Back to Journals » Clinical Ophthalmology » Volume 14
Physician Satisfaction with Anti-Inflammatory Topical Medications for the Treatment of Dry Eye Disease
Authors White DE, Zhao Y, Jayapalan H , Machiraju P, Periyasamy R, Ogundele A
Received 8 November 2019
Accepted for publication 3 March 2020
Published 25 March 2020 Volume 2020:14 Pages 931—938
DOI https://doi.org/10.2147/OPTH.S237832
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Darrell E White,1 Yang Zhao,2 Hemalatha Jayapalan,3 Pattabhi Machiraju,3 Ramu Periyasamy,3 Abayomi Ogundele2
1SkyVision Centers, Westlake, OH, USA; 2Medical Affairs, Sun Pharmaceutical Industries, Princeton, NJ, USA; 3Medical Services, Indegene, Inc, Bangalore, India
Correspondence: Abayomi Ogundele
Medical Affairs, Sun Pharmaceutical Industries, Inc, 2 Independence Way, Princeton, NJ 08540, USA
Email [email protected]
Purpose: Dry eye disease (DED) is one of the most common ophthalmic disorders. Pathogenesis of the disease includes inflammation of the ocular surface and lacrimal gland. Two anti-inflammatory prescription treatments are currently available: cyclosporine ophthalmic emulsion 0.05% (CYC) and lifitegrast 5% ophthalmic solution (LIF). The objective of this survey-based study was to assess physician satisfaction with CYC and LIF for the treatment of DED.
Methods: Physicians currently treating DED patients with CYC or LIF were asked to rate the experiences of their patients currently or formerly using CYC and LIF, and their own perspectives on the two treatments.
Results: Twenty-one physicians participated in the survey, providing responses on behalf of 210 patients. Overall, physicians reported low levels of satisfaction with onset of action of CYC and LIF, and fewer than half considered either drug to be effective in managing symptoms or improving patient quality of life (QoL). Burning sensation and dysgeusia were the most frequently reported side effects. Onset of action and effectiveness after onset were the main switching drivers. Although two-thirds of physicians were satisfied with the overall effectiveness of CYC and LIF, all physicians agreed that more DED treatment options are needed, with > 50% strongly agreeing.
Conclusion: Physicians perceived a gap in DED management with currently available topical anti-inflammatory agents. Although satisfaction with CYC and LIF was high, few physicians considered these medications to be effective in managing symptoms or improving QoL.
Keywords: physician satisfaction, dry eye disease, cyclosporine, lifitegrast, ocular surface
Introduction
Dry eye disease (DED) is a complex, chronic, and progressive condition of the ocular surface. Its pathogenesis includes inflammation of the ocular surface and lacrimal gland. Thus, anti-inflammatory treatment is often required. In the United States (US), DED is estimated to affect 16.4 million adults,1 Currently, two products have been approved by the US Food and Drug Administration to treat DED: cyclosporine ophthalmic emulsion 0.05% (CYC) in 2003, and lifitegrast 5% ophthalmic solution (LIF) in 2016.2–4 CYC is indicated for increasing tear production, while LIF is for the treatment of signs and symptoms of DED.3,4 Both drugs are discussed as treatment options in the 2019 American Academy of Ophthalmology guidelines for managing patients with DED.5
Despite its frequent use, few studies have specifically evaluated the physician experience with CYC and LIF.6–10 Overall, physicians have reported mixed opinions on the efficacy of CYC and LIF for the management of DED. In two Phase III clinical trials, the investigators’ evaluation of global patient response to treatment with CYC was not significantly better than with vehicle alone.6 At the 6-month follow-up visit, 68.5% of patients treated with CYC and 63.0% of patients in the vehicle group had a slight response or better. No case of treatment success (defined as a global evaluation of ≥90% improvement) was reported in either group.
