Back to Journals » Journal of Experimental Pharmacology » Volume 15
Pharmacological Effects of the Lipidosterolic Extract from Kigelia africana Fruits in Experimental Benign Prostatic Hyperplasia Induced by Testosterone in Sprague Dawley Rats
Authors Occhiuto C, Santoro G , Tranchida PQ, Bono G, Occhiuto F
Received 3 August 2022
Accepted for publication 18 January 2023
Published 5 February 2023 Volume 2023:15 Pages 41—50
DOI https://doi.org/10.2147/JEP.S383699
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Nancy Muma
Cristina Occhiuto,1 Giuseppe Santoro,2 Peter Quinto Tranchida,1 Giovanni Bono,3 Francesco Occhiuto1
1Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, Messina, Italy; 2Department of Biomedical and Dental Sciences and Morpho-Functional Images, University of Messina, Messina, Italy; 3A. Imbesi Foundation, University of Messina, Messina, Italy
Correspondence: Francesco Occhiuto, Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, Viale Ferdinando Stagno d’Alcontres, 31, Messina, 98166, Italy, Tel +39 090 676 6453, Email [email protected]
Background: The use of phytotherapics is very frequent in men with prostatic diseases, sexual disorders and infertility, and many associations are commercially available. Various vegetable products used as drugs or nutraceuticals are attributed to possess the capacity to exert benefic effects on the reproductive system, and most of these drugs have a rich and varied lipidosterolic fraction, primarily responsible for the effects related to the male genital sphere. Kigelia africana (Lam.) Benth. (Bignoniaceae) is a plant used in African folk medicine as a vegetal remedy for various diseases, including some disorders of the male reproductive system; however, its potential activities have not yet been fully explored. The aim of the present study was to investigate whether the lipidosterolic hexane extract (LHE) from K. africana fruits, analyzed by comprehensive two-dimensional gas chromatography-mass spectrometry/flame ionization detection (GC×GC-MS/FID), can prevent or reverse benign prostatic hyperplasia (BPH) in rats.
Methods: BPH was induced in experimental groups by daily subcutaneous injections of testosterone propionate (TP) for four weeks. β-sitosterol (β-s) was used as positive control. On day 28, the animals were sacrificed by cervical dislocation after anesthesia. Prostates were excised, weighed, and used for macroscopic and histological studies. Testosterone and dihydrotestosterone (DHT) levels in prostate were measured.
Results: The results showed that LHE significantly reduced the prostatic weight, prostatic index, prostatic levels of testosterone and DHT, and the histopathological alterations (including the epithelial thickness, stromal proliferation, and lumen area) induced by testosterone. These effects were superior to those demonstrated by β-s and appear to be due to a partial antiandrogenic activity of LHE.
Conclusion: The results obtained showed that the LHE can prevent, and reverse testosterone induced prostatic hyperplasia, and support the traditional use of Kigelia africana in some disorders of the reproductive system.
Keywords: Kigelia africana, hexane extract, benign prostatic hyperplasia, GC×GC-MS/FID, histological studies
Graphical Abstract:
Introduction
Benign prostatic hyperplasia (BPH) is a frequent condition seen in senior men, characterized by a non-cancerous prostatic swelling associated with urinary tract disorders including difficulty in starting urination, urinary urgency and frequency, incomplete bladder emptying, urinary retention and histomorphological changes, mainly due to the proliferation of luminal epithelial cells and fibromuscular tissue.1 Androgens have a facilitating role in the development and growth of the prostate. Dihydrotestosterone (DHT) is an important intermediary of prostate growth, which is produced in the prostate from hematic testosterone by 5α-reductase. DHT production increases with aging and leads to hyperplasia and increased growth of the prostate.2 5α-reductase inhibitor drugs (such as finasteride) significantly reduce prostate size and the DHT content in the prostate and have been shown to be effective drugs for the treatment of BPH.3
The use of commercial natural products is very frequent in men with prostatic diseases and sexual disorders. Various vegetable products used as drugs or nutraceuticals are attributed to possess the capacity to exert benefic effects on the reproductive system, and most of these drugs have a rich and varied lipidosterolic fraction, primarily responsible for the effects related to the male genital sphere. The use of phytosterols as treatment for urinary tract symptoms due to a prostatic disease has constantly increased.4 Kigelia africana (Lam.) Benth. (Bignoniaceae) is a plant used in African folk medicine as a vegetal remedy for various diseases (malaria, diarrhea, psoriasis), including some disorders of the male reproductive system (treatment of erectile dysfunction, sexual asthenia and infertility), but its potential activities have not yet been fully explored. The main phytochemical constituents of K. africana fruits are iridoids, naphthoquinones, lignan, flavonoids, as well as phytosterols and fatty acids.5,6
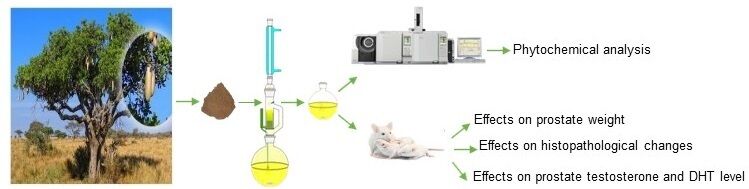
The aim of the present study was to investigate whether the lipidosterolic hexane extract (LHE) from K. africana fruits, identified and quantified (one-point calibration) by comprehensive two-dimensional gas chromatography-mass spectrometry and comprehensive two-dimensional gas chromatography-mass spectrometry/flame ionization detection (GC×GC-MS and GC×GC/FID), can prevent or reverse benign prostatic hyperplasia induced by testosterone in rats.
