Back to Journals » Drug Design, Development and Therapy » Volume 12
Pharmacokinetics of single- and multiple-dose roflumilast: an open-label, three-way crossover study in healthy Chinese volunteers
Authors Huang J, Fu CX , Yang XY, Cui C , Yang S, Kuang Y, Guo CX , Hu P, Pei Q , Yang GP
Received 2 July 2018
Accepted for publication 22 October 2018
Published 26 November 2018 Volume 2018:12 Pages 4047—4057
DOI https://doi.org/10.2147/DDDT.S178862
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Jie Huang,1,2,* Cheng-xiao Fu,1,2,* Xiao-yan Yang,1,2 Chan Cui,1,2 Shuang Yang,1,2 Yun Kuang,1,2 Cheng-xian Guo,1,2 Pei Hu,3 Qi Pei,2,4 Guo-ping Yang1,2
1Center of Clinical Pharmacology, The Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, People’s Republic of China; 2Center for Clinical Drug Evaluation, Central South University, Changsha, Hunan 410013, People’s Republic of China; 3Clinical Pharmacology Research Center, Peking Union Medical College Hospital, Beijing 100032, People’s Republic of China; 4Department of Pharmacy, The Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, People’s Republic of China
*These authors contributed equally to this work
Purpose: To determine the pharmacokinetic properties of the common tablet of roflumilast administered in single and multiple oral doses in Chinese subjects.
Subjects and methods: Both the single- and multiple-dose studies included 12 adults (6 males and 6 females). In this single-center, open-label study, single doses of 0.25, 0.375, and 0.5 mg were administered using a randomized, three-way crossover design, and then, the 0.375 mg dose was continued for 11 days once daily. The pharmacokinetic parameters for roflumilast and roflumilast N-oxide were determined and the safety evaluation included adverse events assessed by monitoring, physical examination, vital sign tests, and clinical laboratory tests.
Results: After every single dose, the time to the maximum concentration (Cmax) of roflumilast (Tmax) was 0.25–2.0 hours; thereafter, the concentration declined, with a mean half-life (t1/2) of 19.7–20.9 hours over the range of 0.25–0.50 mg. As for roflumilast N-oxide, the mean t1/2 was 23.2–26.2 hours. The area under curve from the beginning to 24 hours (AUC0–24 h), the AUC until infinity (AUCinf), and the Cmax of roflumilast and roflumilast N-oxide increased in a dose-proportional manner. After multiple doses, the accumulation index (Rac) on the 11th day of the steady state was ~1.63 for roflumilast and 3.20 for roflumilast N-oxide. No significant sex differences were observed in the pharmacokinetic parameters of roflumilast and roflumilast N-oxide. In addition, there were no serious adverse events across the trial.
Conclusion: Roflumilast was safe and well-tolerated in healthy volunteers, and a linear increase in its Cmax and AUC values was observed at doses ranging from 0.25 to 0.50 mg.
Keywords: pharmacokinetics, roflumilast, roflumilast N-oxide, healthy volunteer, phosphodiesterase 4 inhibitor
Introduction
Roflumilast is a benzamide compound designed for the treatment of asthma and COPD.1–5 A phosphodiesterase 4 (PDE4) inhibitor (Figure 1A) is extensively metabolized by I (cytochrome [CYP]450) and II phase (conjugation) reactions.6 The only major metabolite of roflumilast found in human plasma is roflumilast N-oxide (Figure 1B), whose formation is mainly catalyzed by CYP3A4. Although roflumilast is three times more potent than roflumilast N-oxide upon the inhibition of the PDE4 enzyme in vitro,7 the plasma area under the curve (AUC) of roflumilast N-oxide on average is ~10-fold greater than that of roflumilast.8 After oral administration of a 0.5 mg dose of roflumilast, its absolute bioavailability was ~80%. The maximum concentration (Cmax) for roflumilast and roflumilast N-oxide is usually observed at ~1 hour (range 0.5–2 hours) and 8 hours (range 4–13 hours) after administration, respectively. Food intake does not affect the total inhibitory activity of PDE4,9 but delays the time to the Cmax of roflumilast (Tmax) by 1 hour and decreases Cmax by ~40%. However, the Cmax and Tmax of roflumilast N-oxide are not influenced at all. The effective half-life (t1/2) ranges from 8 to 31 hours after dosing of 0.5 mg roflumilast; thus, these findings support a daily dosing regimen of roflumilast.
