Back to Journals » Drug Design, Development and Therapy » Volume 13
Piceatannol inhibits oxidative stress through modification of Nrf2-signaling pathway in testes and attenuates spermatogenesis and steroidogenesis in rats exposed to cadmium during adulthood
Received 17 December 2018
Accepted for publication 17 April 2019
Published 13 August 2019 Volume 2019:13 Pages 2811—2824
DOI https://doi.org/10.2147/DDDT.S198444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Xiaoxue Shi,1 Lijie Fu2
1Department of Urology Surgery, Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, People’s Republic of China; 2Department of Urological Surgery 2, First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan 650032, People’s Republic of China
Background: Cadmium (Cd) is considered a heavy metal and potential pollutant to the environment.
Purpose: The purpose of this study was to evaluate the protective potential of piceatannol (PT; 10 mg/kg body weight/day) against cadmium (Cd; 5 mg/kg body weight/day)-induced testicular dysfunction in Wistar rats.
Materials and methods: Rats were randomly divided into four groups: control, PT, Cd, and Cd + PT.
Results: Treatment with Cd resulted in a significant decrease in body, testicular, and epididymal weights, sperm quantity and quality, steroidogenic marker–enzyme activities, mRNA- and protein-expression levels of SF1, StAR, and P450 side chain–cleaving enzyme, and serum male sex hormonal levels when compared to controls. Testicular malondialdehyde levels were significantly increased, with a significant reduction in enzymatic and nonenzymatic antioxidants in Cd-treated rats compared to control rats. Testicular histomorphometric results supported the biochemical and molecular alterations observed in the study. In addition, significant downregulation in mRNA- and protein-expression levels of cytosolic Nrf2, HO1, γGCS, GPx, and NQO1, as well as significant upregulation in mRNA- and protein-expression levels of Nrf2 and Keap1 in testicular tissue, were noticed in rats administered Cd. PT treatment inCd-treated rats caused marked alleviation in body and organ weights, sperm analysis, steroidogenesis, serum hormonal levels, histomorphometric changes, and oxidative and antioxidative status in testes when compared to Cd alone–treated rats. Further, treatment of rats with PTl showed a marked improvement in mRNA- and protein-expression levels of Nrf2 and its regulated genes and proteins.
Conclusion: The present study provides compelling evidence that PT treatment results in significant protection against Cd-induced testicular dysfunctions, such as spermatogenesis, steroidogenesis, and oxidative stress in rats, possibly through modification of the Nrf2–Keap1 signalling pathway.
Keywords: piceatannol, cadmium, steroidogenesis, oxidative stress, Nrf2–Keap1 pathway
Introduction
Cadmium (Cd) is a heavy metal and considered a serious health hazard to humans, because of its pervasiveness in the environment. Humans are exposed inevitably to Cd through both occupational and nonoccupational sources. The most noteworthy occupational sources of Cd includes nickel–Cd batteries, Cd-containing pigments, electroplating, and fume inhalation,1 while the nonoccupational sources are water, food, and cigarette smoking.2 Exposure to Cd is detrimental to both humans and wildlife, in particular to the male reproductive system, as it has a long biological life of 20–40 years, even after exposure to low levels.
Cd-induced toxicity in the male reproductive system might be the result of a complex network of causes and interactions. There is increasing evidence that reproductive organs and testes are extremely sensitive to Cd toxicity and may progress to irreversible infertility without affecting any other organ system in the body.3 There is also clear evidence that the blood–testis barrier is more sensitive to Cd toxicity that eventually leads to testicular dysfunction in adult rats.4 Recent findings have also indicated that Leydig cells are increasingly susceptible to Cd toxicity, thereby affecting the steroidogenic capacity of the cells by altering different marker enzymes involved in the steroidogenic pathway.5 The potential toxic role of Cd comes from the fact that high levels of Cd in seminal fluid are linked to asthenozoospermia in infertile males.6 It has also been reported that Cd exposure results in increased abnormal and dead sperm, with decreased sperm density, weight of testes, and epididymides.7,8 Earlier, it was suggested that even low levels of Cd accumulation in semen might result in reduction in sperm quality and quantity and oxidative damage, and thereby lead to male infertility.9 Since Cd has the capacity to generate free radicals and can change the antioxidant defense system, it has a major role in oxidative stress.10
It has been claimed that Cd-induced free radicals can overwhelm antioxidant defenses, leading to increased levels of lipid peroxidation and damage to DNA.11,12 Nrf2 is a member of bZip transcription–factor family, and its role in elimination of free radicals has been evidenced by a study in which Nrf2 deficiency led to Cd-induced oxidative toxicity. During the recent past, several attempts have been made by several researchers using different strategies, such as chelation therapy and elimination by various agents, to minimize the severity of Cd toxicity.10 However, in cases of chronic low-level Cd exposure, chelation therapy has not produced fruitful results, and hence there is a quest for the development of a safer and more affordable therapeutic approach to mitigate Cd-induced testicular toxicity. As a result, there is interest in finding a natural remedy for Cd poisoning.
