Back to Journals » Journal of Pain Research » Volume 13
Phospholipase Cβ3 Expressed in Mouse DRGs is Involved in Inflammatory and Postoperative Pain
Authors Ide S, Kawamata T , Ishida K , Fuseya S, Ishida T, Sugiyama Y, Kawamata M , Tanaka S
Received 8 September 2020
Accepted for publication 20 November 2020
Published 10 December 2020 Volume 2020:13 Pages 3371—3384
DOI https://doi.org/10.2147/JPR.S280565
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
Susumu Ide,1 Tomoyuki Kawamata,1,2 Kumiko Ishida,1 Satoshi Fuseya,1 Takashi Ishida,1 Yuki Sugiyama,1 Mikito Kawamata,1 Satoshi Tanaka1
1Department of Anesthesiology and Resuscitology, Shinshu University School of Medicine, Matsumoto, Nagano, Japan; 2Department of Anesthesiology, Wakayama Medical University, Wakayama, Japan
Correspondence: Susumu Ide; Satoshi Tanaka
Department of Anesthesiology and Resuscitology, Shinshu University School of Medicine, 3-1-1 Asahi, Matsumoto, Nagano 390-8621, Japan
Tel +812-6337-2670
Fax +812-6335-2734
Email [email protected]; [email protected]
Background: Previous studies suggested that phospholipase Cβ 3 (PLCβ 3), which is a common downstream component in the signaling cascade, plays an important role in peripheral mechanisms of perception including nociception. However, detailed profiles of PLCβ 3-expressing dorsal root ganglion (DRG) neurons and involvement of PLCβ 3 in inflammatory and postoperative pain have not been fully investigated.
Purpose: We evaluated neurochemical char0acteristics of PLCβ 3-expressing DRG neurons in mice and then we examined the effects of selective knockdown of PLCβ 3 expression in DRGs on inflammatory and postoperative pain.
Methods: Male C57BL/6-strain mice were used. For the inflammatory model, each mouse received subcutaneous injection of complete Freund’s adjuvant (CFA) in the left hindpaw. For the postoperative pain model, a plantar incision was made in the left hindpaw. PLCβ 3 antisense oligodeoxynucleotide or PLCβ 3 mismatch oligodeoxynucleotide was intrathecally administered once a day for three consecutive days in each model. The time courses of thermal hyperalgesia and mechanical hyperalgesia were investigated. Changes in PLCβ 3 protein levels in DRGs were evaluated by Western blotting.
Results: Immunohistochemical analysis showed that high proportion of the PLCβ 3-positive profiles were biotinylated isolectin B4-positive or transient receptor potential vanilloid subfamily 1-positive. PLCβ 3 protein level in DRGs during CFA-induced inflammation was comparable to that at baseline. Intrathecal administration of PLCβ 3 antisense oligodeoxynucleotide, which significantly suppressed PLCβ 3 expression in DRGs, did not affect pain thresholds in normal conditions but inhibited CFA-induced thermal and mechanical hyperalgesia both at the early and late phases compared to that in mismatch oligodeoxynucleotide-treated mice. Intrathecal administration of PLCβ 3 antisense oligodeoxynucleotide also inhibited surgical incision-induced thermal and mechanical hyperalgesia.
Conclusion: Our results uncover a unique role of PLCβ 3 in the development and maintenance of inflammatory pain induced by CFA application and in those of surgical incision-induced pain, although PLCβ 3 does not play a major role in thermal nociception or mechanical nociception in normal conditions.
Keywords: phospholipase Cβ 3, inflammatory pain, postoperative pain, thermal hyperalgesia, mechanical hyperalgesia
Introduction
Peripheral inflammation leads to the release of chemical mediators including adenosine triphosphate, bradykinin, trypsin/tryptase, endothelin-1, and prostaglandins, which play important roles in peripheral mechanisms of an inflammation-induced state of long-lasting pain.1 These chemical mediators activate G protein-coupled receptors (GPCRs) coupling to Gq/11 expressed on dorsal root ganglion (DRG) neurons. Then, upon ligand binding, GPCRs modulate the excitability of nociceptors through an intracellular signaling network.1 Since phospholipase Cβ (PLCβ) links GPCRs as a common downstream component in the signaling cascade,2 targeting PLCβ may be more effective than targeting individual chemical mediators for reducing pain.
Four major G protein-coupled PLCβ isoforms (PLCβ1, PLCβ2, PLCβ3, and PLCβ4) have been characterized, and PLCβ1, PLCβ3 and PLCβ4, but not PLCβ2, have been shown to be expressed in DRG neurons.3 While PLCβ1 and PLCβ4 are expressed in both DRG neurons and spinal neurons,3 PLCβ3 is preferentially expressed in DRG neurons and keratinocytes4,5 and in the brain6,7 but not in spinal neurons.3 In DRG neurons, PLCβ3 has the highest level of expression among PLCβ1, PLCβ3 and PLCβ4,3 suggesting that PLCβ3 plays an important role in peripheral mechanisms of perception including nociception.
