Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 8
Phosphatidylcholine liposomes as carriers to improve topical ascorbic acid treatment of skin disorders
Authors Serrano G, Almudéver P, Serrano J, Milara J, Torrens A, Expósito I, Cortijo J
Received 19 June 2015
Accepted for publication 28 September 2015
Published 17 December 2015 Volume 2015:8 Pages 591—599
DOI https://doi.org/10.2147/CCID.S90781
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Gabriel Serrano,1,* Patricia Almudéver,2,* Juan-Manuel Serrano,1 Javier Milara,2–5 Ana Torrens,1 Inmaculada Expósito,1 Julio Cortijo2–5
1Sesderma Laboratorios, 2Department of Pharmacology, Faculty of Medicine, University of Valencia, 3Clinical Research Unit, University General Hospital Consortium, 4CIBERES, Health Institute Carlos III, 5Research Foundation of the University General Hospital of Valencia, Valencia, Spain
*These authors contributed equally to this work
Abstract: Liposomes have been intensively investigated as carriers for different applications in dermatology and cosmetics. Ascorbic acid has potent antioxidant and anti-inflammatory properties preventing photodamage of keratinocytes; however, due to its instability and low skin penetration, an appropriate carrier is mandatory to obtain desirable efficacy. The present work investigates the ability of a specific ascorbate phosphatidylcholine (PC) liposome to overcome the barrier of the stratum corneum and deliver the active agent into the dermis to prevent photodamage. Abdominal skin from ten patients was used. Penetration of PC liposomes was tested ex vivo in whole skin, epidermis, and dermis by means of fluorescein and sodium ascorbate. Histology and Franz diffusion cells were used to monitor the percutaneous absorption. Ultraviolet (UV)-high performance liquid chromatography was used to analyze diffusion of sodium ascorbate through the different skin layers, while spectrofluorimetry and fluorescent microscopy were used for fluorescein monitoring. UVA/UVB irradiation of whole skin was applied to analyze the antioxidant capacity by Trolox assay and anti-inflammatory effects by tumor necrosis factor alpha and interleukin 1 beta enzyme-linked immunoassay. PC liposomal formulation improved skin penetration of fluorescein and ascorbate. Fluorescein PC liposomes showed better diffusion through epidermis than dermis while ascorbate liposomes showed better diffusion through the dermis than the epidermis. Ascorbate PC liposomes showed preventive antioxidant and anti-inflammatory properties on whole human skin irradiated with UVA/UVB. In summary, ascorbate PC liposomes penetrate through the epidermis and allow nonstable hydrophilic active ingredients reach epidermis and dermis preventing skin photodamage.
Keywords: skin absorption, liposomes, phosphatidylcholine, sodium ascorbate, fluorescein
Introduction
Skin is an organ subjected to a high degree of oxidative stress from both endogenous (metabolism, inflammation) and exogenous sources (radiation, smoking, chemical air pollutants, wind, etc), the most important being ultraviolet (UV) radiation. The induction of oxidative damage by UV radiation has been demonstrated to occur in lipids, proteins, and DNA.1 Adverse reactions include sunburn in short-term exposure, Langerhans cell depletion, and local immunosuppression caused by longer UV exposure and long-term effects such as cutaneous photoaging and skin cancer. As free radicals can inflict cellular damage, organisms have evolved several lines of defense to protect cells from free radicals and repair DNA damage.2 In skin, the most important lines of defense are enzymatic (eg, superoxide dismutase, catalase, peroxidase) and nonenzymatic (eg, glutathione, α-tocopherol or vitamin E, ascorbate or vitamin C, β-carotene and ubiquinone) antioxidant systems. As the capacity of these systems is limited and they can be overwhelmed by excessive exposure to reactive oxygen species, a promising strategy for enhancing skin protection from oxidative stress would therefore be to support the endogenous skin antioxidant system.