Materials and Methods
Plant Material
Collection of plant material was carried out in accordance with guidelines provided by the University of Messina and West African guidelines. Mature fruits of K. africana were collected, in Gallè village (Mali) in June 2016 and were identified at the Department of Traditional Medicine (DMT), University of Bamako (Mali). The fruits were air-dried, pulverized, and stored in airtight containers under vacuum until the preparation of the extract. A voucher specimen (nr. 03128) was deposited in the Department of Chemical, Biological, Pharmaceutical and Environmental Sciences of the University of Messina (Italy).
Preparation of the Extract
60 g of fruits were exhaustively extracted in a Soxhlet extractor with hexane (500 mL) for 8 hours. The crude extract obtained was then filtered and concentrated to dryness under vacuum with a rotating evaporator (Buchi R-205). The lipidosterolic extractive residue obtained was 1.65g (2.75% of dry drug). The residue was stored in sealed bottles under vacuum at a low temperature. The residue preserved in such a manner remains stable and its chemical composition does not vary over time, as demonstrated by the phytochemical analysis performed at different time intervals. In order to prepare the test solutions, the residues were suspended in Tween 80 at the desired concentrations, immediately before use.
Phytochemical Analysis
The lipidosterolic residue was saponified with a KOH/ethanol solution, and with heating at 80°C Gunder reflux. The unsaponifiable fraction was extracted with diethyl ether. The extraction was repeated twice. The organic solution was dried with anhydrous Na2SO4, filtered then concentrated to dryness with a rotating evaporator. The unsaponifiable dry extract was 0.82g (49.7% of the dry lipid extract). The fatty acid (FA) composition in the saponifiable fraction was investigated by using gas chromatography-quadrupole mass spectrometry, with 11 FAs subjected to identification (as methyl esters).
The unsaponifiable fraction, dissolved in 500 µL of chloroform, was treated with 200 µL of BSTFA [N,O-bis(trimethylsilyl) trifluoroacetamide] (1% TMCS - trimethylchlorosilane) and 200 µL of pyridine, and then heated at 70°C for 20 min. The derivatized sample was then ready for GC injection. The BSTFA + 1% TMCS kit was kindly supplied by Sigma–Aldrich (Milan, Italy).
Qualitative Analysis
Analysis of the unsaponifiable fraction of K. africana lipids was carried out by comprehensive two-dimensional gas chromatography-mass spectrometry (GC×GC-MS), using a Shimadzu GC×GC system, consisting of two independent GC2010 gas chromatographs and a QP2010 Ultra quadrupole mass spectrometer (Shimadzu, Kyoto, Japan). The primary GC system was equipped with an AOC-20i auto-injector, a split-split-less injector (350°C), and a cable extension for the MS connection (due to the presence of the second GC oven).
Data were acquired by using the GCMS solution software ver.4.41 (Shimadzu). Bidimensional chromatograms were generated by using the Chrome-Square software ver. 2.3 (Shimadzu Europe, Duisburg, Germany). Mass spectral identification was performed through the use of a sterols database (in-lab constructed) together with the support of the linear retention indices (LRIs) obtained injecting a C7-C40 alkanes (Sigma–Aldrich) standard solution.