Previous studies showed that itch sensation was reduced but that thermal and cold nociception and thermal hyperalgesia caused by injection of complete Freund’s adjuvant (CFA) were not affected by PLCβ3 whole-body knockout in mice.3,8 In contrast, another previous study showed that development of carrageenan-induced mechanical hyperalgesia was suppressed in PLCβ3 knockdown mice in which PLCβ3 in DRGs was knocked down by antisense oligodeoxynucleotide (ODN).9 Thus, the contribution of PLCβ3 in DRG neurons to inflammatory pain perception is still controversial. Involvement of PLCβ3 in inflammatory pain may vary depending on the degree and stage of inflammation. Postoperative pain is another pain condition in which inflammation around the surgical site is involved but is distinct from pure inflammatory pain because the pathogenesis of postoperative pain may include not only inflammatory but also neuropathic mechanisms.10 The contribution of PLCβ3 in DRG neurons to postoperative pain has not yet been elucidated.
The purpose of this study was to clarify the role of PLCβ3 in the two types of nociceptive pain, that is, inflammatory pain and postoperative pain. Inflammatory pain and postoperative pain share several pain-related phenotypes but might have different underlying signaling mechanisms.11,12 In this study, we investigated the expression profile of PLCβ3 in DRG neurons and the effects of selective knockdown of PLCβ3 expression in DRGs on CFA-induced pain at the early and late phases. We also examined the effects of selective knockdown of PLCβ3 expression in DRGs on surgical incision-induced pain.
Materials and Methods
All of the protocols of this study were approved by the Animal Care and Use Committee of Shinshu University School of Medicine, Matsumoto, Japan (reference No. 240050) on January 22, 2013. Mice were treated in accordance with the Ethics Guidelines for Investigations of Experimental Pain in Conscious Animals as issued by the International Association for the Study of Pain. Every effort was made to minimize animal suffering and to reduce the number of animals used in this study.
Animals
We used male C57BL/6-strain mice (18–20 g, Japan SLC, Hamamatsu, Japan). Mice housed in groups of 4 animals were maintained on a 12-h light/12-h dark cycle with food and water available ad libitum.
Inflammatory Pain Model
Under general anesthesia (1–2% isoflurane in 100% oxygen), each mouse received subcutaneous injection of a 30-μL emulsion of undiluted CFA (Sigma-Aldrich, St. Louis, MO, USA) in the medial left plantar hindpaw using a Hamilton syringe with a 30-gauge needle. After the injection, anesthesia was discontinued and mice were allowed to recover in their cages.
Postoperative Pain Model
Under general anesthesia (2% isoflurane in 100% oxygen), a plantar incision was made according to previous reports.11,13 After sterile preparation of the left hindpaw, a 6-mm longitudinal incision was made with a number 11 scalpel blade through the skin, fascia, and muscle. The incision was started 2 mm from the proximal edge of the heel and extended towards the toes. After wound hemostasis, the skin was sutured with two single sutures of 7–0 nylon. The mice were allowed to recover in cages with sterile bedding after surgery.
Oligodeoxynucleotide Preparation and Intrathecal Administration
The PLCβ3 antisense ODN sequence was directed against a unique region of the mouse PLCβ3 sequence (Gen Bank Accession No. NM008874). The mismatch ODN sequence was designed as the antisense ODN sequence scrambled. The antisense ODN and mismatch ODN sequences used in this study were 5´-TGGTGGTCATCTGGGATGTA-3´ and 5´-TTGCGGTGAGTAGTTCGTAG-3´, respectively. The hemagglutinating virus of the Japan envelope vector system (HVJ Envelope Vector Kit GENOMEONE-Neo; Ishihara Sangyo, Osaka, Japan) was used for in vivo transfection of the ODNs. This HVJ Envelope (HVJ-E) vector has been proven to be an effective ODN delivery system both in vitro and in vivo.14 The ODNs were incorporated into the HVJ-E vector according to the manufacturer’s instructions. The final concentration of the ODNs was adjusted to 2 μg/μL.
For intrathecal administration of the ODNs, mice were anesthetized and implanted with an intrathecal catheter according to our previous report.15 Briefly, under general anesthesia (1–2% isoflurane in 100% oxygen), a polyurethane intrathecal catheter with an inner diameter of 0.35 mm and an outer diameter of 0.84 mm (R-ITC, Pittsburgh, PA, USA) was inserted 5 mm into the mouse lumbar subarachnoid space at the L4/5 intervertebral. To administer drugs intrathecally, the tip of the catheter was located near the lumbar enlargement of the spinal cord. The catheter was tunneled subcutaneously and externalized through the skin in the neck region. The volume of dead space of the intrathecal catheter was 2 μL. After catheter implantation, the effects of intrathecal lidocaine (2%, 2 μL) were examined. Only mice that had shown complete paralysis of the tail and bilateral hind legs after intrathecal lidocaine injection were used in subsequent experiments. Intrathecal drug administration was accomplished using a microinjection syringe (Hamilton, Remo, NV, USA) connected to an intrathecal catheter under brief general anesthesia (1–2% isoflurane in 100% oxygen). The ODNs were administered manually over a 10-s period in a single injection volume of 2 μL (4 μg of the ODN) followed by a flush of physiological saline (3 μL). After discontinuance of general anesthesia, mice fully recovered within 2–3 min.