Numerous antioxidants have been tested for their ability to reduce free radicals to less reactive molecules, thus minimizing oxidative damage to critical cellular constituents.3 The antioxidants for preventing skin oxidative damage that have been studied most intensively are vitamins E and C, coenzyme Q10, and lipoic acid, referred to as “network antioxidants”, which work synergistically to regenerate one another. Although glutathione, coenzyme Q10, and lipoic acid can be synthesized by humans, levels of vitamins C and E depend on their oral intake or topical delivery.4 Vitamin C is important in treating skin pathologies ranging from mild inflammation to skin cancer.5
However, oral supplementation of vitamin C is not thought to increase the skin concentration sufficiently.6 Thus, topical delivery is an attractive alternative. Topical vitamin C treatment can significantly retard UVA-mediated damage to the skin.4,7 It plays a vital role in the metabolism of collagen where it is necessary for the hydroxylation of lysine and proline in procollagen.8 It has been further proven as an anti-inflammatory agent because it decreases the activation of the transcription factor nuclear factor kappa beta, which is responsible for many pro-inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interleukins (IL)-1, IL-6, and IL-8.9 It is also an excellent depigmenting agent owing to inhibition of the enzyme tyrosinase and consequent reduction of melanin production.6 Despite interesting scientific research, topical vitamin C preparations have often been disappointing for several reasons. Most preparations are very unstable on exposure to light and air. Oxidation occurs very rapidly, and once oxidized these preparations are useless. Even when products are stable, many of them do not penetrate the stratum corneum.6
Various types of strategy have been used by different groups to achieve successful antioxidant delivery. The major approaches used are chemical modifications of drug molecules and/or new drug delivery systems, which offer better targeting to the upper skin layer, faster onset, and lower concentrations. Liposomes are small vesicles composed of one or more lipid bilayers. Their unique structure gives them interesting properties such as the possibility to load both hydrophilic and lipophilic substances.10 In this way, they protect the active ingredients included in their internal layer or bilayers from the environment, oxidation, and degradation, improving the stability. It is important to notice that not every liposomal vesicle may protect adequately the active agent. It depends on the structure, which can suffer modifications when including the active agent.11 To date, there has not been a proof of concept concerning the critical parameters in this respect and experimental data are needed in order to substantiate a claim in this regard. Liposomes have been intensively investigated as carriers for different applications in dermatology. The ability of some specific types of liposomal vesicles to diffuse through the different layers of the skin has also been demonstrated allowing a successful treatment of different pathologies while restricting the systemic exposure to drugs.12
The present work evaluates the role of a specific ascorbate phosphatidylcholine (PC) liposome formulation as a carrier to improve the topical ascorbic acid treatment of skin. To this purpose, human skin penetration, inflammation, and oxidation were evaluated.
Materials and methods
Reagents
Liposomes were a grant from Sesderma company (Sesderma S.L, Valencia, Spain). Liposomes were prepared utilizing soybean lecithin of pharmaceutical grade from the supplier Lipoid (Ludwigshafen, Germany); sodium cholate from Wolfgang Mühlbauer (Hamburg, Germany); ethanol 96°, hydrochloric acid 1N, and sodium hydroxide from Panreac (Barcelona, Spain); apyrogenic and double-distilled water and saline from Grifols (Barcelona, Spain); phenoxyethanol from Thor Especialidades (Barcelona, Spain); sodium fluorescein from Sigma Chemical (Madrid, Spain); and sodium ascorbate from Fagron Ibérica (Barcelona, Spain). Tissue-Tek optimal cutting temperature (OCT) compound was obtained from Sakura (Leiden, the Netherlands).
Liposome preparation and characterization
Liposomes were prepared by Sesderma laboratories (Valencia, Spain) according with the patented procedure.
To manufacture the sodium ascorbate liposomes, we proceeded as follows: firstly, we dissolved the phospholipids in ethanol 96° and the sodium cholate in apyrogenic and double-distilled water, and then mixed both the solutions. Secondly, a solution of sodium ascorbate at 250 mg/mL was prepared in saline. Furthermore, the lipid and aqueous phases were mixed and agitated with a food mixer. A pH of 6.5 was achieved by adding a small amount of hydrochloric 1N. The suspension was incubated at room temperature for 10 minutes and then filtered through the Minisart® syringe filter with 0.2 μm pore size.