Nowadays, there is increasing global interest in the use of plant-based drugs in the prevention and treatment of different pathological conditions.13,14 Piceatannol (PT; trans-3,4,3ʹ,5ʹ-tetrahydroxystilbene) is a naturally occurring hydroxylated analogue of resveratrol with an additional phenolic group present at the 3ʹposition, and is found in various fruit, vegetables, and wine typically consumed in the human diet. It has been reported that red grapes,15 white grapes,15 apple juice,16 red wine,16 sweet wine,16 and green tea15 contain PT at concentrations of 390 ng/g, 48 ng/g, 18 ng/mL, 15 ng/mL, 8 ng/mL and 55 µg/g, respectively. Further, PT has been shown to be more stable and effective than resveratrol.17,18 It has stated that piceatannol has the potential to scavenge hydroxyl radicals and superoxide anions.19 Further, the antioxidant property of piceatannol was revealed in a study in which it activates Nrf2 and its target gene heme oxygenase 1 (HO1) in a dose dependent manner.20
Considering the fact that Cd has the potential to cause oxidative toxicity in testis and piceatannol has the free radical scavenging activity and anti-oxidative property, the present study was undertaken to know the ameliorative effect of piceatannol in Cd-induced testicular toxicity. The current study also sheds light on the possible mechanisms by which piceatannol alleviates Cd-induced testicular dysfunctions. In addition, this study broadened its scope by including spermatogenesis, steroidogenesis and testicular histo-morphometry.
Materials and methods
Chemicals and reagents
Cd chloride (CdCl2) was purchased from Sinopharm Chemical Reagent Co. Ltd., Beijing, China. Piceatannol (PT) was purchased from Beijing Solar Bio-Science & Technology Co. Ltd., Beijing, China (Purity >98%). Androstenedione (≥98% purity), dihydroepiandrosterone (98% purity), NAD (≥98% purity) and NADPH (97% purity) were purchased from Sigma Chemical Company (St Louis, MO, USA). All other reagents used in the experiment are of analytical grade and were procured from local commercial sources.
Animals
Adult male healthy Wistar rats weighing between 225±20g were obtained by Shandong Peng Yue Experimental Animal Breeding Co. Ltd., Shandong, China. Animals were housed in specific-pathogen-free environment within polypropylene cages with a controlled temperature (23±2 °C), humidity (50±10%), and with a light/dark cycle of 12/12 h. Animals were allowed ad libitum free access to standard commercially available rodent feed and water. All animal experimental procedures were performed in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved (Approval number: K201809) by ethical committee in hospital of Zhengzhou University, China.
Experimental design
Initially, animals were acclimatized to the laboratory conditions for a period of one week before initiation of the experiment. Thirty-two male rats were randomly divided into four groups with eight in each: group I served as control and administered distilled water; group II was given 10 mg/kg body weight/day piceatannol dissolved in distilled water; group III received 5 mg/kg/body weight/day CdCl2 dissolved in distilled water); and group IV treated with 5 mg/kg/body weight/day CdCl2+10 mg/kg body weight/day PT. Rats in group IV administered with PT 30 min before CdCl2 administration. The treatment was performed through oral administration using oral gastric gavage and the treatment is lasted for 28 days. The doses of CdCl2 and PT administered in the present study were based on earlier studies.21
Sample preparation
After the completion of experimental period, animal’s body weights were recorded. Animals were given slight anaesthesia to collect blood from the animals, and the collected blood was centrifuged at 2500 rpm for 15 min at 4 °C to collect serum for hormonal estimation. Rats were euthanized, testes and epididymides were dissected out and weighed to nearest milligram possible. Cauda epididymis was used for the sperm analysis. Testes were used for the biochemical, expression and histo-morphometric studies determination.
Sperm parameters analysis
Cauda epididymis was taken out and chopped in in 5.0 ml of Ham’s F12 medium and counted the sperms by using a Neubauer Chamber as method described by Belsey et al.22 Progressive sperm motility was evaluated within 5 min following their isolation from cauda epididymis at 37°C using the method described earlier.22 The percentage of viable sperm was evaluated using 1% tryphan blue reagent.23 The number of morphologically abnormal sperms (1000 spermatozoa) were counted using a high magnification in order to count sperm malformation rate. Morphology abnormal sperms included for counting were head, mid-piece and tail morphologies.
Testicular steroidogenic marker enzyme activities and serum hormonal levels
The testicular tissue homogenate supernatant was used to estimate the activities of 3β- hydroxysteroid dehydrogenase (3β- HSD) and 17β hydroxysteroid dehydrogenase (17βHSD) using the method of Bergmeyer.24 The enzyme activities were expressed as nmol of nicotinamide adenine dinucleotide (NAD) converted to nicotinamide adenine dinucleotide reduced (NADH) per mg protein per min (3βHSD) or nmol of nicotinamide adenine dinucleotide phosphate reduced (NADPH) converted to nicotinamide adenine dinucleotide phosphate (NADP) per mg protein per min (17βHSD). Serum levels of testosterone, luteinizing hormone (LH) and follicle stimulating hormone (FSH) of the rats were estimated by enzyme linked immune-sorbent assay (ELISA) using specific kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the manufacturer’s protocols.
Testicular histopathology
Testicular tissues were fixed immediately after dissection in 4% paraformaldehyde, and then washed, dehydrated in ascending concentrations of alcohols, embedded in paraffin, sliced into 5μm thick sections and stained in hematoxylin-eosin stain. The sections were visualized and pictures taken by using a Olympus phase contrast microscope (Tokyo, Japan). In each animal, the round or nearly round seminiferous tubules were randomly selected and measured for morphometric analysis.25 The diameters of 15 seminiferous tubules (both longest and shortest for each tubule) were determined and the same tubules were used to measure the epithelium height using Image Pro Plus program associated with an Olympus BX-40 microscope. The total seminiferous tubular length expressed in meter was analyzed by dividing the seminiferous tubule volume to the squared radius of the tubule and is multiplied to pi value. These values were expressed in per testis as well as per gram testis.