Quantitative Analysis
The flow-modulation GC×GC-FID applications were carried out on a GC2030 Nexis gas chromatograph. Semi-quantification was performed using an external standard solution of stigmasterol trimethylsilyl ether (purity 95%), at a concentration of 50 ppm (v/v), in chloroform. Hence, single-point calibration was performed. The procedure used to obtain the silanization of the standard compound has been reported previously.7 Absolute quantities of the sterols and triterpenic alcohols contained in the unsaponifiable fraction of K. africana were determined.
Animals and Experimental Design
Male Sprague-Dawley rats, weighing 210–240 g, were used (Envigo RMS of Udine, Italy). The animals were kept under standard laboratory conditions (12 h light: 12 h dark and at 23 ± 2°C with a relative humidity of 40–60%). They were allowed unrestricted access to water and standard laboratory diet (rat pellets). Animal care and experimental procedures were approved by the animal welfare Committee of the University of Messina (ID 16/2016) and the Italian Ministry of Health (authorization number 814/2016 PR). The animals were handled throughout the experiment according to international laboratory animal use and care guidelines.8
BPH was induced in experimental groups by daily subcutaneous injections of testosterone propionate (TP, Sigma Chemical Co., St. Louis, MO), for four weeks. β-sitosterol (β-s) was used as positive control. A first series of experiment was conducted to investigate the ability of LHE to prevent BPH, using five groups of 5 rats for group: testosterone group (5 mg/kg b.w., solubilized in soy oil, for 4 weeks); LHE groups (administered daily orally at a dose of 10 or 100 mg/kg b.w. suspended in Tween 80, along with the TP injections, for 4 weeks); β-s group (administered daily orally at a dose of 1 mg/kg b.w. together with the TP injections, for 4 weeks); negative control group (no treatment); vehicle control group (vehicle, Tween 80, by oral gavage and soy oil by subcutaneous injection). The dosage of 10 and 100 mg/kg of LHE was chosen based on the positive results obtained from our preliminary research. The second series of experiments was carried out, in two groups of 5 animals, to investigate the ability of LHE to reverse BPH. The rats were treated daily with testosterone. After 2 weeks of testosterone-treatment, the rats were orally treated with LHE (100 mg/kg b.w) or β-s (1 mg/kg b.w) together with the TP injections for other 2 weeks. The control animals were treated with only vehicle (Tween 80, by oral gavage and soy oil by subcutaneous injection). All rats were weighed prior to the start of the experimentation and subsequently were weighed weekly during the experiment. The administered dose/animal was calculated based on the most recent recorded body weight of individual animals. Twenty-four hours after the last administration, the animals were weighted and sacrificed by cervical dislocation after anesthesia. Whole prostates were immediately removed, and weighted. Relative organ weights (prostate index) were calculated as the ratio of prostate weight (g) to body weight (g) x 100. Ventral prostate lobe was fixed with 10% neutral buffered formalin and embedded in paraffin for histological studies. The remainder of each prostate was stored at −20°C and used to evaluate testosterone and DHT levels.
Macroscopic Examination
After excision, entire prostates were examined for any macromorphological alterations, and the prostate sizes were compared in all the experimental groups.
Histological Examination
After 24 hours of fixation in formalin, prostates were embedded in paraffin and sectioned (5-µm thick sections were cut) using a Rotary Microtome RM2125RT (Leica Instruments Nussoloch Germany). The sections were stained with haematoxylin and eosin for microscopic observation of structural changes in the prostates, using a Nikon Ci-L microscope (Nikon Instruments Tokyo Japan). Each section was examined, and photomicrographs were taken. All low magnification images were obtained with a 10X objective, while the high magnification micrographs with a 40X objective.
Determination of Testosterone and DHT Concentrations in Prostate
Testosterone and DHT concentrations in the prostate were determined using enzyme-linked-immunosorbent assay (ELISA) and values were expressed for mg of total protein in prostate. In summary, prostates were homogenized (1/10 w/v) with a tissue lysis/extraction reagent that contained a protease inhibitor mixture (Sigma). Homogenates were centrifuged at 12.000 rpm, g-force 11,270, for 30 min at 4°C, and the protein concentration in the supernatant fractions were determined using Bradford reagent (Sigma).