Thermal Testing
Mice were placed in plastic chambers on a glass surface and allowed to habituate for 30 min. Paw withdrawal latency (PWL) to noxious heat stimuli was assessed by applying a focused radiant heat source (model number 37370; Ugo Basil, Comerio, Italy) to unrestrained mice.16 PWLs were recorded as an average of 3 trials per animal with an interval between trials of more than 5 min. The intensity of the heat was adjusted so that the basal PWL was 10–15 s. The cut-off time was 20 s in order to avoid tissue damage. The person performing the behavioral tests was blinded to the treatment.
Mechanical Testing
Mice were placed on a stainless mesh floor covered with a clear plastic cage top. For testing mechanical responses, calibrated von Frey filaments (0.068-, 0.166-, 0.407-, 0.692-, 1.202-, 2.041-, and 3.630-g forces) were applied to the plantar surface in unrestrained mice. Each filament was applied at least 30 s between applications. Testing was initiated with a 1.202-g force. Depending on the initial response, subsequent filaments were applied by the “up-down method” to determine the paw withdrawal threshold (PWT).11,17
Immunohistochemistry
We used polyclonal antibodies raised against the following molecules: goat anti-PLCβ3 (1.0 μg/mL, Frontier Science, Sapporo, Japan), guinea-pig anti-transient receptor potential vanilloid subfamily 1 (TRPV1) (0.1 μg/mL, provided by Dr. Watanabe, Hokkaido University, Japan), and mouse anti-neurofilament 200 kD (NF200, a marker of myelinated neurons) (1:4000, N0142, Sigma-Aldrich). The specificities of the goat anti-PLCβ3 antibody and the mouse anti-NF200 antibody have been confirmed previously.7,16,18 Our preliminary study using TRPV1-deficient mice also confirmed the specificity of the guinea-pig anti-TRPV1 antibody. In addition, we used biotinylated isolectin B4 (IB4) (1:100, L3759, Sigma-Aldrich). Mice were deeply anesthetized with urethane (1.25 g/kg intraperitoneally) and perfused transcardially with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB). L4 and L5 DRGs were removed. The samples were immersed in 4% PFA in 0.1 M PB for 2 h for post-fixation and then cryoprotected in 25% sucrose in 0.01 M phosphate-buffered saline (PBS) overnight at 4°C. The samples were placed in Tissue-Tek embedding medium (Sakura, Tokyo, Japan) and rapidly frozen. The frozen samples of DRGs were cut into 16-μm sections using a sliding cryostat (LEICA, Tokyo, Japan). The tissue sections were thaw-mounted onto gelatin-coated slides for processing. The sections were washed in 0.01 M PBS for 30 min followed by 0.2% Triton X-100 (Sigma-Aldrich) in 0.01 M phosphate-buffered saline (PBS-t) for 30 min and incubated for 60 min at room temperature in a blocking solution consisting of 1% normal donkey serum and 0.01 M PBS-t. Then the sections were incubated with mixtures of primary antibodies in the blocking solution overnight at 4°C. After rinses with 0.01 M PBS-t, the slices were incubated with Alexa Fluor 488-, Alexa 597-, or Alexa 649-labelled species-specific secondary antibodies (Invitrogen, Carlsbad, CA, USA) at a dilution of 1:500 in PBS-t for 2 h at room temperature. Photographs were taken with a confocal laser-scanning microscope (ECLIPSE C1, Nikon, Tokyo, Japan).
Immunoblot Analysis
The mice were deeply anesthetized with isoflurane and sacrificed by decapitation. The L4, L5 and L6 DRGs were rapidly removed and homogenized in the presence of 150 μL of Extraction Buffer 1 (ProteoExtract®, Merck, Germany) containing a protease inhibitor cocktail on ice. The crude homogenates were incubated for 10 min and centrifuged at 1,000 g for 5 min at 4°C. Supernatants of the homogenates were collected, and the protein concentration was determined using a DC protein assay (Bio-Rad Laboratories, Hercules, CA, USA). The samples were heated for 10 min at 100°C with sodium dodecylsulfonate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Equal amounts of the proteins (10 μg) were loaded onto a 4–15% SDS-PAGE separation gel and transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA). The membranes were incubated with rabbit anti-PLCβ1 (1.0 μg/mL, Frontier Science), guinea-pig anti-PLCβ3 (1.0 μg/mL, Frontier Science), or rabbit anti-PLCβ4 (1.0 μg/mL, Frontier Science) in PBS containing 10% skim milk overnight at 4°C. Concentrations of actin, a housekeeping protein, were also measured using a rabbit anti-actin antibody (1:5,000, A2066, Sigma-Aldrich). Immunoreaction was visualized with an enhanced chemiluminescence plus Western blotting detection system (Amersham Biosciences, Piscataway, NJ, USA). The reaction product was visualized using an image analyzer (LAS-3000mini, Fuji Film, Tokyo, Japan). Previous studies showed that the PLCβ1, PLCβ3 and PLCβ4 antibodies used in this study recognized single protein bands corresponding to expected molecular weights (150 kDa, 157 kDa and 134 kDa, respectively).7,19,20 The person performing the experiments was blinded to the treatment. The amount of protein that can be obtained from 3 DRGs (left L4/5/6) of one mouse is very small and not sufficient for immunoblot analysis. Therefore, L4, L5, and L6 DRGs isolated from 2 mice were pooled for each sample, and data from Western blot analysis of PLCβ3 in DRGs were analyzed from 8 mice per group.