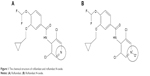  | Figure 1 The chemical structure of roflumilast and roflumilast N-oxide. |
However, to our knowledge, there is little literature about the pharmacokinetics of roflumilast and roflumilast N-oxide in healthy Chinese subjects.10,11 As a result, it is important to investigate the pharmacokinetics of these two compounds. This study was designed to evaluate the pharmacokinetics of roflumilast and roflumilast N-oxide in healthy Chinese volunteers.
Subjects and methods
Eligibility
A total of 12 healthy Chinese volunteers including six males and six females were screened for inclusion. The volunteers were aged between 18 and 45 years, with a body mass index (BMI) of 19–24 kg/m2. The minimum body weight was 45 and 50 kg for females and males, respectively. None of the volunteers had significant cardiac, hepatic, renal, pulmonary, neurologic, gastrointestinal, or hematologic disorders, as confirmed by performing a physical examination. Besides, those who had abused alcohol in the past 6 months or those who smoked heavily (more than five cigarettes a day) 3 months prior to the study were excluded. The study was conducted at a Phase I clinical center in the Third Xiangya Hospital of Central South University. It was conducted in accordance with the ethical standards for human studies of the Declaration of Helsinki and its amendments,12 the International Conference on Harmonization Guideline for Good Clinical Practice,13 and the Guideline for Good Clinical Principles recommended by the SFDA.14 The study protocol and informed consent form were approved by the ethics committee of The Third Xiangya Hospital of Central University. All participants were informed of the aim and risks by a clinical investigator, and each submitted written informed consent before participating in the study.
Study drug
Roflumilast tablets (0.25 mg, No 150101C, expiration date: January 2017; 0.375 mg, No 150101B, expiration date: January 2017; 0.5 mg, No 150101A, expiration date: January 2017) were manufactured and provided by Sichuan Baili Pharmaceutical Industry (Sichuan, P.R. China).
Study design and drug administration
This was a randomized, single-center, open-label trial based on a flowchart (Figure 2) wherein the pharmacokinetics of single and multiple oral administration of roflumilast were studied. A single-dose pharmacokinetic test was in the form of 3×3 Latin square crossover design. The subjects were admitted to the I phase ward one night before each cycle trial and fasted for more than 10 hours before the treatment. Then, a single dose of 0.25, 0.375, and 0.5 mg was administrated in the morning on the first day of each study period. The single dose was 0.25, 0.375, or 0.5 mg coupled with 250 mL of boiled water. The patients were not allowed to drink water 2 hours before and after dosing. Four and 10 hours after dosing, standard lunch and dinner, respectively, were allowed. After the completion of the single-dose test, multiple doses of 0.375 mg were administrated once daily for 11 days continuously.
Blood collection
For the single-dose test, blood samples were collected from the cubital vein before dosing and 0.25, 0.5, 0.75, 1, 1.5, 2.5, 3, 4, 6, 8, 10, 12, 14, 24, 30, 36, 48, 72, and 96 hours after dosing. The washout period for each group was 10 days. For the multiple-dose test, blood samples were collected before dosing and 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 14, 24, 30, 36, 48, 72, 96 hours after dosing on the 11th day.
Sample preparation
A volume of 5 mL of blood was collected into heparinized tubes and centrifuged at 2,700 rpm at 4°C for 10 minutes. The collected plasma was transferred to Eppendorf tubes, which were maintained at −70°C until the concentration analysis.
Analytical assays
High-performance liquid chromatography–tandem mass spectrometry was used to determine the concentration of N-oxide in the samples at different time points after administration.15 An LC–MS/MS quantitation method was developed to simultaneously determine roflumilast and roflumilast N-oxide in human plasma with rather low limits of quantitation (0.02 ng mL−1 for roflumilast and 0.04 ng mL−1 for roflumilast N-oxide). Human plasma samples were prepared by solid-phase extraction, which ensured high recovery and slight matrix effect for both analyses. In each validation batch, the calibration standards were analyzed and the calibration curve was linear within the range of 0.02–10 ng mL−1 and 0.04–50 ng mL−1 for roflumilast and roflumilast N-oxide, respectively. The regression coefficients of all the calibration curves were >0.99. The average bias among lower quality control (LQC), middle quality control (MQC), and higher quality control (HQC) samples was <15% compared with the nominal concentration, and the coefficient of variation (CV) of each concentration level was <15% as well, both for inter-run precision and intra-run precision. The developed method was successfully applied for the pharmacokinetic research in Chinese healthy volunteers after oral administration of 0.25, 0.375, and 0.5 mg roflumilast tablet.