For immunohistochemistry analysis, deparaffinized and rehydrated sections were heated with 10 mM sodium citrate buffer (pH 6.0) for antigen retrieval with the help of a microwave. After washing, the sections blocked by bovine serum albumin (BSA) and were incubated overnight at 4 °C with primary antibodies (3β- (ab65156) and 17βHSDs (ab97971) (Abcam, Cambridge, UK, 1:100)). Once this incubation is over then the sections were incubated with appropriate horseradish peroxidase (HRP) secondary antibody (ab65156). The sections were stained and counterstained with 3ʹ-Diaminobenzidine and hematoxylin, respectively. Finally, the sections viewed under Olympus phase contrast microscope (Tokyo, Japan).
For immunofluorescence analysis, once the sections were deparaffinized, rehydrated and antigen retrieved then blocked with 5% BSA. The sections were incubated with primary antibodies (StAR (ab96637) and P450scc (ab175408)) at a dilution of 1:100 in PBS at 4°C overnight. The sections underwent three washings with phosphate buffered saline (PBS) followed by incubation with secondary antibodies conjugated with Alexa Fluor® 488 (ab150077) (1:500, Abcam, Cambridge, UK) for both StAR and P450scc at room temperature for 60 min. Finally, the sections were mounted with Ultra Cruz (Santa Cruz, CA, USA) mounting medium with 4ʹ,6-diamino-2-phenylindole (DAPI) and then images were captured with the help of a fluorescence microscope (Leica DM IRB, Germany).
Testicular oxidative and anti-oxidative status
Fresh 10% testicular homogenate supernatant samples were used for the estimation of oxidative and antioxidant status in testis. The levels of malondialdehyde (MDA) and the enzyme activities of superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) were analysed using the commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The experiments were done as per manufacturer’s instructions.
Gene expressions analysis
Total testicular tissue RNA was extracted using a total RNA Extraction Kit (Sangon Biotech Co., Ltd. Shanghai, China) according to the protocol of manufacturer. Quantification of testicular steroidogenic factor-1 (SF1), steroidogenic acute regulatory protein (StAR), P450 side chain cleaving enzyme (P450scc), Nrf2, GPx, quinone oxidoreductase 1 (NQO1), Kelch sample related protein-1 (Keap1), γ-glutamyl cysteine synthetase (γGCS) and HO1 gene expressions were quantified by real time PCR. The purity of the total RNA was based on OD values at 260/280 nm, cDNAs were synthesized by Sangon Biotech Co., Ltd (Shanghai, China). Real-time polymerase chain reaction (RT-PCR) data was calculated using the gene expression formula 2−ΔΔCt. All values were normalized by β-actin. RT-PCR was conducted following Giribabu et al.26 The primers used in the study were commercially synthesized by Sangon Biotech Institute Co., Ltd., China. The PCR primer pairs used in the present study are as follows.
The primers sequence of the target genes are as follows:
SF1- F:5′GGAATTCAAGCTGGAGACCGGACCA3′,
R:5′CCCGCTCGAGCTCTGGTACATTGGGCCC3′;
StAR- F:5′TTGGGCATACTCAACAACCA3′,
R:5′ATGACACCGCTTTGCTCAG3′;
P450scc- F:5′ AGGTGTAGCTCAGGACTTCA3′,
R:5′ AGGAGGCTATAAAGGACACC3′;
Nrf2- F:5′AGCACATCCAGACAGACACCA3′,
R:5′TATCCAGGGCAAGCGACTC3′;
Keap1- F:5′AGCAGGCTTTTGGCATCAT3′,
R:5′CCGTGTAGGCGAACTCAATTAG3′;
GPx- F:5′GGAGAATGGCAAGAATGAAGAG3′,
R:5′CGGGGACCAAATGATGTACT3′;
Nqo1- F:5′GGTGAGAAGAGCCCTGATTGT3′,
R:5′CTCCCCTGTGATGTCGTTTC3′;
Hmox1- F:5′CGAAACAAGCAGAACCCAGT3′;
R:5′ACCAGCAGCTCAGGATGAGTA3′
γGCS- F:5′TTCAGGTGACATTCCAAGCC3′,
R:5′AAATCACTCCCCAGCGACA3′.
Western blot analysis
For Western blot analysis, the testicular tissues were lysed with radio immune-precipitation assay (RIPA) lysate buffer and protease inhibitor PMSF (RIPA lysate and PMSF, Beyotime Institute of Biotechnology, shanghai, China). The protein concentration in the sample was analyzed by BSA protein assay kit (Thermo Fisher Scientific, USA). A protein homogenate sample of 30 μg was loaded onto 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred to Polyvinylidene fluoride membrane (EMD Millipore, USA). The transferred membranes were then incubated with anti-Nrf2 (1:800; ab137550), anti-HO1 (1:1800; ab13243), anti- Keap-1(1:1000; ab218815), anti-NQO1(1:1000; ab97385), anti-GPx (1:1000; ab59546), anti-γGCS (1:1000; ab53179), and anti-β –actin (1:2000; ab8227) primary antibody diluted in phosphate buffered saline/tween (PBST) (Abcam, Cambridge, UK) overnight at 4 ºC, and then non-specific binding was blocked with 5% BSA for 1 h at room temperature. Wash three times with PBST buffer and then incubate the membranes with HRP -conjugated secondary antibody (1:4000; ab205718) (Abcam, Tokyo, Japan) diluted in PBST for 1h at room temperature. Finally, the membranes were rinsed 3 times with PBST and then Enhanced Chemi-Luminescence Western Blotting Substrate Kit (BioVision, USA) was used to visualize the immune-reactive bands. The quantification of protein expression was measured by densitometry using Image J software (version 1.46; National Institutes of Health, USA).