Acute Toxicity
The acute toxicity was assessed by daily clinical observation of the behavior and of physiological functions of all animals during the treatment period using the Irwin test.9
Statistical Analysis
All data are expressed as mean ± SD. Statistical analysis was performed using both one-way variance analysis (ANOVA) and Dunnett’s t-test. Values of p < 0.05 were considered as statistically significant.
Results
Phytochemical Examination
The phytochemical analyses showed that the lipidosterolic extract from K. africana fruits, contained numerous sterols. In particular, 17 sterols were identified (Figure 1): β-sitosterol, stigmasterol, campesterol, cholesterol, lathosterol, campestanol, clerosterol, Δ−5-avenasterol, Δ−7-stigmastenol (4-α-methylsterols), citrostadienol (4-des-methylsterols), β-amyrin, α-amyrin, cycloartanol, 24-Me-cycloartanol, parkeol (4,4-dimethylsterols), erythrodiol and uvaol (triterpenic dialcohols). The absolute quantities and the relative percentage abundances of the sterols and triterpenic alcohols contained in the unsaponifiable fraction of K. africana are reported in Table 1. In the lipidosterolic extract, the overall content of phytosterols was found to be 24 mg (1.46%), with an individual content of β-sitosterol of 13.6 mg (0.83%). The results of the phytochemical analysis also showed that the lipid extract from K. africana fruits contains numerous fatty acids such as long chain and unsaturated fatty acids. The most abundant FAs, in a decreasing order, were linolenic, linoleic and oleic acids. Saturated FAs were present in low quantities and were represented mainly by palmitic and stearic acid (Table 2).
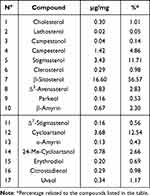 |
Table 1 Absolute Quantities and Percentual Relative Abundances of the Sterols and Triterpenic Alcohols Contained in the Unsaponifiable Fraction of K. africana Fruits |
 |
Figure 1 Comprehensive 2D GCxGC-MS chromatographic expansion highlighting the sterols contained in the lipid extract of the fruits of K. africana. |
Effects of LHE on Prostate Weight
Testosterone-induced BPH group showed a significant increase in relative prostate weight (P < 0.01) compared with the vehicle control group (Table 3). The co-treatment (for 4 or 2 weeks) with plant extract at a concentration of 10 mg/kg reduced prostatic index in an insignificant manner whereas at 100 mg/kg (which contains an almost equivalent amount of β-s) the prostatic index was significantly reduced (p < 0.05) as compared to the testosterone group. In addition, also PI values for the (β-s) groups were all significantly lower than those of the testosterone group (p < 0.05).
 |
Table 3 Effects of Lipidosterolic Hexane Extract from K. africana Fruits and of β-Sitosterol on Body and Prostate Weights in Testosterone Treated Rats for Subcutaneously Route |
Macroscopic Examination
Macroscopic examination of the prostate revealed that rats treated with only testosterone showed increases in prostate size compared visually with the control group. The prostate was enlarged, especially in the transition area, with fibroadenomatous nodules, while the fibromuscular part was peripherally displaced without tissue infiltration. On the other hand, treated rats with LHE or β-s displayed a marked reduction in prostate sizes and did not show other morphological alterations, compared visually with the testosterone-group (Figure 2). Considering that the weight gain of the prostate is a valid parameter for the evaluation of its size, these results are in agreement with the statistical comparison of the various groups relating to the weight of the prostate shown in Table 3, which, in fact, shows that the weight of the prostate was significantly increased in the TP group compared to the control group, while it was significantly reduced by the administration of LHE and β-s.
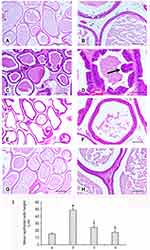
Effects of LHE on Histopathological Changes in Hyperplastic Prostates
In the control group, histological examination of the prostate showed tubular glands containing secreted material (corpora amylacea), of variable diameter and irregular lumen with secretory luminal cells lined with a single layer of low columnar epithelium. The interglandular fibromuscular matrix rich in vessels contained a normal strip of smooth muscle fibers.
In the testosterone group (Figure 3), the density of interglandular fibromuscular stromal was significantly increased, the gland walls were thickened, the intraglandular epithelial cells proliferated to significantly higher levels and the prostatic glandular cavities were also markedly enlarged than in the vehicle control group. In the plant extract groups (for 4 or 2 weeks) and in the (β-s) groups fibromuscular stromal and both epithelial cell proliferation and glandular cavity enlargement were markedly reduced. The morphological data were confirmed by a statistical evaluation of the mean epithelial height: a significant increase in the testosterone alone group and a significant decrease in both treated groups, more evident in the extract + testosterone group (Figure 3I) were demonstrated.