In a retrospective chart review of 35 patients who had a repeat trial of CYC after previously discontinuing treatment within 12 weeks, physicians reported that clinical benefit was achieved in 80% of patients and 43% persisted with CYC beyond 1 year of treatment.7 In a larger US survey of 100 eye care providers, 42.1% of respondents (40/95) reported that CYC treatment failed in 40% or more of their patients with DED; four respondents (4.2%) reported an 80–100% failure rate.8 More recently, a survey of key stakeholders across the 28 European Union members (75 ophthalmologists) reported that the majority of health care providers had a positive perception of the efficacy and safety of cyclosporine A formulations for the treatment of DED.9 Finally, a survey of 76 ophthalmologists reported that 28% of respondents desired better anti-inflammatory activity than is currently offered by CYC and LIF.10
There is a paucity of data from the physician perspective on quality of life (QoL) outcomes, despite the well-documented impact of dry eye symptoms on patients’ everyday activities, such as using a computer, reading, and driving, and the known detrimental impact of DED on work productivity.11–13 The primary objectives of this study were to evaluate physicians’ perspectives and satisfaction with CYC and LIF in terms of efficacy, tolerability, and impact on patient QoL, as well as to identify the main reasons of switching.
Materials and Methods
Study Design
This was a cross-sectional survey study conducted between October 2018 and January 2019 across 20 centers in the US. A recruitment target of 20 physicians was set, with the aim of obtaining responses pertaining to 200 patients diagnosed with DED. Physicians were eligible to participate in the survey if they were currently treating patients with DED using CYC or LIF and had switched patients from CYC or LIF to other drugs within the past 6 months. Participating physicians were able to access the surveys via a secure web-based link and could complete them in intervals to lessen the burden of fatigue. The physician survey was developed with clinical experts. This study was Health Insurance Portability and Accountability Act (HIPAA) compliant, approved by an institutional review board (Sterling Institutional Review Board, Atlanta, Georgia), and conducted in accordance with the Declaration of Helsinki.
Survey
A two-part survey was developed to elicit physicians’ perspectives on CYC and LIF as treatments for DED and the experiences of their patients currently or formerly using either drug (Table 1). In Part 1, physicians were asked to complete a minimum of 10 patient questionnaires, rating their experiences with 5 CYC users and 5 LIF users. Of the 5 patients in each drug group, 1 or 2 were required to be patients recently switched from CYC or from LIF. Physicians were asked to rate the experiences of their patients in terms of 19 items over four domains: 1) satisfaction (onset of action, effectiveness of the drug after onset of action, ease of use), 2) frequency of side effects (burning sensation, itching, dysgeusia, blurred vision, discharge), 3) limited activities (reading, driving, working on computer, watching TV, work productivity, social activities), and 4) reasons for switching (inability to relieve dry eye symptoms, onset of action, effectiveness of the drug after onset, ease of use, limited activities). Each item was assessed on a 5-point scale.
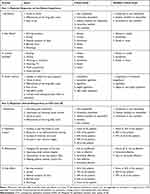 |
Table 1 Survey Design |
In Part 2, physicians were asked to rate their own experiences with CYC and LIF more generally, in terms of 17 items over four domains: 1) satisfaction (stimulating tear production, lubricating/hydrating ocular surface, onset of action, effectiveness of the drug after onset of action), 2) objective measures of treatment success (increase in tear film break up time [TBUT], reduction in corneal/conjunctival staining, increase of Schirmer test score, increase in tear meniscus height), 3) effectiveness (managing the symptoms of dry eye, improving ocular surface integrity, improving patient QoL, improving contact lens tolerance), and 4) side effects (burning sensation, itching, dysgeusia, blurred vision, discharge). All items were assessed on a 5-point scale. One additional item asked physicians to respond to the question, “do you think more prescription treatment options are needed for the management of dry eye?” Responses were rated on a 5-point scale from 1 (strongly disagree) to 5 (strongly agree). Physicians were required to obtain written informed consent from their patients before including their experiences in the study.