Statistical analysis of results
The statistical analysis was performed with the help of non-parametric Kruskal-Wallis test. Post-hoc analysis was carried out with Dunn’s test. The experimental data were expressed as median with upper and lower quartiles. A value of p<0.05 was considered statistically significant. The data were analyzed using SPSS v25 software (SPSS, Inc., Chicago, IL, USA).
Results
Body weight and tissue somatic indices of testis and epididymis
A significant decrease was observed in final body weight (P=0.03; Table 1) and tissue somatic indices of testis (P=0.003; Table 1) and epididymis (P=0.014; Table 1) in Cd-treated group of rats compared to the control group of rats. While, a significant increase in TSI of testes (P=0.004) was observed with no significant difference in final body weight (P=0.100) and indices of epididymis was observed in rats administered with both piceatannol (PT) and CdCl2 when compared to Cd-alone treated group rats. No significant difference in the final body weight (P=0.81), tissue somatic indices of testis (P=0.631) and epididymis (P=0.749) was observed in PT treated rats when compared to control rats. On the other hand, no significant difference was observed in initial body weight (P=0.698) and tissue somatic indices of seminal vesicles (P=0.524; Table 1), prostate gland (P=0.859; Table 1) and vas deferens (P=0.463; Table 1) in any one of the experimental groups.
 |
Table 1 Effect of piceatannol treatment on body weight and tissue somatic index (TSI) values for testes, epididymis, seminal vesicles, prostate gland, and vas deferens of control and Cd-treated rats |
Sperm parameters analysis
Sperm count (P=0.030) and percentage of motile (P=0.003) and viable (P=0.003) sperm significantly decreased, whereas sperm-morphology abnormalities, such as head abnormal (P=0.003), mid-piece abnormal (P=0.003), and tail abnormal (P=0.003) significantly increased in Cd-treated rats compared to control rats (Table 2; Figure 1). On the other hand, rats treated with PT + Cd showed a significant increase in sperm count (P=0.004) and percentage of motile (P=0.004) and viable sperm (P=0.004) and a decrease in sperm-morphology abnormalities, such as head (P=0.004), mid-piece (P=0.004), and tail (P=0.004), were observed compared to Cd-treated rats. However, no statistical significance was observed in these sperm parameters (sperm count, P=0.737; percentage motility, P=0.934; percentage viability, P=0.935; percentage head abnormal sperm, P=0.682; percentage mid-piece abnormal sperm, P=0.622; and percentage tail abnormal sperm, P=0.936) of PT-treated rats compared to control rats (Table 2).
 |
Table 2 Effect of PT treatment on epididymal sperm count, motility, viability, head, and mid-piece and tail abnormal sperm of control and Cd-treated rats |
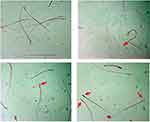 |
Figure 1 Effect of piceatannol treatment on abnormal sperm (arrow). |
Testicular steroidogenesis
Activity levels of 3βHSD (P=0.003) and 17βHSD (P=0.003) significantly decreased in testes of Cd-treated rats compared to control rats, while in rats administered PT + Cd there was a significant increase in 3βHSD (P=0.004) and 17βHSD (P=0.004) compared to rats administered Cd alone. However, no significant difference was observed in these parameters (3βHSD, P=1; 17βHSD, P=0.297) in PT alone–treated rats compared to control rats (Table 3).
 |
Table 3 Effect of PT treatment on activities of 3βHSD, 17βHSD, and mRNA levels of SF1, STAR, and P450SCC in control and Cd-treated rats |
Also, steroidogenic marker–gene SFI (P=0.0001), StAR (P=0.0001), and P450scc (P=0.0001) mRNA-expression levels were significantly decreased in testes of Cd-treated rats compared to control rats, while in rats administered PT + Cd there was a significant increase in mRNA-expression levels of SFI (P=0.025), StAR (P=0.004), and P450scc (P=0.004) compared to rats administered Cd alone. Further, a significant increase was observed in SFI (P=0.002), StAR (P=0.002), and P450scc (P=0.002) mRNA-expression levels in PT alone–treated rats compared to control rats (Table 3).
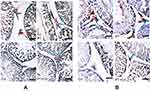
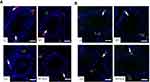
Further immunohistochemistry results confirmed the biochemical analysis of 3βHSD and 17βHSD and showed decreased protein expression levels of 3βHSD (Figure 2A) and 17βHSD (Figure 2B) in Leydig cells of Cd-treated rat testes compared to control rat testes. However, rats administered PT + Cd showed higher expression of 3βHSD and 17βHSDs compared with Cd-alone administered rats. The results of immunofluorescence studies revealed decreases in protein expression of StAR (Figure 3A) and P450scc (Figure 3B) in Leydig cells of Cd-treated rat testes compared to control rats. Meanwhile, PT + Cd–administered rats showed higher distribution of StAR and P450scc than to rats administered Cd alone. No significant difference was observed in 3βHSD, 17βHSD, StAR, or P450scc distribution in PT alone–treated rats compared with control rats.