To prepare the fluorescein liposomes, we did as follows: the lipid phase was done in the same way as in the sodium ascorbate liposomes. Fluorescein was then dissolved in apyrogenic and double-distilled water with the aid of sodium hydroxide to form sodium fluorescein. The final concentration of fluorescein was 1 mg/mL. The two phases were mixed and agitated with a food mixer. A solution of water and phenoxyethanol (conservative) was added to the liposome suspension. Liposome pH was between 9 and 8.5. A final pH of approximately 8.5 was achieved by adding a small amount of hydrochloric 1N. The suspension was incubated at room temperature for 10 minutes and then filtered through the Minisart® syringe filter with 0.2 μm pore size.
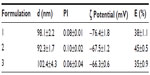
Size determination, polydispersity index, and zeta potential were done with the Delsa Nano C Particle Analyzer (Beckman Coulter Alcobendas, Madrid, Spain). The particle size determination was performed by a method of dynamic light scattering and the zeta potential by an electrophoretic light scattering method based on the Doppler effect. The polydispersity index was used as a measure to describe the uniformity of the size distribution. A value of polydispersity index equal or below to 0.12 indicates that the population is homogenous. The zeta potential is used as the index of the surface charge of the particles. If the zeta potential is high (30–150 mV), the liposomes are stable due to high electrostatic repulsion between particles and, on the contrary, a low zeta potential value increases the probability of particles colliding and thus forming particle aggregates.
The encapsulation efficiency (E%) was calculated using the formula: E% = EA/TA × 100 where EA represents the amount of encapsulated sodium ascorbate and TA the total amount of sodium ascorbate initially added to solution. E% in the liposomes was measured by determining the amount of sodium ascorbate remaining after dialysis against distilled water. Samples were injected into a dialysis cell (Slide-A-Lyzer® Dialysis Cassettes, Pierce, NJ, USA) with a hydrophilic cellulose membrane (10,000 Molecular weight cut-off [MWCO]). After 4 hours of dialysis, the dialyzing medium was changed and left overnight.
After 24 hours of dialysis, samples were withdrawn for high performance liquid chromatography (HPLC) analysis (the reference solution was a sample that was not dialyzed). The experiment was repeated three times for each sample.
Human skin samples for permeation studies
The study was approved by the Research Clinical Ethical Committee of the University General Hospital of Valencia. Signed informed consent was obtained for each patient. Ten Caucasian patients (40–50-years-old) free of any topical treatment gave the consent to use the leftover skin after plastic surgical abdominal resection procedure. Skin was used for percutaneous absorption ex vivo assays. Skin was fatty cleaned and frozen at -20ºC up to 3 months. The ex vivo studies were performed in Franz diffusion cells using epidermis, dermis, and whole skin as membranes. Epidermis and dermis were obtained using the method of Kligman.13 Briefly, it consists of the separation of the upper layers (stratum corneum and viable epidermis) after the skin has been immersed in distilled water at 60°C for 45 seconds.
Franz diffusion cells system
The passive diffusion across human abdominal skin was investigated using Franz diffusion jacketed cells with flat grounded (ground O-ring) joints composed of clear borosilicate glass with an orifice diameter of 10 mm and a receptor volume of 5 mL (Franz cell apparatus, PermeGear Inc., Hellertown, PA, USA). The Franz diffusion cells were mounted on a Franz cell stirrer with acrylic cell holders, synchronously stirred using magnetic Teflon stir bars at a constant speed of 500 rpm at 220 V/50 Hz (Franz cell stirrer, PermeGear Inc.), and thermostabilized. Skin sections were mounted with the epidermal side of the skin (if present) exposed to ambient conditions while dermal side was bathed by phosphate-buffered saline (PBS) pH 7.4 at 36±1°C in the receptor medium. To achieve higher reproducibility, the skin sample was prehydrated with the receptor medium for 60 minutes before applying the formulation. All bubbles were carefully removed between the underside of the skin and solution in the receptor compartment.
