion into myometrial vascular spaces and extravillous trophoblast proliferation are frequent and specific findings with implications for diagnosis and pathogenesis. Int J Gynecol Pathol. 2016;35(6):497–508. doi:10.1097/PGP.0000000000000250
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.