Serum hormonal levels
Serum testosterone (P=0.003) levels were significantly decreased, with significant increases in serum hormonal levels of FSH (P=0.003) and LH (P=0.003) in rats treated with Cd compared with control rats. In contrast, PT and Cd treatment of animals caused a significant rise in testosterone levels (P=0.004) with significant falls in levels of FSH (P=0.006) and LH (P=0.004) compared to Cd-treated animals. Moreover, PT only–treated animals presented no significant change (serum testosterone, P=0.873; FSH, P=0.630; LH, P=0.631) in levels of serum hormones compared to control rats (Table 4).
 |
Table 4 Effect of PT treatment on serum hormonal levels of testosterone, FSH, and LH in control and Cd-treated rats |
Testicular oxidative and anti-oxidative status
Testicular MDA content (P=0.003) was significantly increased with significant decreases in activities of SOD (P=0.003), catalase (P=0.003) and GPx (P=0.002) in Cd-treated rats compared to control rats. In rats treated with both PT and Cd, a significant decrease in MDA levels (P=0.004) and significant increases in activities of SOD (P=0.004), catalase (P=0.012), and GPx (P=0.024) in testes were observed compared to rats treated with Cd alone. In PT only–treated animals, there was no significant change in MDA levels (P=1) or antioxidant-enzyme activities (SOD, P=0.297; catalase, P=0.467; GPx, P=0.106) compared to control rats (Table 5).
 |
Table 5 Effect of PT treatment on testicular LPO, SOD, catalase, and GPx activity of control and Cd-treated rats |
Nrf2-keap1 signalling gene and protein expression
mRNA levels of Nrf2 (P=0.002), NQO1 (P=0.002), HO1 (P=0.002), γGCS (P=0.002), and GPx (P=0.002) were significantly decreased and associated with a significant increase in mRNA levels of Keap1 (P=0.002) in testes of Cd-treated rats compared to normal control rats. Conversely, mRNA levels of Nrf2 (P=0.010), NQO1 (P=0.004), HO1 (P=0.004), γGCS (P=0.004), and GPx (P=0.006) were significantly upregulated with significant downregulation in mRNA levels of Keap1 (P=0.004) in testes of rats treated with PT + Cd compared to rats treated with Cd alone. In PT-treated animals, there was significant upregulation in mRNA levels of Nrf2 (P=0.002), NQO1 (P=0.002), HO1 (P=0.002), γGCS (P=0.002), and GPx (P=0.002) in association with decreased mRNA levels of Keap1 compared to controls (Table 6).
 |
Table 6 Effect of PT treatment on mRNA levels of NRF2, KEAP1, NQO1, HO1, γGCS, and GPx in testes of control and Cd-treated rats |
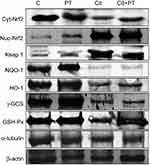
Western blotting results supported the results of mRNA-expression levels and showed decreased protein-expression levels of cytosolic Nrf2 (P=0.003), NQO1 (P=0.002), HO1 (P=0.003), γGCS (P=0.002), and GPx (P=0.001) associated with significantly increased expression levels of protein nuclear Nrf2 (P=0.001) and Keap1 (P=0.001) in testes of Cd-treated rats compared with normal control rats. Moreover, PT + Cd–treated rats showed significantly increased cytosolic Nrf2 (P=0.004), NQO1 (P=0.004), HO1 (P=0.004), γGCS (P=0.004), and GPx (P=0.008) expression levels with decreased nuclear and (P=0.036) and Keap1 (P=0.004) expression levels compared with Cd alone–treated rats. However, in PT only–treated animals, no statistical significance was observed in Western blotting results of cytosolic Nrf2 (P=0.333), NQO1 (P=0.065), HO1 (P=0.296), γGCS (P=0.148), or Keap1 (P=108), but there were significant increases in nuclear Nrf2 (P=0.004) and GPx (P=0.006) compared to controls (Table 7; Figure 4).
 |
Table 7 Effect of PT treatment on Western blotting results of cytosolic Nrf2, nuclear Nrf2, Keap1, NQO1, HO1, γGCS, and GPx in testes of control and Cd-treated rats |
Histopathological changes
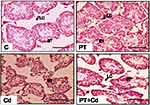
Microscopy of testes is depicted in Figure 5. Control and PT-treated rat testicular morphology is presented with mature seminiferous tubules, intact epithelial membrane, complete series of spermatogenic cells, and lumen full of sperm. In contrast to that, testicular transverse sections of Cd-treated rats showed degenerative, atrophied tubules with incomplete series of spermatogenic cells and rupture in epithelial membrane. Moreover, administration of PT + Cd showed restoration evidenced by intact basement membrane with different spermatogenic stages of cells and an increase in the number of germ cells compared to Cd alone–treated rats.
Further morphometric evaluation of testicular architecture in Cd-treated rats revealed significant reductions in seminiferous tubular diameter (P=0.003), seminiferous tubular length (P=0.003), seminiferous tubular epithelial height (P=0.003; Figure 10D) and total tubular length/g testes (P=0.003) compared to control rats. PT-treated Cd-intoxicated rats showed marked increases in seminiferous tubular length (P=0.004) and epithelial height (P=0.004) with no significant increase in tubular diameter (P=0.109) or total tubular length/g testes (P=0.108) compared to Cd-treated rats. These parameters (seminiferous tubular diameter, P=0.262; seminiferous tubular length, P=0.81; seminiferous tubular epithelial height, P=0.262; total tubular length/g testis, P=0.748) were not significantly altered in PT-administered rats compared to control rats (Table 8; Figure 5).
 |
Table 8 Effect of PT treatment on tubular diameter, epithelium height, total tubular length, and tubular length/g in testes of control and Cd-treated rats |
Discussion
Humans exposure to Cd is inevitable, due to its prevalence in nature, exposure through occupational and nonoccupational sources, and long biological life. An analogue of the well-known antioxidant resveratrol, PT has been demonstrated to be an effective antioxidant. In this study, we evaluated the effect of administration of Cd on testicular functions, such as spermatogenesis, steroidogenesis, oxidant and antioxidant status, serum hormone levels, and histomorphometry in rats. In addition, we also included the PT and Cd + PT groups to demonstrate the role of PT in Cd-induced changes in testicular function. The results obtained in the present study revealed that treatment with PT induced remarkable attenuation of Cd-induced testicular dysfunction in rats.