Analysis
Responses on the 5-point scale were converted to a 3-point scale by combining the two most favorable categories and the two least favorable categories. Outcomes were reported descriptively. Continuous measures were presented as means and standard deviations (SD); categorical measures were presented as counts and percentages.
Results
A total of 21 physicians participated in the study, including 13 general ophthalmologists, four optometrists, and four corneal specialists. Thirteen physicians (62%) reported treating an average of 50–100 patients with DED each month, and two (10%) reported treating >100. Overall, six physicians (29%) reported that >50% of their patients were currently being treated with CYC; for LIF, only one physician reported current use in >50% of patients (data not shown). In Part 1 of the study, physicians provided responses for 210 patients, including 75 current CYC users, 75 current LIF users, 30 patients switched from CYC to LIF, and 30 patients switched from LIF to CYC (Table 2). Current CYC users were slightly older than current LIF users (49% were aged ≥60 years compared with 40% for LIF), and had a longer duration on their current treatment (43% had been using CYC for >12 months, compared with 28% for LIF).
 |
Table 2 Demographics of Patients Included in Physician Responses |
Part 1: Physician Responses on Behalf of Patients
Treatment satisfaction was lowest for onset of action: physicians were satisfied (“somewhat satisfied” or “very satisfied”) with time to onset of action in only 37% of their patients currently using CYC (n=28) and 65% of their patients currently using LIF (n=49) (Figure 1A). Physicians were satisfied with the ability of CYC and LIF to manage DED after onset of action in ≥75% of current users (n=56 and n=58 for CYC and LIF, respectively). Satisfaction with ease of use was 67% for current CYC users (n=50) and 60% for current LIF users (n=45).
Across the majority of activity limitation categories, physicians reported that >50% of current users of CYC and LIF were “rarely” or “never” limited by dry eye symptoms while on medication. The two exceptions were both among current CYC users: physicians reported that reading and working on the computer were “rarely” or “never” limited in only 37% (n=28) and 47% (n=35) of current CYC users, respectively.
Burning sensation was the side effect most commonly observed by treating physicians: 27% of current CYC users (n=20) and 13% of current LIF users (n=10) “always” or “usually” experienced a burning sensation upon instillation of the medication (Figure 1B). Physicians reported that 19% of LIF current users and 5% of CYC current users “always” or “usually” experienced dysgeusia. Blurred vision, itching, or discharge were less frequent (<10%).
For patients who had a recent treatment change from CYC, physicians reported that onset of action and effectiveness after onset were “very important” or “extremely important” factors in the decision to switch in more than half of patients, followed by inability to relieve dry eye symptoms and limited visual activities (Figure 2). Among the LIF switchers, effectiveness after onset (40%) tended to the most important factor followed by limited visual activities (33%). For both drugs, ease of use and side effect profile were the least common reasons for switching.
Part 2: Physician Perspectives on CYC and LIF
Around two-thirds of physicians were “satisfied” or “very satisfied” with the ability of CYC and LIF to stimulate tear production (67% for both drugs) and lubricate and hydrate the ocular surface (76% for CYC, 71% for LIF) (Figure 3A). Over 80% of physicians were satisfied with the effectiveness of CYC (81%) or LIF (86%) after onset of action. However, onset of action was less than satisfactory for both drugs (10% for CYC, and 48% for LIF).
CYC was considered to be successful at increasing TBUT and reducing corneal/conjunctival staining in ≥75% of patients by 24% and 33% of physicians, respectively (Figure 3B). The same responses for LIF were reported by 29% (n=6) and 38% (n=8) of physicians, respectively. Among physicians who performed the Schirmer test (n=9), CYC and LIF were judged to be successful in increasing the test score in ≥75% of patients by 22% (n=2) and 44% (n=4) of the physicians, respectively. Among those who measured increase in tear film meniscus height (n=19), CYC and LIF were seen to be successful in ≥75% of patients by 26% (n=5) and 37% (n=7) of the physicians.