Effects of LHE on Prostate Testosterone and DHT Concentrations
The results obtained showed that in the prostate, the levels of testosterone (4.75 ± 0.30 ng/mL, P < 0.01) and DHT (420.35 ± 25.42 pg/mL, P < 0.01) were increased in the testosterone group as compared to the vehicle control group, in the β-s co-treated group (for 4 weeks at 1 mg/kg) a decrease in testosterone (2.88 ± 0.22 ng/mL, P < 0.01) and DHT (315.39 ± 34.20 pg/mL, P < 0.05) was observed. Similar to the results of β-s treated group, but with a greater potency, in the LHE-co-treated groups (for 4 weeks or 2 weeks) at 100 mg/kg, the level of testosterone (2.03 ± 0.6 ng/mL, P < 0.01) and DHT (245.24 ± 42.67 pg/mL, P < 0.01) were significantly reduced compared with those of the BPH group.
Acute Toxicity
Signs of acute toxicity effects like difficult or labored breathing, lacrimation, hair erection, convulsion, agitation, or dizziness were not observed during the whole observation period and all animals survived the experimentation period. Similarly, no macroscopic lesions of the autopsy organs were observed in all the groups treated up to highest dose of lipidosterolic hexane extract (100 mg/kg body weight) or β-sitosterol (1 mg/kg).
Discussion
Benign prostatic hyperplasia is one of the most common conditions in senior men. 5α-reductase inhibitors (such as finasteride) are frequently used for the treatment of BPH because they reduce the level of DHT. α-blockers drugs are also used for BPH, as they relax the smooth muscles of the prostate. However, both classes of drugs show different types of side effects including erectile and ejaculatory dysfunctions decreased libido, hypotension, and nasal congestion, which limit their therapeutic use. Therefore, phytomedicines are considered as a valid alternative to use, because of reduced side effects. Several plant extracts, including Serenoa repens, Pygeum africanum, and Secale cereal, have been evaluated in clinical trials for the treatment of BPH. The lipidosterolic extract of Serenoa repens (Permixon®), is frequently used for the treatment of BPH. This extract contains phytosterols (including β-sitosterol, stigmasterol, campesterol), aliphatic alcohols and a mixture of fatty acids including linoleic, oleic, palmitic and lauric acids, to which the pharmacological action is attributed, mainly due to the inhibition of 5α-reductase.
The results of the phytochemical analysis showed that the extract from K. africana fruits has a very similar chemical composition to that of the extract of Serenoa repens in fact, it contains numerous sterols as β-sitosterol, stigmasterol, campesterol, and unsaturated fatty acids as linolenic, linoleic, and oleic. Phytosterols are regarded as the most important contributors to the pharmacological activity of various vegetable products used as phytotherapics concerning prostatic hyperplasia. Positive effects of these phytochemicals on malign prostate hyperplasia and pro-apoptotic effects on prostate cancer cells have also been reported.10 Amongst the diverse group of phytosterols, β-sitosterol has widely been described as exceptionally active in several clinical studies on BPH,11 proving to be capable of preventing and reversing this condition. This clinical activity has been attributed due to a partial 5α-reductase inhibition, by this individual phytosterol,12 which has not been reported in the same manner from other associates of this compound group. In addition, also unsaturated fatty acids, like oleic, linolenic, and linoleic acids can play an important role in the inhibition of the 5α-reductase enzyme, thus, suggesting a competitive mechanism of inhibition with the sterols.13
The increase of relative prostate weight (prostate index) is considered as significant index of BPH.
The results obtained in relation to the prostate index showed that the LHE can prevent, and reverse testosterone induced prostatic hyperplasia. As can be seen from Table 3, the prostate index values are lower in the 2-week treatment (reversal) groups than in the 4-week (prevention) respective treatment groups and this indicates that LHE is more effective in reversing than preventing BPH.
Prostatic growth is used as one of important marker of BPH. Testosterone and DHT have a fundamental role in the growth of male reproductive organs. In the prostate, testosterone is turned by the 5α-reductase enzyme, into the active form dihydrotestosterone, that is commonly associated with BPH. Our results, in particular the reduction of DHT content in rats with BPH, indicating the capacity of the LHE to inhibit the 5α-reductase activity in the prostate.