The present study showed that treatment with Cd resulted in a significant decrease in body weight, which is in agreement with earlier findings of Cd-induced inhibition of protein synthesis and growth.27,28 Administration of Cd resulted in a significant decrease in weights of testes and epididymides. The results are in agreement with earlier reports.29 The decrease in testis weight may be an indication of generation of free radicals. Free radicals cause oxidation and thus deterioration in DNA, proteins, and lipids, which leads to testicular atrophy.30,31 Histological evaluation of testes revealed severe effects in the form of degenerative changes in seminiferous tubules with loss of spermatogenesis after treatment of rats with Cd. Cd damages reproductive functions in rats by causing severe testicular degeneration, seminiferous tubule damage, and necrosis.32 When massive cellular loss occurs in the tubular epithelium, a sharp decline in morphometric parameters of testes can be verified.29 Rats administered Cd showed significant decreases in testicular seminiferous tubule diameter and epithelial height along with conspicuous decreases in tubular length. These results are in agreement with earlier findings of Cd-induced reduction in length of seminiferous tubules and diminished epithelium height.29 França and Godinho,33stated that total seminiferous tubule length is directly correlated with the size of testes. In fact, pathological damage to the seminiferous epithelium may result in deterioration of germ and Sertoli cells, which in turn progresses to impaired spermatogenesis.29
Spermatozoa are present with high amounts of polyunsaturated fatty acids and extremely sensitive to peroxidation damage. As such, oxidant stress appears to play a major role in Cd-induced male reproductive toxicity. Since spermatozoa are exceedingly sensitive to oxidant stress, it can cause damage to the quality and quantity of sperm after exposure to Cd. As expected, the results revealed that Cd exposure resulted in decreased sperm density and motile and viable sperm with increased sperm morphological abnormalities. These results are in agreement with the findings of Kumar and Singh,34 who found a significant decrease in sperm motility and count with increase in sperm deformity. Previous studies speculated that Cd might disrupt the blood–testis barrier and thereby make its way to testes to alter testicular function in male rats. The present results are in line with El-Missiry and Shalaby,35 who proposed that Cd-induced testicular lipid peroxidation, necrosis, and apoptosis in rats is correlated with changes in circulatory androgen levels and fertility.
Testosterone is a crucial androgen synthesized in Leydig cells of testes and plays critical roles in the development of male reproductive tissue and spermatogenesis. The synthesis of testosterone is a complex process in which several proteins are involved: the mitochondrial membrane protein StAR plays the role of transporting cholesterol from cytoplasm to mitochondria, and then the rate-limiting enzyme P450scc cleaves cholesterol into pregnenolone.36 The pregnenolone gets transported to smooth endoplasmic reticulum, where under the influence of a cascade of such enzymes as 3β- and 17βHSDs, the synthesis of testosterone ensues.36 Further, adult testes express high levels of SF1 in the Leydig cells, which is not only considered a major transcriptional regulator of various steroidogenic genes but also a regulator of gonadal function.37 The role of SF1 in steroidogenesis was revealed in a study in which testes with SF1-deleted Leydig cells showed defects in steroidogenic expression.38 In the current study, Cd treatment resulted in significant decreases in testicular mRNA-expression levels of SF1, StAR, and P450scc and protein-expression levels of StAR, P450scc, 3β- and 17βHSDs compared to controls. The diminution in steroidogenic markers was also supported by previous research.5 Expression levels of steroidogenic markers also supported Cd induced decline in levels of serum testosterone in the study. Alterations in serum hormonal levels, such as decreased testosterone and increased FSH and LH is an indication of an intact hypothalamic–testicular axis and damage to the testes. Elmallah et al,39suggested that Cd-mediated testicular toxicity could possibly be due to increased oxidant stress.
n this study, Cd toxicity caused testicular oxidant stress by an increase in levels of MDA as an index of lipid peroxidation and decrease in GSH content and activities of SOD, catalase, and GPx as a measure antioxidant defense in testes. These results are in agreement with earlier research.30 The enzyme SOD plays a vital role in protection from oxidant damage produced by ROS in terms of dismutation of highly toxic superoxide anion radicals into less toxic hydrogen peroxide, which is then neutralized into oxygen and water by catalase. Further, GPx catalyzes the reduction of lipid peroxides and hydrogen peroxide using glutathione40 to protect against accumulation of lipid peroxides and other oxidants, thereby preventing oxidant damage. The observed reduction in activities of antioxidant defenses demonstrates the failure of the primary antioxidant system to act against Cd-induced oxidant stress. Despite the fact that Cd induces oxidant damage to the testes, the precise mechanism by which it causes insult is still unclear.
Nrf2 is an important transcription factor that promotes cell survival and maintains redox homeostasis by regulating antioxidant-response element (ARE) DNA sequences of phase II antioxidant genes, such as NQO1, HO1, γGCS, and GPx.41 The Nrf2/ARE signaling pathway is a crucial antioxidant pathway that is widely distributed in testicular cells and throughout the body. Under normal biological conditions, Keap1, a chaperone molecule, binds and inhibits Nrf2, which upon activation decouples from Keap1 and translocates to the nucleus42 to regulate its target genes. The results of the present study indicated that Cd treatment decreases Nrf2 and its regulated mRNA and protein expressions of NQO1, HO1, γGCS, and GPx and increases Keap1 mRNA- and protein-expression levels in testis. The findings observed in the present study are in agreement with He et al.21 The present results clearly indicated that exogenous Cd can suppress the intrinsic antioxidant capacity of testicular cells by inhibiting expression levels of Nrf2 and its regulated genes and thereby induce oxidant stress.