Safety assessment
Safety was assessed according to interviews and adverse event (AE) monitoring. Vital sign tests, physical examinations, clinical laboratory tests, and electrocardiography were performed for each subject at screening and at study completion. AEs were assessed by direct observation, by using spontaneous reports, and by nonspecific inquiry and their clinical significance was determined by the monitoring physician. All information, including symptoms and signs before and after dosing were recorded in case report forms by investigators regardless of whether a relationship with the study drug was suspected.
Pharmacokinetic analysis
First, the pharmacokinetic analysis was performed by noncompartmental analysis using WinNonlin version 6.3 (Pharsight Corporation, Mountain View, CA, USA). Plasma roflumilast and roflumilast N-oxide concentrations vs time data after single-dose administration included Cmax and Tmax. The AUC from the beginning to the last values (AUClast) was determined using the linear trapezoidal method as was the AUC0–24 h, which indicated the AUC from the beginning to 24 hours. The first-order rate decline constant (λz) for roflumilast plasma concentrations in the terminal phase of the concentration–time curve was determined using log-linear regression, and the t1/2 was estimated from ln2/λz. AUCinf was determined using the following equation: AUClast + Clast/λz. Clearance rate (CL/F) was estimated as dose divided by AUCinf, and the volume of distribution (V/F) was determined by dividing the apparent CL/F by λz. The multiple-dose study provided Tmax at steady state (Tmax,ss), Cmax at steady state (Cmax,ss), and minimum plasma concentration at steady state (Cmin,ss). The accumulation index was determined as AUC0–24 h (day 11)/AUC0–24 h (day 1), while the degree of fluctuation (DF) was calculated as (Cmax,ss − Cmin,ss)/Cavg, where Cavg was the average steady-state drug concentration during multiple-dosing intervals, which was calculated as AUCtan,SS/tan, where tan was the dosing interval for 24 hours. The t1/2, CL/F, and V/F at steady state were determined using the same method as that used for the single-dose study.
In addition, pharmacokinetic analyses were performed by compartmental analysis using WinNonlin version 6.3. Nonlinear least squares regression analysis was performed using the plasma roflumilast and roflumilast N-oxide concentration vs time data. One and two compartment models with zero-order input and first-order elimination from the central compartment were fitted to the data. Data were weighted by the reciprocal of the observed plasma. The best-fitting model was selected by observing the residual plot, and by using Akaike’s information criterion. The model estimated the apparent volume of the central compartment (V1/F), Tlag, K21, K12, K01, and K10. Data are reported as medians (SD).
Statistical analyses
Statistical analyses were performed using SPSS version 18.0. All data were expressed as mean ± SD values. Log-transformed pharmacokinetic parameters AUC0–24 h, AUCinf, and Cmax were analyzed to determine dose proportionality using the power model, PK=A×(dose)β, where PK was the pharmacokinetic parameter, A was the intercept, and β was the dose-proportionality coefficient. In case the 90% CI for the dose-proportionality parameter β was included in the estimation interval, the pharmacokinetic parameters would correspond to perfect dose proportionality. Pharmacokinetic parameters were compared among dose levels by using ANOVA, including sex-related differences. A P-value of <0.05 was considered significant.
Results
Demographic characteristics
Twelve healthy Chinese volunteers (Table 1) with the following characteristics were enrolled in this study: age, 21±3 years (range, 18.0–27.0 years); weight, 61.3±5.4 kg (range, 47.0–73.5 kg); and height, 171±4 cm (range, 153–176 cm). All participants completed the study and were included in the PK analysis.
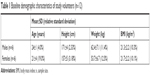  | Table 1 Baseline demographic characteristics of study volunteers (n=12) |
Noncompartmental analysis of single-dose administration
The mean plasma concentration vs time profiles for roflumilast and roflumilast N-oxide after administration of a single dose of 0.25, 0.375, or 0.50 mg were shown in Figure 3. The shape of the curves was similar between the various dose groups. As shown in Table 2, the mean Tmax values for roflumilast among the three dose groups were within 0.25–2.0 hours. The mean t1/2 values of roflumilast and roflumilast N-oxide were ~20 and 25 hours, respectively. The differences in t1/2 and CL/F among these dose groups were not statistically significant (P>0.05). Furthermore, the differences in these main pharmacokinetic parameters between male and female patients were not statistically significant (P>0.05) (Table 3).