CYC was considered “very effective” or “extremely effective” by 33%, 24%, 24%, and 26% of physicians with respect to managing symptoms of dry eye, managing corneal staining, improving patient QoL, and improving contact lens tolerance, respectively (Figure 3C). The corresponding numbers for LIF were 43%, 33%, 33%, and 24%, respectively. Physicians reported that treatment effectiveness with both drugs was similar for the aqueous deficient and evaporative dry eye subtypes (data not shown).
With respect to side effects, burning sensation was the primary concern among physicians: 43% and 29% in ≥75% of CYC- and LIF-treated patients, respectively. Four physicians (19%) reported dysgeusia in ≥75% of patients treated with LIF, compared with one physician (5%) reported CYC. For both drugs, only one physician (5%) reported itching and blurred vision in ≥75% of current users and no physician reported for discharge (data not shown).
Physicians unanimously agreed that additional prescription treatment options are needed for the management of dry eye, with 52% (n=11) strongly agreeing.
Discussion
Topical anti-inflammatory agents are routinely prescribed for DED; however, few studies have evaluated the perceptions and experiences of physicians prescribing these treatments. To our knowledge, this cross-sectional study was the first to comprehensively assess treatment satisfaction and effectiveness, frequency of side effects, and activity limitations among eye care providers prescribing CYC and LIF. Although more than two-thirds of physicians were satisfied with the effectiveness of CYC and LIF after onset of action, including the ability of the drugs to increase tear production and lubricate and hydrate the ocular surface, satisfaction with onset of action was low, particularly for CYC (10%). This may have important implications for patient adherence and persistence. Although this survey did not directly evaluate these outcomes, it is notable that 36% of CYC users and 45% of LIF users had been on their current treatment for 6 months or less.
Fewer than two in five physicians considered LIF and CYC to be successful in ≥75% of patients based on the four objective measures of treatment success, and fewer than half considered either treatment to be very or extremely effective in managing dry eye symptoms and improving QoL. In both parts of the survey, physician responses were consistent with the known tolerability issues of CYC and LIF,6,14 with burning sensation being the most frequently encountered side effect. Dysgeusia has been reported in 14.5% of patients treated with LIF across five clinical trials.14 Our results suggested that the real-world incidence of dysgeusia may be considerably higher; several physicians reported dysgeusia in ≥75% of their patients.
The growing prevalence and burden of DED, particularly the potential negative impact of dry eye symptoms on patient QoL, confirm the condition as a major cause of ocular morbidities. Indeed, dry eye is one of the most common reasons for patients to visit an eye care specialist seeking treatment.15 Chronic inflammation of the ocular surface can be a consequence as well as a cause of DED. Topical medications such as CYC and LIF can help to break or slow the self-perpetuating inflammatory cycle, but responses are heterogeneous, due in part to the influence of non-modifiable risk factors for DED such as sex- and age-related changes in aqueous tear and lipid production.16,17
This study had some limitations. First, there was potential for selection bias since specific details of how the patients were selected by the physicians and the timing of patient visits were not captured in the survey. In addition, since the majority of physicians who participated in the survey were ophthalmologists, the sample may not be representative of the entire population of eye care professionals with patients who use CYC or LIF. Second, the definition, duration, and severity of dry eye were not assessed and therefore the influence of severity on physician experiences was unclear. Third, the number of physicians included in the study was relatively small and all responses were subjective. Thus, the generalizability of the responses may be limited. Finally, this was a descriptive study and therefore no formal comparisons between the two treatments could be undertaken.
Conclusions
In conclusion, this survey study identified gaps in the management of DED as perceived by physicians currently using topical anti-inflammatory medications in their patients. Limitations of CYC and LIF include delayed onset of action, suboptimal symptom relief, and lack of improvement in patient QoL. There was a strong belief among physicians that additional treatment options are needed.