Diet-derived antioxidants, such as PT, play a critical role in maintaining the health of humans. Halliwell,43observed a positive correlation between Cd toxicity and a marked fall in several dietary antioxidants. A natural analogue of resveratrol, PT is widely consumed in the human diet in the form of various fruit, vegetables, and wines. It has been demonstrated that PT is more effective than resveratrol.17,18 Murias et al,44demonstrated that the radical-scavenging activity of PT was 1,250-fold that of resveratrol. The results of the present study showed that PT treatment of Cd-intoxicated rats caused a significant increase in the activities of SOD, catalase, and GPx and decreased levels of lipid peroxidation compared to Cd-treated rats. As a consequence of oxidant-stress mitigation and alleviation in antioxidant status, the treatment of Cd rats with PT ameliorated body weight and weights of testes and epididymides, as well as steroidogenesis, spermatogenesis, and histomorphometric alterations in testes. Further, in order to know whether PT can inhibit the insult caused by Cd treatment in terms of activation of the Nrf2–ARE signaling pathway, expression levels of related genes in the Nrf2–ARE signaling pathway were evaluated in the present study. Significant upregulation in mRNA- and protein-expression levels of cytosolic Nrf2, NQO1, HO1, γGCS, and GPx with down regulation in nuclear Nrf2 and Keap1 mRNA- and protein-expression levels were observed in testes of rats treated with Cd and PT compared to Cd alone–treated rats. It has been reported that PT increased the expression of HO1, an enzyme with antioxidant properties, in a dose-dependent manner through activation of NRF2.20 The results of the present study suggested that PT can regulate the Nrf2–Keap1 pathway against Cd-induced oxidant damage of testes in rats.
In conclusion, PT treatment provided significant protection against Cd-induced testicular dysfunction in rats, as evidenced by significant improvement in steroidogenesis, spermatogenesis, and histomorphometric changes and alleviation of oxidant stress through modification of the Nrf2–Keap1 signaling pathway. This study provides impetus for future investigations to explore the mechanism(s) of PT on Cd-induced reproductive toxicity.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Klaassen CD, Liu J, Diwan BA. Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol. 2009;238(3):215–220. doi:10.1016/j.taap.2009.03.026
2. Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118(2):182–190. doi:10.1289/ehp.0901234
3. Takiguchi M, Yoshihara S. New aspects of cadmium as endocrine disruptor. Environ Sci. 2006;13(2):107–116.
4. Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-beta3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144(4):1139–1142. doi:10.1210/en.2002-0211
5. Alkhedaide A, Alshehri ZS, Sabry A, Abdel-Ghaffar T, Soliman MM, Attia H. Protective effect of grape seed extract against cadmium-induced testicular dysfunction. Mol Med Rep. 2016;13(4):3101–3109. doi:10.3892/mmr.2016.4928
6. Taha EA, Sayed SK, Ghandour NM, et al. Correlation between seminal lead and cadmium and seminal parameters in idiopathic oligoasthenozoospermic males. Cent European J Urol. 2013;66(1):84–92. doi:10.5173/ceju.2013.01.art28
7. Monsefi M, Alaee S, Moradshahi A, Rohani L. Cadmium-induced infertility in male mice. Environ Toxicol. 2010;25(1):94–102. doi:10.1002/tox.20468
8. Adamkovicova M, Toman R, Cabaj M, et al. Effects of subchronic exposure to cadmium and diazinon on testis and epididymis in rats. TheScientificWorldJournal. 2014;2014:632581. doi:10.1155/2014/632581
9. Wu HM, Lin-Tan DT, Wang ML, et al. Cadmium level in seminal plasma may affect the pregnancy rate for patients undergoing infertility evaluation and treatment. Reproductive toxicology (Elmsford. N.Y.). 2008;25(4):481–484.
10. Al-Azemi M, Omu FE, Kehinde EO, Anim JT, Oriowo MA, Omu AE. Lithium protects against toxic effects of cadmium in the rat testes. J Assist Reprod Genet. 2010;27(8):469–476. doi:10.1007/s10815-010-9426-3
11. Bagchi D, Vuchetich PJ, Bagchi M, et al. Induction of oxidative stress by chronic administration of sodium dichromate [chromium VI] and cadmium chloride [cadmium II] to rats. Free Radic Biol Med. 1997;22(3):471–478.
12. Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192(2–3):95–117.
13. Ramana KV, Singhal SS, Reddy AB. Therapeutic potential of natural pharmacological agents in the treatment of human diseases. Biomed Res Int. 2014;2014:573452. doi:10.1155/2014/573452
14. Chikezie PC, Ibegbulem CO, Mbagwu FNJRJOP. Bioactive principles from medicinal plants. Res J Phytochem.2015;9(3):88–115.
15. Vinas P, Campillo N, Martinez-Castillo N, Hernandez-Cordoba M. Solid-phase microextraction on-fiber derivatization for the analysis of some polyphenols in wine and grapes using gas chromatography-mass spectrometry. J Chromatogr A. 2009;1216(9):1279–1284. doi:10.1016/j.chroma.2008.12.058
16. Vinas P, Martinez-Castillo N, Campillo N, Hernandez-Cordoba M. Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography-mass spectrometry for the analysis of polyphenols in herbal infusions. Fruits and Functional Foods. J Chromatogr A. 2011;1218(5):639–646.
17. Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat Res. 2012;750(1):60–82. doi:10.1016/j.mrrev.2011.11.001
18. Setoguchi Y, Oritani Y, Ito R, et al. Absorption and metabolism of piceatannol in rats. J Agric Food Chem. 2014;62(12):2541–2548. doi:10.1021/jf404694y
19. Camont L, Collin F, Couturier M, et al. Radical-induced oxidation of trans-resveratrol. Biochimie. 2012;94(3):741–747. doi:10.1016/j.biochi.2011.11.005
20. Kershaw J, Kim KH. The therapeutic potential of piceatannol, a natural stilbene, in metabolic diseases: a review. J Med Food. 2017;20(5):427–438. doi:10.1089/jmf.2017.3916
21. He L, Li P, Yu LH, et al. Protective effects of proanthocyanidins against cadmium-induced testicular injury through the modification of Nrf2-Keap1 signal path in rats. Environ Toxicol Pharmacol. 2018;57:1–8. doi:10.1016/j.etap.2017.11.002
22. Belsey MA, Eliasson R, Gallegos AJ, Moghissi KS, Paulsen CA, Prasad MRN. World Health Organization: Laboratory Manual for the Examination of Human Semen and Semen-cervical Mucus Interaction. Singapore: Press Concern 1980.
23. Talbot P, Chacon RSJJOEZ. A triple‐stain technique for evaluating normal acrosome reactions of human sperm. J Exp Zool. 1981;215(2):201–208.
24. Bergmeyer HU,Bernt E, Schmidt F, Stork H. D-glucose determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press. 1974;3:1196–1198.
25. Wing TY, Christensen AK. Morphometric studies on rat seminiferous tubules. Am J Anat. 1982;165(1):13–25. doi:10.1002/aja.1001650103
26. Giribabu N, Karim K, Kilari EK, Salleh N. Phyllanthus niruri leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis and apoptosis and enhances kidney cell proliferation in adult male rats with diabetes mellitus. J Ethnopharmacol. 2017;205:123–137. doi:10.1016/j.jep.2017.05.002
27. Waalkes MP, Diwan BA. Cadmium-induced inhibition of the growth and metastasis of human lung carcinoma xenografts: role of apoptosis. Carcinogenesis. 1999;20(1):65–70. doi:10.1093/carcin/20.1.65
28. El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem Toxicol. 2004;42(10):1563–1571. doi:10.1016/j.fct.2004.05.001
29. de Souza Predes F, Diamante MA, Dolder H. Testis response to low doses of cadmium in Wistar rats. Int J Exp Pathol. 2010;91(2):125–131. doi:10.1111/j.1365-2613.2009.00692.x
30. Kheradmand A, Alirezaei M, Dezfoulian O. Biochemical and histopathological evaluations of ghrelin effects following cadmium toxicity in the rat testis. Andrologia. 2015;47(6):634–643. doi:10.1111/and.12311
31. Selim ME, Rashed El HA, Aleisa NA, Daghestani MH. The protection role of heat shock protein 70 (HSP-70) in the testes of cadmium-exposed rats. Bioinformation. 2012;8(1):58–64. doi:10.6026/97320630008058
32. Burukoglu D, Baycu C. Protective effects of zinc on testes of cadmium-treated rats. Bull Environ Contam Toxicol. 2008;81(6):521–524. doi:10.1007/s00128-007-9211-x
33. França LR, Godinho CLJBOR. Testis morphometry, seminiferous epithelium cycle length, and daily sperm production in domestic cats (Felis catus). Biol Reprod. 2003;68(5):1554–1561.
34. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci. 2015;8(4):191–196. doi:10.4103/0974-1208.170370
35. El-Missiry MA, Shalaby F. Role of beta-carotene in ameliorating the cadmium-induced oxidative stress in rat brain and testis. J Biochem Mol Toxicol. 2000;14(5):238–243. doi:10.1002/1099-0461(2000)14:5<238::AID-JBT2>3.0.CO;2-X
36. Payne AH, Youngblood GL, Sha L, Burgos-Trinidad M, Hammond SH. Hormonal regulation of steroidogenic enzyme gene expression in Leydig cells. J Steroid Biochem Mol Biol. 1992;43(8):895–906. doi:10.1016/0960-0760(92)90317-C
37. Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18(3):361–377. doi:10.1210/edrv.18.3.0301
38. Buaas FW, Gardiner JR, Clayton S, Val P, Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development. 2012;139(24):4561–4570. doi:10.1242/dev.087247
39. Elmallah MIY, Elkhadragy MF, Al-Olayan EM, Abdel Moneim AE. Protective effect of fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. Int J Mol Sci. 2017;18:5. doi:10.3390/ijms18050957
40. Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830(5):3289–3303. doi:10.1016/j.bbagen.2012.11.020
41. Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16(2):123–140. doi:10.1111/gtc.2011.16.issue-2
42. Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi:10.1016/j.redox.2012.10.001
43. Halliwell B. Establishing the significance and optimal intake of dietary antioxidants: the biomarker concept. Nutr Rev. 1999;57(4):104–113. doi:10.1111/j.1753-4887.1999.tb06933.x
44. Murias M, Jager W, Handler N, et al. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: structure-activity relationship. Biochem Pharmacol. 2005;69(6):903–912. doi:10.1016/j.bcp.2004.12.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.