The liposomal formulations were applied onto the skin surface area of 0.9 cm2 in a nonoccluded condition. A minimum of three diffusion cells was used for each product. All studies were done in conditions of light protection of the Franz cells and samples. After 20 hours, the experiments were stopped and the diffusion setup was dismantled.
Biopsy processing for histology and staining
At defined incubation times, whole skin samples were extracted from Franz cell and fixed in 4% buffered formaline pH 3.7 at 4°C for 24 hours. Then, a progressive OCT inclusion was done using PBS in three steps for 1 hour each: OCT:PBS (1:3), OCT:PBS (1:1), and OCT. Cryostat (Leica CM1900) at -15ºC was used to prepare 5 μm skin sections. Oil red stain was conducted in prewarmed Oil Red O solution for 8–10 minutes in 60ºC oven and then Gill’s hematoxylin for 30 seconds. An aqueous mounting medium for oil red stains and a specific fluorescent mounting medium (DAKO, Nottingham, UK) were used for optic and fluorescence microscopy, respectively.
Ascorbate HPLC analysis
Ascorbate was measured in the receptor medium of Franz cells under skin preparations at different times. Ascorbate liposomes or free solution of ascorbate was added on human skin preparation as described above for up to 20 hours. Fluid samples from the receptor medium containing ascorbate were diluted immediately with mobile phase (50:50 v/v) and were analyzed by HPLC. A Shimadzu system (Shimadzu, Madrid, Spain): LC-10ADvp isocratic pump, Shimadzu SIL-10ADvp auto-injector, and a Shimadzu controller system with UV detection (λ=254 nm). The separation was done at room temperature and included the stationary phase, column Spherisorb ODS1 with precolumn C18 (Waters, Madrid, Spain), and mobile phase, potassium hydrogen phosphate buffer pH 2.6. The flow rate was 1.0 mL/min and the injection volume was 100 μL. The HPLC method was validated for accuracy (relative error obtained was <10%), precision (2.7% as the highest variation coefficient), and linearity over the concentration range analyzed (r2>0.996). The determination limit was 0.1 μg/mL. Concentration of ascorbate diffusing across the skin was expressed as μg/cm2 of skin.
Fluorescein analysis and fluorescence microscopy in skin permeation studies
Fluorescein liposomes skin absorption was analyzed by collecting fluorescein samples directly from the receptor medium of Franz cells, protected from light, and measuring the concentration by fluorometry with a plate spectrophotometer (PerkinElmer Inc., Waltham, MA, USA; victor 3). A standard curve was constructed with known concentrations of fluorescein to calculate fluorescein concentration as μg/cm2 of skin.
A Fluorescence Microscope Leica® DM6000B, equipped with a photographic camera DFC480 and a Leica GFP filter, was used to monitor fluorescein liposomes skin absorption. Excitation of the sample was carried out with 470/40 nm blue light. The fluorescent emission obtained was of 525/50 nm, in green light.
Skin antioxidant capacity, TNFα, and IL-1β measures
Total antioxidant status of the patients was estimated using a Trolox™ Equivalent Antioxidant Capacity assay in human skin tissue samples, based on the scavenging of the 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical (ABTS•), a soluble chromogen that is converted into a colorless product when it reacts with the ferryl myoglobin radical (a product from the reaction between metmyoglobin and hydrogen peroxide) according to the manufacturer’s protocol (cat. n° CS0790, Sigma-Aldrich, Madrid, Spain). The concentration of Trolox, a water-soluble vitamin E analog, was used to construct a standard curve. Antioxidant capacity was expressed as mM of Trolox by interpolation to the standard curve. TNFα and IL-1β cytokines were measured by ELISA kit assay according to the manufacturer’s instructions (B&D assay ELISA kit, Madrid, Spain).