Distribution of PLCβ3 in DRG Neurons
To examine the distribution of PLCβ3, we performed multiple immunostainings of PLCβ3, TRPV1, IB4 and NF200 in naïve mouse DRGs. Only neurons with clearly visible nuclei were counted using a computerized image analysis system (EZ-C1 3.90, Nikon). The proportion of colocalization of PLCβ3-positive profiles with TRPV1-positive or IB4-positive neurons was determined by counting 300–600 neuronal profiles from 4–7 DRG sections for each mouse. Because a stereological approach was not used in this study, quantification of data may have yielded biased estimates of the actual numbers of cells and neurons. To prevent duplicate counting of neuronal cell bodies, sections that were 112 μm apart were counted for each DRG. An assistant who was unaware of the treatment groups of sections performed all of the counting.
Time Course of Hyperalgesia and PLCβ3 Expression Following CFA-Induced Inflammation
In order to know the time course of hyperalgesia after CFA injection, the following study was performed in naïve mice. After obtaining basal PWL and PWT, a 30-μL emulsion of undiluted CFA (Sigma-Aldrich) was injected in the medial left plantar hindpaw using a Hamilton syringe with a 30-gauge needle. To examine the involvement of PLCβ3 both in the early and late phases of CFA-induced inflammatory pain, PWLs and PWTs were measured before the injection and at 4 h, 6 h, 12 h, 24 h, 2 days, 3 days, and 7 days after the CFA injection. In some mice, the expression of PLCβ3 proteins in L4/5/6 DRGs was examined using Western blot analysis before and 24 h, 3 days, and 7 days after CFA injection.
Involvement of PLCβ3 in CFA-Induced Hyperalgesia
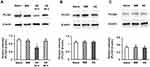
First, we examined the subtype specificity of the PLCβ3 antisense ODN in naïve mice. We tested effects of the PLCβ3 antisense ODN on not only expression of PLCβ3 but also expression of PLCβ1 or PLCβ4. Mice received single daily intrathecal administration of the PLCβ3 antisense ODN or the mismatch ODN for 3 consecutive days. Expression of PLCβ1, β3 and β4 proteins in L4/5/6 DRGs was examined using Western blot analysis 24 h and 48 h after the last administration of the antisense ODN or mismatch ODN.
Next, to examine the role of PLCβ3 in the development of CFA-induced hyperalgesia, mice received single daily intrathecal administration of the PLCβ3 antisense ODN or the mismatch ODN for 3 consecutive days before CFA injection. On the last day of ODN administration, CFA was injected into the plantar surface of the left hindpaw. Behaviors were assessed before ODN administration, before CFA injection and at 4 h, 6 h, 12 h, 24 h, 2 days, and 3 days after CFA injection. In some mice, expression of PLCβ3 was assessed 24 h after the last administration of the ODN. In addition, to examine the role of PLCβ3 in the late phase of CFA-induced hyperalgesia, mice received the PLCβ3 antisense ODN or the mismatch ODN once daily at 4 days, 5 days, and 6 days after CFA injection. Behaviors were assessed before CFA injection and at 1 day, 3 days, 7 days, and 8 days after the injection. In some mice, expression of PLCβ3 was assessed 24 h after the last administration of the ODN.
Involvement of PLCβ3 in Postoperative Pain
In order to know the time course of hyperalgesia after plantar incision, the following study was performed in naïve mice. After obtaining the basal PWL and PWT, a plantar incision was made in the left hindpaw. PWLs and PWTs were measured before the incision and at 2 h, 4 h, 6 h, 24 h, 2 days, 3 days, 5 days, and 7 days after the incision.
To examine the role of PLCβ3 in pain behaviors after plantar incision, mice received single daily intrathecal administration of the PLCβ3 antisense ODN or the mismatch ODN for 3 consecutive days before plantar incision. On the last day of ODN administration, a plantar incision was made in the left hindpaw. Behaviors were assessed before the ODN administration, before the incision and at 2 h, 4 h, 6 h, 24 h, 2 days, 3 days, 5 days, and 7 days after the incision.
Statistical Analysis
All results are expressed as means ± standard deviation (SD). A one-way analysis of variance for repeated measures followed by Dunnett’s test was used for within-group comparison. A two-way analysis of variance for repeated measures followed by Tukey’s test was used for between-group comparison. For immunoblot analysis, the intensity of bands was determined using an image densitometer (NIH Image 1.63; National Institutes of Health, Bethesda, MD, USA) and was normalized to the intensity of β-actin. The relative intensities of the bands were compared using the Mann–Whitney U-test. P < 0.05 was considered statistically significant. Statistical analysis was performed using Statview 5.0 software (Abacus Concepts, Berkeley, CA, USA) and NP Multi (Nagata T, Tokyo, Japan).
Results
Distribution of PLCβ3 Expression in DRG Neurons
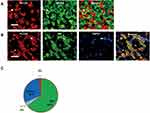
In naïve mice, most of the PLCβ3-positive neurons did not show NF200 immunoreactivity (Figure 1A). NF200-positive neurons were observed in 1.5% of all PLCβ3-positive DRG neurons (5 of the 333 PLCβ3-positive neurons). We performed triple immunostaining with PLCβ3, TRPV1, and IB4. A total of 1571 PLCβ3-positive DRG neurons were analyzed from 18 fluorescent slices from 4 mice. The results showed that 52% of TRPV1-expressing DRG neurons were PLCβ3-positive and 96% of IB4-expressing DRG neurons were PLCβ3-positive. 64%, 30% and 4% of PLCβ3-expressing DRG neurons were IB4-positive, TRPV1-positive, and both IB4- and TRPV1-positive, respectively (Figures 1B and C and Supplementary Figure 1).