As shown in Table 4, the power model estimating the linearity of a single dose ranging from 0.25 to 0.50 mg was analyzed. The 90% CIs for the slope of the relational equation were 0.869–1.103 (Cmax), 0.930–1.099 (AUC0–24 h), and 0.890–1.085 (AUCinf) for roflumilast, whereas those for roflumilast N-oxide were 1.020–1.198 (Cmax), 0.984–1.116 (AUC0–24 h), and 0.852–1.059 (AUCinf), all of which were within the estimation interval (0.8, 1.25). The pharmacokinetic parameters of roflumilast in the human body at a dose range of 0.25–0.50 mg conformed to the linear pharmacokinetic process.
  | Table 4 Dose proportionality following 0.25–0.5 mg doses |
Noncompartmental analysis of multiple-dose administration
After multiple doses of roflumilast 0.375 mg was administered over 11 days, the pharmacokinetic parameters Tmax (0.75 vs 0.50, P>0.05), CL/F (8.6 vs 9.4, P>0.05), and Cmax (11.4 vs 9.2, P>0.05) for roflumilast were similar to those observed in the single-dose study. For roflumilast N-oxide, some pharmacokinetic parameters were relatively higher, such as AUC0–24 h (618.0 vs 192.0, P<0.05), AUCinf (1,320.0 vs 493.0, P<0.05), AUClast (1,410.0 vs 458.0, P<0.05), and Cmax (35.4 vs 10.4, P<0.05). In addition, the accumulation index (Rac) was around 1.63 for roflumilast and 3.20 for roflumilast N-oxide. The DF was ~4.85 for roflumilast and 0.56 for roflumilast N-oxide (Table 5 and Figure 4).
Compartmental analysis of single- and multiple-dose administration
According to statistical analysis and “goodness-of-fit” criteria, a weighted (1/Y2) two-compartment pharmacokinetic model was adopted to describe roflumilast activity in plasma, and a one-compartment pharmacokinetic model was adopted to describe roflumilast N-oxide activity in plasma following three single oral doses of 0.25, 0.375, and 0.5 mg as well as multiple oral doses of 0.375 mg. The compartment pharmacokinetic parameters are summarized in Table 6.
Safety and tolerability
As seen in Table 7, during the course of the single- and multiple-dose studies, a total of 48 treatment-related adverse events were noted, of which 19 were considered as certainly related to treatment; 5, probably related to treatment; and 24, possibly related to treatment. Seven TRAEs were reported by four subjects while receiving the 0.25 mg single dose, six by six subjects while receiving the 0.375 mg single dose, 10 by six subjects while receiving the 0.50 mg single dose, and 25 by 12 subjects while receiving 0.375 mg multiple doses. Dizziness (53.8%), nausea (53.8%), diarrhea (30.8%), and headache (23.1%) were the more common TRAEs. Forty-six TRAEs were of mild degree, whereas only one TRAE presenting as diarrhea was of severe degree, and one TRAE presenting as acute gastroenteritis was of moderate degree. All these TRAEs resolved without sequelae.
  | Table 7 Number of subjects (%) reporting treatment-related adverse events (TRAEs) following single and multiple doses of roflumilast |
Discussion
COPD is a crucial factor causing morbidity and mortality and has a substantial global economic and social burden. The present approach for the management of COPD involves initial treatment with bronchodilators, followed by inhaled steroids when required. However, many patients still experience exacerbations despite access to the currently available therapy.16
A previous study17 confirmed that oral roflumilast at doses of 0.10, 0.25, and 0.50 mg once daily showed statistically significant effects with dose-related increases from baseline based on forced expiratory volume in 1 second in patients with asthma. It is clear that roflumilast at 0.50 mg once daily is more effective than lower doses with well tolerance. In a randomized double-blind controlled trial of roflumilast treatment for acute exacerbations of COPD,18 patients treated with roflumilast over 4 weeks experienced a statistically significant greater reduction in the percentage of sputum neutrophils and sputum myeloperoxidase concentration, which tended to have beneficial effects on lung function.