In summary, our results demonstrate that the LHE can prevent, and reverse testosterone induced prostatic hyperplasia by a significant reduction of the prostatic weight, prostatic index, prostatic levels of testosterone and DHT, and by a marked reduction of the histopathological alterations (including the epithelial thickness, stromal proliferation, and lumen area) induced by testosterone. These effects appear to be due to a partial antiandrogenic activity of LHE.
In addition, the results obtained show also that the effects of LHE were superior to those demonstrated by β-s; therefore, it can be speculated that although phytosterols seem to contribute more significantly to the effects described above, synergism with other constituents of the lipidic extract such as fatty acids and antioxidants as α-tocopherol (a compound with established antioxidant properties) probably plays an important additional role. Therefore, the lipid extract of the fruits of K. africana could potentially be considered as a typical phytotherapic product, the activity of which is due to the synergistic interaction of numerous constituents of the extract.
Conclusion
In conclusion, we evaluated the effects of the lipidosterolic hexane extract from K. africana fruits, on prostate size and DHT and testosterone levels in prostate tissue in a testosterone-induced prostatic hyperplasia rat model. The results obtained show that the LHE can prevent, and reverse testosterone induced prostatic hyperplasia, and support the traditional use of Kigelia africana in some disorders of the reproductive system.
Abbreviations
BPH, Benign prostatic hyperplasia; GC×GC-MS/FID, gas chromatography-mass spectrometry/flame ionization detection. LHE, lipidosterolic hexane extract; β-s, β-sitosterol; TP, testosterone propionate; DHT, dihydrotestosterone.
Acknowledgments
We declare that part of the abstract of this manuscript was presented at the following conference: https://pinpick.it/wpcontent/uploads/documenti/BIBLIOGRAFIA/programma_ed_abstract.pdf.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pais P. Potency of a novel saw palmetto extract, SPET-085, for inhibition of 5alpha-reductase II. Adv Ther. 2010;27:555–563. doi:10.1007/s12325-010-0041-6
2. Carson C, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61:2–7. doi:10.1016/S0090-4295(03)00045-1
3. Paba S, Frau R, Godar SC, Devot P, Marrosu F, Bortolato M. Steroid 5α reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr Pharm Des. 2011;17:151–167. doi:10.2174/138161211795049589
4. Buck AC. Is there a scientific basis for the therapeutic effects of Serenoa repens in benign prostatic hyperplasia? Mechanisms of action. J Urol. 2004;172:1792–1799. doi:10.1097/01.ju.0000140503.11467.8e
5. Kokwaro JO. Medicinal Plants of East Africa.
6. Bello I, Shehu MW, Musa M, Zaini Asmawi M, Mahmus R. Kigelia africana (Lam) Benth. (Sausage tree): phytochemistry and pharmacological review of a quintessential African medicinal plant. J Ethnopharmacol. 2016;189:253–276. doi:10.1016/j.jep.2016.05.049
7. Tranchida PQ, Salivo S, Bonaccorsi I, Rotondo A, Dugo P, Mondello L. Analysis of the unsaponifiable fraction of lipids belonging to various milk-types by using comprehensive two-dimensional gas chromatography with dual mass spectrometry/flame ionization detection and with the support of high-resolution time-of-flight mass spectrometry for structural elucidation J. Chromatogr. 2013;1313:194–201.
8. National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; 2010.
9. Irwin S. Comprehensive observational assessment: a systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi:10.1007/BF00401402
10. Ifere GO, Barr E, Equan A, et al. Differential effects of cholesterol and phytosterols on cell proliferation, apoptosis and expression of a prostate specific gene in prostate cancer cell lines. Cancer Detect Prev. 2009;32:319–328. doi:10.1016/j.cdp.2008.12.002
11. Prager N, Bickett K, French N, Marcovici G. A randomized, double-blind, placebo-controlled trial to determine the effectiveness of botanically derived inhibitors of 5-alpha-reductase in the treatment of androgenetic alopecia. J Altern Complement Med. 2002;8:143–152. doi:10.1089/107555302317371433
12. Gutendorf B, Westendorf J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology. 2001;166:79–89. doi:10.1016/S0300-483X(01)00437-1
13. Liang T, Liao S. Inhibition of steroid 5 alpha-reductase by specific aliphatic unsaturated fatty acids. Biochem J. 1992;285:257–262. doi:10.1042/bj2850557
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.