Acknowledgment
This paper was presented at the American Academy of Optometry (AAOPT) 98th Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published on the website of the AAOPT: www.aaopt.org/detail/knowledge-base-article/physician-satisfaction-with-anti-inflammatory-topical-medications-for-the-treatment-of-dry-eye-disease-339197-3238632. The authors thank Alan Pedder, MSc, for writing assistance.
Funding
This study was sponsored by Sun Pharmaceutical Industries, Inc. (Princeton, NJ, USA).
Disclosure
All authors were either clinical trial investigators or researchers sponsored or employed by Sun Pharmaceutical Industries, Inc. Dr Darrell White reports personal fees from Sun Pharmaceutical, personal fees from Allergan, personal fees from Takeda/Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi:10.1016/j.ajo.2017.06.033
2. Restasis Approval Package. Available from: www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-023_Restasis_Approv.pdf.
3. Galor A, Zheng DD, Arheart KL, et al. Dry eye medication use and expenditures: data from the medical expenditure panel survey 2001 to 2006. Cornea. 2012;31(12):1403–1407. doi:10.1097/ICO.0b013e31823cc0b7
4. Novartis Pharmaceuticals Corporation. Xiidra (Lifitegrast Ophthalmic Solution 5%) [Package Insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation;2019.
5. Akpek EK, Amescua G, Farid M, et al. American academy of ophthalmology preferred practice pattern cornea and external disease panel. dry eye syndrome preferred practice pattern®. Ophthalmology. 2019;126(1):PP286–P334. doi:10.1016/j.ophtha.2018.10.023
6. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 study group. Ophthalmology. 2000;107(4):631–639. doi:10.1016/S0161-6420(99)00176-1
7. Mah F, Milner M, Yiu S, Donnenfeld E, Conway TM, Hollander DA. PERSIST: physician’s evaluation of Restasis® satisfaction in second trial of topical cyclosporine ophthalmic emulsion 0.05% for dry eye: a retrospective review. Clin Ophthalmol. 2012;6:1971–1976.
8. Williamson JF, Huynh K, Weaver MA, Davis RM. Perceptions of dry eye disease management in current clinical practice. Eye Contact Lens. 2014;40(2):111–115. doi:10.1097/ICL.0000000000000020
9. Labbé A, Baudouin C, Ismail D, et al. Pan-European survey of the topical ocular use of cyclosporine A. J Fr Ophthalmol. 2017;40(3):187–195. doi:10.1016/j.jfo.2016.12.004
10. William CS, Henry H, Jeanette AS, et al. Dry eye treatments and preference survey. J Eye Dis Disord. 2018;3:118.
11. Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–415. doi:10.1016/j.ajo.2006.11.060
12. Patel VD, Watanabe JH, Strauss JA, Dubey AT. Work productivity loss in patients with dry eye disease: an online survey. Curr Med Res Opin. 2011;27(5):1041–1048. doi:10.1185/03007995.2011.566264
13. Uchino M, Uchino Y, Dogru M, et al. Dry eye disease and work productivity loss in visual display users: the Osaka study. Am J Ophthalmol. 2014;157(2):294–300. doi:10.1016/j.ajo.2013.10.014
14. Nichols KK, Donnenfeld ED, Karpecki PM, et al. Safety and tolerability of lifitegrast ophthalmic solution 5.0%: pooled analysis of five randomized controlled trials in dry eye disease. Eur J Ophthalmol. 2019;29(4):394–401. doi:10.1177/1120672118791936
15. Bradley JL, Özer Stillman I, Pivneva I, Guerinm A, Evans AM, Dana R. Dry eye disease ranking among common reasons for seeking eye care in a large US claims database. Clin Ophthalmol. 2019;13:225–232. doi:10.2147/OPTH.S188314
16. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003
17. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