As seen in Table 8, Bethke et al11 used a two-period, two-sequence crossover study in 15 subjects receiving immediate-release tablets of roflumilast 0.25 or 0.5 mg to investigate the single-dose pharmacokinetics of the drug. However, that study was based on Caucasians. Moreover, the previous literature,10 which described a parallel-group study conducted in healthy Chinese subjects, did not show considerable dose-proportional pharmacokinetic characteristics because of interindividual variability. This article used a 3×3 Latin square crossover design to investigate single-dose pharmacokinetics while reducing interindividual differences. The report11 showed that after a single oral dose of 0.25 and 0.5 mg roflumilast, the Cmax and AUCinf of roflumilast and roflumilast N-oxide increased along with the dose of the drug. In our study, after single oral administration of 0.25, 0.375, and 0.5 mg of roflumilast, the Cmax values were 6.38, 9.24, and 12.50 ng mL−1 and the AUCinf values were 33.80, 48.7, and 65.10 h ng mL−1 for roflumilast, respectively, while the Cmax values were 6.74, 10.4, and 14.7 ng mL−1 and AUCinf values were 333.0, 493.0, and 649.0 h ng mL−1 for roflumilast N-oxide, respectively, confirming the linear pharmacokinetic characteristics of the drug. The Cmax of roflumilast was attained within 1.0 hours in healthy volunteers and the AUCinf of roflumilast N-oxide is around 10 times more than that of roflumilast, which is in accordance with the previous literature.19
It appears that after single oral administration of roflumilast, the degree of roflumilast exposure in Chinese patients was slightly higher than that in the foreign population.11 Population pharmacokinetic models have demonstrated the interracial variability in exposure to roflumilast and roflumilast N-oxide between Caucasians and other racial populations (African Americans, Hispanics, and Japanese).20 The racial differences in drug pharmacokinetics can be caused by biological factors (weight and genetics) or nonbiological factors.21 Thus, it is necessary to explore the interracial variability of exposure to roflumilast and roflumilast N-oxide between Chinese individuals and Caucasians. The recommended roflumilast dose of 0.5 mg once daily was approved by the US Food and Drug Administration and the European Medicine Agency.22 However, with reference to the prescribing information for DALIRESP® (roflumilast),6 some clinicians recommend that the roflumilast dose for Chinese patients should be <0.5 mg.18 Table 7 shows that the AUCinf of roflumilast and roflumilast N-oxide in Chinese patients was higher than those in Caucasians at the same doses (0.25 and 0.5 mg). After oral administration of 0.375 mg roflumilast, the peak and systemic exposure to roflumilast and roflumilast N-oxide in Chinese patients were similar to that observed in Caucasians administered 0.5 mg roflumilast, implying that the dose of 0.375 mg might be more appropriate for Chinese COPD patients.11 Thus, the 0.375 mg dose was used for the multiple-dose study, considering the safety.
The results of the present study showed that most of the major pharmacokinetic parameters of roflumilast and roflumilast N-oxide did not statistically differ between males and females, with slightly higher levels of roflumilast Cmax and roflumilast N-oxide AUC0–24 h being observed in healthy female subjects than in male subjects.
The most common (more than 2%) adverse reactions of roflumilast tablets (DALIRESP) are diarrhea, weight loss, nausea, headache, back pain, influenza, insomnia, dizziness, and loss of appetite. In this study, when healthy volunteers were administered a single oral dose and multiple oral doses of roflumilast, the major AEs were dizziness (53.8%), nausea (53.8%), diarrhea (30.8%), headache (23.1%), upper respiratory tract infection (23.1%), elevated creatine kinase level (15.4%), and elevated total bilirubin level (15.4%). Some subjects also showed loss of appetite, fatigue, chest tightness, and right lower back pain, which was consistent with the description in the drug instructions. Except for the severe diarrhea that occurred in one subject and the acute gastroenteritis, which was moderate and occurred in one subject, the other AEs were mild. In a previous report11 including 19 subjects, 2 subjects exited the study because of AEs such as erectile dysfunction, visual flicker, and blurred vision. A total of 15 subjects reported 59 AEs after receiving 0.5 mg roflumilast, with the most common AEs being headache (46.7%), nausea (20%), and abdominal discomfort (20%). Thus, the results presented in the literature were consistent with those obtained in our paper.
It is worth mentioning that our study only included 12 volunteers, which a drawback. Second, reasonable markers should be used to describe dose–response, and the results obtained remain to be tested in patients with COPD and asthma, because healthy volunteers are not representative of a patient population. Finally, the influence of renal impairment on the pharmacokinetics of oral roflumilast in Chinese subjects should be studied.
Conclusion
Roflumilast was safe and well-tolerated in healthy Chinese volunteers and showed linear characteristics for Cmax and AUC values at single doses ranging from 0.25 to 0.5 mg. No significant sex differences were observed in the pharmacokinetic parameters of roflumilast and roflumilast N-oxide.