Time Courses of Hyperalgesia and PLCβ3 Expression in DRGs After CFA-Induced Inflammation
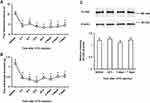
After an intraplantar CFA injection, ipsilateral PWL in response to noxious heat stimuli and ipsilateral PWT in response to mechanical stimuli by von Frey filaments were significantly decreased compared to basal values (before injection) (P < 0.01). Significant decreases in PWL and PWT were evident 4 h after CFA injection and lasted up to 7 days (Figure 2A and B). On the other hand, the relative intensity of PLCβ3/β-actin in L4/5/6 DRGs was not significantly changed at 24 h, 3 days, or 7 days after CFA injection compared to the basal value (P = 0.24, 0.24, 1.00, respectively) (Figure 2C).
Specificity of the PLCβ3 Antisense ODN
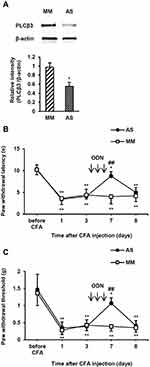
PLCβ3 antisense or mismatch ODNs were administered intrathecally once a day for 3 consecutive days. Intrathecal PLCβ3 antisense ODN, but not mismatch ODN, significantly decreased the relative intensity of PLCβ3 to β-actin in L4/5/6 DRGs compared to that in naïve mice (P = 0.02 and 0.31, respectively) (Figure 3A). PLCβ3 antisense ODN significantly decreased the relative intensity of PLCβ3/β-actin to about 60% of that in naïve mice at 24 h after the last administration of the ODN. The relative intensity in antisense-treated mice at 48 h after the last administration of the ODN was comparable to that in naïve mice. On the other hand, PLCβ3 antisense and mismatch ODNs did not change the relative intensity of PLCβ1/β-actin and that of PLCβ4/β-actin compared to levels in naïve mice (Figure 3B and C). In addition, PLCβ3 antisense ODN had no effect on either the relative intensity of PLCβ3/β-actin in keratinocytes or that in the cerebellum (data not shown).
Effects of the PLCβ3 Antisense ODN on CFA-Induced Hypersensitivity to Thermal and Mechanical Stimuli at the Early Phase
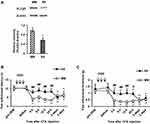
To examine the involvement of PLCβ3 in the early phase of CFA-induced inflammatory pain, the PLCβ3 antisense ODN or mismatch ODN was intrathecally administered once a day for 3 consecutive days before CFA injection. Intrathecal administration of the PLCβ3 antisense ODN significantly decreased the relative intensity of PLCβ3/β-actin at 24 h after CFA injection compared to that with intrathecal administration of the PLCβ3 mismatch ODN (0.56 ± 0.10 and 0.97 ± 0.14, respectively; P < 0.05) (Figure 4A). In behavioral analyses, intrathecally administered PLCβ3 antisense ODN or mismatch ODN did not affect the basal PWL and PWT (Figure 4B and C). Mice treated with the PLCβ3 mismatch ODN (mismatch-treated mice) showed significant decreases in PWLs and PWTs from 4 h to 3 days after CFA injection compared to those before CFA injection (**P < 0.01). Mice treated with the PLCβ3 antisense ODN (antisense-treated mice) showed significantly higher PWTs and PWLs from 4 h to 24 h after CFA injection (##P < 0.01; #P < 0.05) compared to those in the mismatch-treated mice. At 2 days and 3 days after CFA injection, PWTs and PWLs in the antisense-treated mice were comparable to those in the mismatch-treated mice. On the other hand, the antisense-treated mice showed significant decreases in PWTs from 4 to 24 h (**P < 0.01) but not in PWLs after CFA injection compared to those before CFA injection.
Effects of the PLCβ3 Antisense ODN on CFA-Induced Hypersensitivity to Thermal and Mechanical Stimuli at the Late Phase
To examine the involvement of PLCβ3 in the late phase of CFA-induced hypersensitivity, the PLCβ3 antisense ODN or mismatch ODN was intrathecally administered for 3 consecutive days (days 4, 5 and 6 after CFA injection) after CFA-induced hypersensitivity had been established. Intrathecal administration of the PLCβ3 antisense ODN significantly decreased the relative intensity of PLCβ3/β-actin at 7 days after CFA injection compared to that with intrathecal administration of the PLCβ3 mismatch ODN (0.55 ± 0.09 and 0.97 ± 0.10, respectively; P < 0.05) (Figure 5A). The PLCβ3 antisense ODN, but not the mismatch ODN, significantly reversed the decreased PWLs and PWTs at 7 days after CFA injection (Figure 5B and C). At 8 days after CFA injection, PWLs and PWTs in the antisense-treated mice were comparable to those in the mismatch-treated mice.