An amount of 100 mg/mL ascorbate liposomes, empty liposomes, or a 100 mg/mL ascorbate water solution was placed on the skin surface area of 0.9 cm2 in Franz cells for 20 hours. After ascorbate exposure, skin surface was washed with phosphate buffer and skin was irradiated with solar simulator (+UVA/UVB ranging 270–700 nm) with an intensity of 50 J/cm2 for 2 hours with 1.7 mW/cm2 (Sun Simulator UVA/UVBCUBE400; Hoenle system, München, Germany) according to the OCDE 432 phototoxic test with modifications,14 whereas controls were kept in the dark. After another 24 hours of incubation in Franz cells, human skin was retired and cut in small pieces and homogenized with Ultra-Turrax (IKA, Staufen, Germany) in 500 μL of PBS. Skin tissue solution was assayed for antioxidant capacity and for TNFα and IL-1β cytokines.
Statistics
Statistical analysis was performed with the software program GraphPad Prism 5. One-way ANOVA data analysis followed by the Bonferroni multiple comparison test was performed when appropriate on the permeability coefficients (Kp) and different groups of treatments to establish differences between the calculated means.
Results
Analysis of liposome formulation and human skin penetration
All liposome formulations used in this study showed a liposome diameter in the range of 80–120 nm, with a polydispersity index below 0.12 and a zeta potential between 30 and 150 mV. The final concentration of sodium ascorbate was 100±11 mg/mL resulting in an E% of ~40% (Table 1).
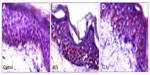
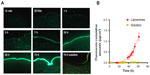
Whole human skin penetration assays were evaluated by histology and measuring cumulative amounts of active agent. Oil red staining skin histology was performed to label lipids from liposomes in different skin layers (Figure 1). Empty liposomes penetrated, per se, through the epidermis to the dermis with a sustained presence during 48 and 72 hours as visualized in red color (Figure 1). Parallel experiments performed with fluorescein labeled liposomes revealed a similar pattern of penetration (Figure 2). Fluorescein (lipophilic fluorescence substance) was used to monitor and quantify liposome–lipophilic active agent penetration through different layers of whole human abdomen skin. Fluorescein liposomes were added to the surface of whole human skin at different times (Figure 2) followed by surface skin wash and visualization of skin fluorescein penetration by fluorescence microscopy. Fluorescein quantification from the receptor medium of Franz cells was evaluated to analyze liposome whole skin penetration (Figure 2). We concluded that fluorescein liposomes reached epidermis after 3 hours of exposure (fluorescence microscopy images in Figure 2A) and that their content, fluorescein in the free form, in receptor medium of Franz cells started reaching dermis progressively after 20 hours (Figure 2B).
Human skin penetration profile of ascorbate liposomes
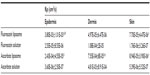
To study the differences between ascorbate liposomes and fluorescein liposomes permeation through human skin, various cutaneous preparations from human abdomen were assayed: whole skin, epidermis (without stratum corneum), and dermis (without stratum corneum and epidermis). As shown in Figure 3, whole skin penetration (samples collected from the receptor medium of Franz cells) of fluorescein and ascorbate, after corresponding liposomes applications, was small, reaching 8.94E-05±3.90E-06 kp (cm2/s) and 7.70E-05±4.47E-06 kp (cm2/s), respectively, being significantly higher than the solutions containing the free forms (Table 2), which indicates a local deposition onto the horny layer and epidermis, allowing only small amounts to get to the dermis (and consequently in the receptor medium of Franz cells). Next, human epidermis explants were evaluated. The penetration quantity of fluorescein coming from the liposomal formulation was higher than that of the ascorbate in the liposomal preparation reflecting the lipophilic nature of epidermis (Figure 3 and Table 2). By contrast, in the dermis samples, ascorbate liposomes diffused significantly more than the fluorescein liposomes which reflected the more hydrophilic nature of the dermis (Figure 3 and Table 2).