Acknowledgments
We thank Xin Zheng and Xinge Cui for providing samples determination assistance. This study was supported by Pharmaceutical Preparation Optimization and Early Clinical Evaluation Engineering Technology Research Center of Hunan Province (No 2015TP2005) and Hunan Natural Science Foundation (2017JJ3464).
Disclosure
The authors report no conflicts of interest in this work.
References
Karish SB, Gagnon JM. The potential role of roflumilast: the new phosphodiesterase-4 inhibitor. Ann Pharmacother. 2006;40(6):1096–1104. | ||
Taegtmeyer AB, Leuppi JD, Kullak-Ublick GA. Roflumilast – a phosphodiesterase-4 inhibitor licensed for add-on therapy in severe COPD. Swiss Med Wkly. 2012;142:w13628. | ||
Pinner NA, Hamilton LA, Hughes A. Roflumilast: a phosphodiesterase-4 inhibitor for the treatment of severe chronic obstructive pulmonary disease. Clin Ther. 2012;34(1):56–66. | ||
Spina D. Phosphodiesterase-4 inhibitors in the treatment of inflammatory lung disease. Drugs. 2003;63(23):2575–2594. | ||
Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365(9454):167–175. | ||
FDA. DALIRESP (roflumilast) tablets (Initial U.S. Approval: 2011). Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022522s006lbl.pdf. Accessed March 31, 2015. | ||
Susuki-Miyata S, Miyata M, Lee B-C, et al. Cross-talk between PKA-Cβ and p65 mediates synergistic induction of PDE4B by roflumilast and NTHi. Proc Nat Acad Sci. 2015;112(14):E1800–E1809. | ||
Hermann R, Nassr N, Lahu G, et al. Steady-state pharmacokinetics of roflumilast and roflumilast N-oxide in patients with mild and moderate liver cirrhosis. Clin Pharmacokinet. 2007;46(5):403–416. | ||
Hauns B, Hermann R, Hünnemeyer A, et al. Investigation of a potential food effect on the pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46(10):1146–1153. | ||
Li Q, Wang Y, Liu L, Ma P, Ding L. Pharmacokinetics of roflumilast and its active metabolite roflumilast N-Oxide in healthy Chinese subjects after single and multiple oral doses. Eur J Drug Metab Pharmacokinet. 2017;42(3):371–381. | ||
Bethke TD, Böhmer GM, Hermann R, et al. Dose-proportional intraindividual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J Clin Pharmacol. 2007;47(1):26–36. | ||
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. | ||
European Agency for the Evaluation of Medicinal Products (EMEA). International Conference on Harmonisation World Health Organization. Guideline for Good Clinical Practice [EMEA Web site]. ICH topic E 6 (R1). Geneva, Switzerland: WHO; 2002. Available from: http://www.emea.europa.Eu/pdfs/human/ich/013595en.pdf. Accessed September 26, 2007. | ||
State Food and Drug Administration. Good Clinical Practice Guideline. Available from: http://www.sda.gov.cn/WS01/CL0053/24473.html. Accessed August 6, 2003. | ||
Cui X, Huang J, Zheng X. Simultaneous determination of roflumilast and its metabolite in human plasma by LC–MS/MS: Application for a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1029–1030:60–67. | ||
Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. | ||
Bateman ED, Izquierdo JL, Harnest U, et al. Efficacy and safety of roflumilast in the treatment of asthma. Ann Allergy Asthma Immunol. 2006;96(5):679–686. | ||
Mackay AJ, Patel ARC, Singh R, et al. Randomized double-blind controlled trial of roflumilast at acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(5):656–659. | ||
Lahu G, Hünnemeyer A, Diletti E, et al. Population pharmacokinetic modelling of roflumilast and roflumilast N-oxide by total phosphodiesterase-4 inhibitory activity and development of a population pharmacodynamic-adverse event model. Clin Pharmacokinet. 2010;49(9):589–606. | ||
Lahu G, Nassr N, Hünnemeyer A. Pharmacokinetic evaluation of roflumilast. Expert Opin Drug Metab Toxicol. 2011;7(12):1577–1591. | ||
Roflumilast (Daliresp) for COPD. Med Lett Drugs Ther. 2011;53:59–60. Available from: https://secure.medicalletter.org/article-share?a=1369b&p=tml&title=Roflumilast%20(Daliresp)%20for%20COPD&cannotaccesstitle=1. Accessed November 6, 2018. | ||
Kim K, Johnson JA, Derendorf H. Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol. 2004;44(10):1083–1105. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.