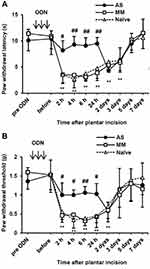
Effects of the PLCβ3 Antisense ODN on Surgical Incision-Induced Hypersensitivity to Thermal and Mechanical Stimuli
After a plantar incision, ipsilateral PWL in response to noxious heat stimuli and ipsilateral PWT in response to mechanical stimuli by von Frey filaments were significantly decreased compared to basal values (before injection) (P < 0.01) (Figure 6A and B). Significant decreases in PWL and PWT were evident 2 h after plantar incision and lasted up to 3 days and 2 days, respectively. To examine the involvement of PLCβ3 in postoperative pain, the PLCβ3 antisense ODN or mismatch ODN was intrathecally administered once a day for 3 consecutive days before plantar incision. Mismatch-treated mice showed significant decreases in PWLs and PWTs after plantar incision compared to those before plantar incision, and these significant decreases were comparable to those in naïve mice. Antisense-treated mice showed significantly higher PWLs and PWTs from 2 h to 24 h after plantar incision than those in the mismatch-treated mice (P < 0.05). Because PLCβ3 was not upregulated even in a long-lasting pathological state by CFA injection, we did not investigate incision-induced changes of PLCβ3 expression by Western blot analysis.
Discussion
The main findings of our study are as follows. First, most of the PLCβ3 was expressed in TRPV1-positive and/or IB4-binding primary afferents, which are considered nociceptive (Figure 1). Second, the amount of PLCβ3 expressed in lumbar DRGs was not changed after peripheral inflammation induced by CFA injection into the hindpaw (Figure 2). Third, knockdown of PLCβ3 in lumbar DRGs before CFA injection, which did not affect basal PWL in response to thermal stimuli and PWT in response to mechanical stimuli, reversed the thermal hyperalgesia and mechanical hyperalgesia seen at 1 day (early phase) and 7 days (late phase) after CFA injection (Figures 4 and 5). The knockdown effect observed in Western blot analysis lasted until 24 h after the last administration of the antisense ODN but was wearing off at 48 h (Figure 3), consistent with the time course of behavioral analysis in CFA-induced inflammatory hyperalgesia. Depression of PLCβ3 suppressed thermal and mechanical hyperalgesia in the postoperative pain model (Figure 6). Taken together, these results suggest that PLCβ3 in DRG neurons is involved in the transmission of inflammatory and postoperative pain.
Expression of PLCβ3 in DRG Neurons
We investigated the distribution of PLCβ3-expressed DRG neurons for clarifying the mechanisms of inflammation-induced hyperalgesia via the PLCβ3 signal pathway. Previous studies showed that PLCβ3 is expressed in DRG neurons, keratinocytes and cerebellar Purkinje cells3,6,21 but not in spinal neurons.3 Previous studies have suggested that, among the small- to medium-sized DRG neurons, PLCβ3 is mainly expressed in IB4-binding DRG neurons, which are unmyelinated non-peptidergic cells.3,21 However, more detailed information about the expression profile of PLCβ3 in DRG neurons was not obtained in those previous studies. In this study, we found that approximately half of TRPV1-positive DRG neurons expressed PLCβ3. We also found that 64%, 30% and 4% of the PLCβ3-expressing DRG neurons were IB4-positive, TRPV1-positive, and both IB4- and TRPV1-positive, respectively (Figure 1B and C).
Since TRPV1-positive and IB4-positive DRG neurons are considered to be different types of nociceptors,22,23 the expression pattern of PLCβ3 in DRG neurons suggests that PLCβ3 particularly modulates the activity of nociceptors contributing to pain perception. A recent study showed that inflammation-induced mechanical hyperalgesia and thermal hyperalgesia are selectively mediated by different types of DRG neurons.24 MrgprD-positive DRG neurons, which are thought to be mostly IB4-positive DRG neurons, and calcitonin gene-related peptide-positive DRG neurons, which are thought to be mostly TRPV1-positive DRG neurons, are selectively involved in inflammation-induced mechanical hyperalgesia and thermal hyperalgesia, respectively.24 Therefore, knockdown of PLCβ3 expressed in IB4-positive and TRPV1-positive DRG neurons might alleviate both mechanical hyperalgesia and thermal hyperalgesia during inflammation.
Roles of PLCβ3 in Inflammatory Pain
In the present study, intrathecal administration of the PLCβ3 antisense ODN selectively inhibited PLCβ3 expression in DRG neurons but not PLCβ1 or PLCβ4 expression (Figure 3). Intrathecally administered substances probably spread to not only DRGs but also the spinal cord. Since PLCβ3 is not expressed in spinal neurons,3 alleviation of thermal and mechanical hyperalgesia after intrathecal administration of the PLCβ3 antisense ODN is likely to be due to inhibition of PLCβ3 expression in DRG neurons.
Intrathecal administration of the PLCβ3 antisense ODN for three days significantly decreased the relative intensity of PLCβ3/β-actin to approximately 60% of that in naïve mice but did not affect basal PWLs and PWTs under non-inflammation conditions (Figure 4). These results suggested that PLCβ3 is not involved in nociception without inflammation or tissue damage. In contrast, intrathecal administration of the PLCβ3 antisense ODN for three days significantly reversed PWLs and PWTs in pathophysiological nociceptive pain states. Expression of PLCβ3 in DRGs was not up-regulated at the early and late phases of CFA-induced inflammation compared to the baseline level under normal conditions (Figure 2C). The level of activation of PLCβ3 was not determined in the current study because activation-specific antibodies were not used. PLCβ3 may be activated in a pathological state such as inflammation. It is possible that inflammation-induced activation of PLCβ3 in DRG neurons is required to augment transmission of nociceptive signals from peripheral nerve endings.