Antioxidant and anti-inflammatory properties of the ascorbate liposome in human skin exposed to UVA/UVB
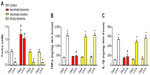
Whole human abdomen skin preparations were mounted in Franz cells and treated with ascorbate liposomes or ascorbate solution (100 mg/mL) during 20 hours. After washing the surface, skin was irradiated by UVA/UVB for 2 hours followed by incubation during 24 hours in Franz cells. Irradiated skin showed a decrease of the antioxidant capacity of human skin, elucidated by a decrease of the amount of Trolox in whole human skin (Figure 4A). Pretreatment with ascorbate liposomes prevented the effect of UVA/UVB irradiation, maintaining similar Trolox levels than nonirradiated skin. However, ascorbate solution did not prevent the loss of antioxidant capacity induced by UVA/UVB which indicates the necessity of adequate liposome carriers to reach damaged skin layers. Similar results were found for inflammatory cytokines. UVA/UVB exposure enhanced human skin inflammation, increasing TNFα and IL-1β amounts in whole human skin tissue. In contrast to ascorbate solution, ascorbate liposomes pretreatment prevented the increase of TNFα and IL-1β induced by UVA/UVB irradiation (Figure 4B and C) which is in agreement with the antioxidant results of ascorbate liposomes.
Discussion
In this study, we provide new evidence on topical bioavailability of novel ascorbate liposomes, as well as their antioxidant and anti-inflammatory properties, which protect skin from UVA/UVB irradiation and may be of potential value to attenuate different skin disorders.
During recent years, the topical delivery of liposomes has been applied to different applications and in different disease models. After topical application of liposomal formulations, such formulations can significantly increase the rate and extent of drug absorption into epidermis.15 On the other hand, different advantages such as their ease of preparation, enhanced absorption of active ingredients by skin, and continuous supply of agents in a sustained period of time make these carriers also suitable for cosmetic applications.16 Our results showed that PC liposomes are versatile as they can be used to encapsulate both hydrophilic and lipophilic molecules. Sodium fluorescein (hydrophilic, anionic) is a model of low lipophilicity active agent and sodium ascorbate (unstable) as a hydrophilic molecule. To be effective, an active drug must cross the stratum corneum barrier. To achieve this, there are different factors to pay attention to: initial concentration of drug, diffusion coefficient, molecular size, partition coefficient, pH ionization degree of the drug, anatomy of the stratum corneum, and vehicle–drug interactions. According to previous studies, the hydrophobic liposome structure interacts with corneocytes and improves access to epidermis.17 Hydrophobic liposomes allow the active component (ascorbate or fluorescein) to reach the epidermis. However, it should be mentioned that the liposomes are loaded with active agent both inside and outside of their phospholipid membrane; therefore, the physicochemical characteristics and mainly the n-octanol/water partition coefficient (log Pow) of the active agent will also be factors that influence the transepidermal absorption. In the case of the target compounds, fluorescein is more lipophilic and has a higher molecular weight than ascorbate, which explains that the diffusion of ascorbate in the epidermis is lower with respect to fluorescein due to the low lipophilicity of ascorbate. Once dermis was reached, liposome diffusion was lower due to the higher hydrophilicity of this milieu and the degradation of the ingredients by enzymatic means. Just the fraction released from the vesicles can penetrate easily through the dermis. These results were verified by comparing the absorption profiles of the liposomal suspensions with aqueous solutions containing the ingredients at the same concentrations than in dermis. Therefore, once penetrated through the epidermis, the characteristics of the active ingredient play a decisive role in the degree of diffusion across the dermis. In this case, the ascorbate presents the hydrophilic characteristics suitable for getting through this layer. Hence, although the liposomal fluorescein has a faster profile to cross the epidermis, ascorbate is the molecule of choice to achieve deeper targets such as the dermis and/or hypodermis (see Figure 3 in “Results” section).