At present, it is not clear how PLCβ3 in DRGs mediates signal transmission of inflammatory pain. One possible explanation is that continuous activation of PLCβ3 under the condition of inflammation promotes signal transmission of pain through PLCβ3-expressing primary afferents, resulting in hyperalgesia. Our results are consistent with the results of a previous study showing that intrathecal administration of PLCβ3 antisense ODN decreased mechanical hyperalgesia induced by injection of carrageenan into the hindpaw.9 These results support the notion that PLCβ3 has a role in signal transmission under the condition of inflammation. However, the results of our behavioral analysis are not consistent with the results of another previous study showing that PWL to thermal stimuli and PWT to mechanical stimuli after CFA injection in PLCβ3 whole-body knockout mice were similar to those in wild-type mice.8 Functional compensation might occur in such PLCβ3-deficient mice. On the other hand, since PLCβ3 is exclusively expressed in nociceptors,9,21,24 it could be expected that ablation of PLCβ3 would result in a decrease in basal pain sensitivity. However, this was not the case in our study. Although the reason for this is not clear, the results of our study suggest that targeting PLCβ3 does not affect such basal pain sensitivities but only reverses mechanical and thermal hyperalgesia in inflammatory pain states. In addition, since PLCβ3 is mostly expressed in nociceptors, targeting PLCβ3 may reduce pain perception in inflammatory pain states without affecting other sensory modalities such as touch, pressure and vibration.
Roles of PLCβ3 in Postoperative Pain
We first demonstrated that intrathecal administration of the PLCβ3 antisense ODN alleviated postoperative thermal and mechanical hyperalgesia, indicating that PLCβ3 in DRG neurons is also involved in postsurgical hyperalgesia. We performed behavioral studies at the early phase but not at the late phase because the duration of postoperative hyperalgesia is relatively short compared to that caused by CFA. Postoperative pain is distinct from pure inflammatory pain or neuropathic models of hyperalgesia.10 This is partly because TRPV1 protein upregulation occurs after inflammation but apparently not after incision, though TRPV1 mediates heat hyperalgesia after both incision and inflammation.12 Our results suggest that PLCβ3 has an important role in the signal transmission of both CFA-induced inflammatory pain and incisional pain. On the other hand, it has been reported that TRPV1 was functionally up-regulated specifically in isolated IB4-positive DRG sensory neurons following skin incision injury and that TRPV1 was involved in heat hyperalgesia.25 Therefore, it is possible that the PLCβ3 antisense ODN alleviated incisional thermal hyperalgesia through downregulation of PLCβ3 in TRPV1-positive DRG neurons. The mechanism of postsurgical mechanical hyperalgesia has not been clearly elucidated. Further study is needed to investigate the role of PLCβ3 in postoperative hypersensitivity.
Clinical Implication
The mechanisms of early phase of hyperalgesia, that is, development of hyperalgesia induced by inflammation are partially different from those of late phase hyperalgesia, that is, maintenance of hyperalgesia. For example, the interleukin-1β and tumor necrosis factor-α/nuclear factor-kappa B/cyclooxygenase-2 pathways are involved in the development of inflammation-induced hyperalgesia but not the maintenance of the hyperalgesia.26 Acid-sensitive ion channel 3 is involved in the development but not in the maintenance of mechanical hyperalgesia in CFA-induced inflammatory pain.27 Our results showed that the PLCβ3 antisense ODN had anti-hyperalgesic effects both at the early and late phases of CFA-induced inflammatory pain. In addition, the PLCβ3 antisense ODN inhibited thermal and mechanical hyperalgesia in the postoperative pain model. In addition to inflammatory pain as a type of tissue injury-induced pain such as osteoarthritis, postoperative pain is also another type of tissue injury-induced pain that is commonly encountered in a clinical setting. Nociceptor-specific deletion of Gq/11, which is upstream of PLCβ3, and systemic administration of a nonselective PLC inhibitor reduce CFA-induced inflammatory pain and neuropathic pain,28 suggesting that PLCβ3 may play an important role in various stages of inflammatory pain including postoperative pain. Targeting PLCβ3 may thus lead to a new insight into drug discovery of analgesics for relieving various types of pathophysiological pain states.
Conclusion
Our results indicate that PLCβ3 in DRG neurons is involved in the development and maintenance of inflammatory pain and postoperative pain, although PLCβ3 does not play a major role in thermal sensitivity and tactile sensory threshold in normal conditions without inflammation. PLCβ3 may be an attractive target for the treatment of thermal and mechanical hyperalgesia in inflammatory pain and postoperative pain.
Funding
This study was supported by our institutional grant and Grants-in-Aid for Scientific Research (23390375 to Tomoyuki Kawamata and 25861369 to Susumu Ide) from the Japan Society for Promotion of Science (Tokyo, Japan).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi:10.1016/j.neuron.2007.07.008
2. Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335.