Vitamin C, unique in its high reactivity with all aggressive oxygen radicals, is a major and the only essential antioxidant in the aqueous cell compartment. Most animals synthesize their own vitamin C, but only humans and other primates lack the enzyme α-glucono-γ-lactone oxidase, essential for vitamin C synthesis. As active transport of vitamin C from the gastrointestinal tract is limited, even massive oral doses do not increase its skin concentration to optimal levels.6 The skin contains relatively low amounts of ascorbic acid, ~41 ng/mg (dry weight) for the entire skin, with the stratum corneum containing only 7 ng/mg (dry weight).18 Its level in the skin, especially in the epidermis, also decreases with age.19,20 Skin levels of vitamin C can also be severely depleted by UV radiation and environmental pollutants. Even minimal UV exposure (a 1.6 minimal erythema dose) decreases the level of vitamin C to 70% of the normal level and exposure to 10 ppm of ozone in city pollution decreases its epidermal level by 55%.6 Topical application has been shown to increase significantly its cutaneous levels.
In fact, we observed a significant increase of ascorbate penetration with the PC liposome formulation in whole skin as well as epidermis and dermis which may be of potential value to provide adequate antioxidant properties to the skin at risk of oxidative or inflammatory damage.
Photoaging is accelerated aging of the skin resulting from environmental damage. The process is mainly due to UV irradiation and can be prevented. It accounts for most of the aging symptoms on sun-exposed skin. Knowledge of the mechanisms involved in photoaging provides the key approach for prevention and treatment of aged skin. In this regard, topical retinoids, particularly tretinoin, isotretinoin, and tazarotene, are medical therapies with proven benefits for the treatment of photodamaged skin derived from randomized clinical evidence.21 However, antioxidants have also been shown to have beneficial effects on reversing photoaged skin damage. Antioxidants are a very heterogeneous group of compounds varying from those similar to physiological species to numerous substances derived from nutritive plants, particularly polyphenols, catechins, flavonoids, and acyclic carotenoids, which also demonstrate strong antioxidant activity. Some substances of natural origin, such as curcumin, capsaicin, and gingerol, have also been shown to reduce photocarcinogenesis, not only because of their antioxidant activity but also because of their capacity to prevent inflammation, gene mutation, and immunosuppression.
In this work, we provide the first evidence on the preventive anti-inflammatory and antioxidant profile of ascorbate PC liposomes in human whole skin irradiated with UVA/UVB. These results demonstrate that an adequate ascorbate formulation can stabilize ascorbate and allows targeting the deeper layers of the skin so that it can produce its beneficial action. In contrast to the ascorbate solution, ascorbate PC liposomes prevented the loss of antioxidant skin capacity induced by sun simulator UVA/UVB. Antioxidant properties of ascorbate PC liposomes were similar to those anti-inflammatory properties since ascorbate PC liposomes were able to prevent the increase of TNFα and IL-1β enhanced by UVA/UVB exposure. Previous reports have shown that UV radiation can induce TNFα and IL-1β release from human keratinocytes as part of its cytotoxic actions.22 However, to our knowledge the effects of ascorbate on UVA/UVB-induced whole human skin damage have been not reported previously, probably due to the difficulty of an appropriate ascorbate skin delivery carrier as well as complexity of obtaining intact whole human skin. Regardless, important limitations of this work have to be highlighted. Thus, for example, ex vivo assays cannot reflect what happens in human sun exposition since we used a sun simulator, and laboratory-controlled conditions can be different to those expected in human skin photodamage.
Conclusion
In summary, we showed a new ascorbate PC liposome formulation with appropriate skin penetration, and antioxidant and anti-inflammatory properties against UVA/UVB photodamage. Although the PC liposomes assayed in this work showed promising results, other clinical assays are to be done to extract definitive conclusions.
Acknowledgments
This work was supported by grants SAF2011-26443 (JC), FIS CP11/00293 (JM), FIS PI14/01733 (JM), CIBERES (CB06/06/0027), ADE10/00020 (Spanish Government), as well as research grants from Regional Government Prometeo II/2013/014 (JC, JM) “Generalitat Valenciana”. Sesderma laboratories partially support this work.
Disclosure
Gabriel Serrano, Juan-Manuel Serrano, Ana Torrens and Inmaculada Expósito are employees of Sesderma SL company. The other authors report no conflicts of interest in this work.
References
Svobodova A, Walterova D, Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:25–38. | |
Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–120. | |
Antoniou C, Kosmadaki MG, Stratigos AJ, Katsambas AD. Photoaging: prevention and topical treatments. Am J Clin Dermatol. 2010;11:95–102. | |
Fuchs J. Potentials and limitations of the natural antioxidants RRR-alpha-tocopherol, L-ascorbic acid and beta-carotene in cutaneous photoprotection. Free Radic Biol Med. 1998;25:848–873. | |
Godic A, Poljsak B, Adamic M, Dahmane R. The role of antioxidants in skin cancer prevention and treatment. Oxid Med Cell Longev. 2014; 2014:860479. | |
Burke KE. Interaction of vitamins C and E as better cosmeceuticals. Dermatol Ther. 2007;20:314–321. | |
Darr D, Combs S, Dunston S, Manning T, Pinnell S. Topical vitamin C protects porcine skin from ultraviolet radiation-induced damage. Br J Dermatol. 1992;127:247–253. | |
Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54:1135S–1140S. | |
Sorice A, Guerriero E, Capone F, Colonna G, Castello G, Costantini S. Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini Rev Med Chem. 2014;14:444–452. | |
Verma DD, Verma S, Blume G, Fahr A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: a skin penetration and confocal laser scanning microscopy study. Eur J Pharm Biopharm. 2003;55:271–277. | |
Sharma VK, Sarwa KK, Mazumder B. Fluidity enhancement: a critical factor for performance of liposomal transdermal drug delivery system. J Liposome Res. 2014;24:83–89. | |
Pierre MB, Dos Santos Miranda Costa I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch Dermatol Res. 2011;303:607–621. | |
Kligman AM, Christophers E. Preparation of isolated sheets of human stratum corneum. Arch Dermatol. 1963;88:702–705. | |
Ceridono M, Tellner P, Bauer D, et al. The 3T3 neutral red uptake phototoxicity test: practical experience and implications for phototoxicity testing – the report of an ECVAM-EFPIA workshop. Regul Toxicol Pharmacol. 2012;63:480–488. | |
Yoo J, Shanmugam S, Song CK, et al. Skin penetration and retention of L-ascorbic acid 2-phosphate using multilamellar vesicles. Arch Pharm Res. 2008;31:1652–1658. | |
Raj S, Jose S, Sumod US, Sabitha M. Nanotechnology in cosmetics: opportunities and challenges. J Pharm Bioanal Sci. 2012;4:186–193. | |
Betz G, Imboden R, Imanidis G. Interaction of liposome formulations with human skin in vitro. Int J Pharm. 2001;229:117–129. | |
Pugliese PT. The skin’s antioxidant systems. Dermatol Nurs. 1998;10:401–116:quiz 17–18. | |
Leveque N, Muret P, Mary S, et al. Decrease in skin ascorbic acid concentration with age. Eur J Dermatol. 2002;12:XXI–XXII. | |
Leveque N, Robin S, Makki S, Muret P, Rougier A, Humbert P. Iron and ascorbic acid concentrations in human dermis with regard to age and body sites. Gerontology. 2003;49:117–122. | |
Kohl E, Steinbauer J, Landthaler M, Szeimies RM. Skin ageing. J Eur Acad Dermatol Venereol. 2011;25:873–884. | |
Marionnet AV, Chardonnet Y, Viac J, Schmitt. Differences in responses of interleukin-1 and tumor necrosis factor alpha production and secretion to cyclosporin-A and ultraviolet B-irradiation by normal and transformed keratinocyte cultures. Exp Dermatol. 1997;6:22–28. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.