3. Han SK, Mancino V, Simon MI. Phospholipase Cβ 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi:10.1016/j.neuron.2006.09.036
4. Haase I, Liesegang C, Binting S, Henz BM, Rosenbach T. Phospholipase C-mediated signaling is altered during HaCaT cell proliferation and differentiation. J Invest Dermatol. 1997;108:748–752. doi:10.1111/1523-1747.ep12292135
5. Ando T, Xiao W, Gao P, et al. Critical role for mast cell stat5 activity in skin inflammation. Cell Rep. 2014;6:366–376. doi:10.1016/j.celrep.2013.12.029
6. Watanabe M, Nakamura M, Sato K, Kano M, Simon MI, Inoue Y, Patterns of expression for the mRNA corresponding to the four isoforms of phospholipase Cβ in mouse brain. Eur J Neurosci. 1998;10:2016–2025. doi:10.1046/j.1460-9568.1998.00213.x
7. Nomura S, Fukaya M, Tsujioka T, Wu D, Watanabe M. Phospholipase Cβ3 is distributed in both somatodendritic and axonal compartments and localized around perisynapse and smooth endoplasmic reticulum in mouse Purkinje cell subsets. Eur J Neurosci. 2007;25:659–672.
8. Imamachi N, Park GH, Lee H, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi:10.1073/pnas.0905605106
9. Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-β3 signals upstream of PKCε in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi:10.1016/j.pain.2007.01.027
10. Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America. 2005;23:1–20. doi:10.1016/j.atc.2004.11.009
11. Ishida K, Kawamata T, Tanaka S, Shindo T, Kawamata M. Calcitonin gene-related peptide is involved in inflammatory pain but not in postoperative pain. Anesthesiology. 2014;121:1068–1079. doi:10.1097/ALN.0000000000000364
12. Pogatzki-Zahn EM, Shimizu I, Caterina M, Raja SN. Heat hyperalgesia after incision requires TRPV1 and is distinct from pure inflammatory pain. Pain. 2005;115:296–307. doi:10.1016/j.pain.2005.03.010
13. Banik RK, Brennan TJ. Trpv1 mediates spontaneous firing and heat sensitization of cutaneous primary afferents after plantar incision. Pain. 2009;141:41–51. doi:10.1016/j.pain.2008.10.004
14. Kaneda Y, Nakajima T, Nishikawa T, et al. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther. 2002;6:219–226. doi:10.1006/mthe.2002.0647
15. Furuse S, Kawamata T, Yamamoto J, et al. Reduction of bone cancer pain by activation of spinal cannabinoid receptor 1 and its expression in the superficial dorsal horn of the spinal cord in a murine model of bone cancer pain. Anesthesiology. 2009;111:173–186. doi:10.1097/ALN.0b013e3181a51e0d
16. Kawamata T, Ji W, Yamamoto J, Niiyama Y, Furuse S, Namiki A. Contribution of transient receptor potential vanilloid subfamily 1 to endothelin-1-induced thermal hyperalgesia. Neuroscience. 2008;154:1067–1076. doi:10.1016/j.neuroscience.2008.04.010
17. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi:10.1016/0165-0270(94)90144-9
18. Baiou D, Santha P, Avelino A, et al. Neurochemical characterization of insulin receptor-expressing primary sensory neurons in wild-type and vanilloid type 1 transient receptor potential receptor knockout mice. J Comp Neurol. 2007;503:334–347. doi:10.1002/cne.21389
19. Fukaya M, Uchigashima M, Nomura S, Hasegawa Y, Kikuchi H, Watanabe M. Predominant expression of phospholipase Cβ1 in telencephalic principal neurons and cerebellar interneurons, and its close association with related signaling molecules in somatodendritic neuronal elements. Eur J Neurosci. 2008;28:1744–1759. doi:10.1111/j.1460-9568.2008.06495.x
20. Nakamura M, Sato K, Fukaya M, et al. Signaling complex formation of phospholipase Cβ4 with metabotropic glutamate receptor type 1α and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20:2929–2944.
21. Shi TJ, Liu SX, Hammarberg H, Watanabe M, Xu ZQ, Hokfelt T. Phospholipase Cβ3 in mouse and human dorsal root ganglia and spinal cord is a possible target for treatment of neuropathic pain. Proc Natl Acad Sci U S A. 2008;105:20004–20008. doi:10.1073/pnas.0810899105
22. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824.
23. Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108:143–155. doi:10.1016/S0306-4522(01)00377-3
24. Cavanaugh DJ, Lee H, Lo L, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi:10.1073/pnas.0901507106
25. Barabas ME, Stucky CL. TRPV1, but not TRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity. Mol Pain. 2013;9:9. doi:10.1186/1744-8069-9-9
26. Narita M, Shimamura M, Imai S, et al. Role of interleukin-1β and tumor necrosis factor-α-dependent expression of cyclooxygenase-2 mRNA in thermal hyperalgesia induced by chronic inflammation in mice. Neuroscience. 2008;152:477–486. doi:10.1016/j.neuroscience.2007.10.039
27. Karczewski J, Spencer RH, Garsky VM, et al. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol. 2010;161:950–960. doi:10.1111/j.1476-5381.2010.00918.x
28. Tappe-Theodor A, Constantin CE, Tegeder I, et al. Gα(q/11) signaling tonically modulates nociceptor function and contributes to activity-dependent sensitization. Pain. 2012;153:184–196. doi:10.1016/j.pain.2011.10.014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.