Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 10
Pharmacokinetics of evocalcet in secondary hyperparathyroidism patients receiving hemodialysis: first-in-patient clinical trial in Japan
Authors Shigematsu T , Shimazaki R, Fukagawa M, Akizawa T
Received 13 April 2018
Accepted for publication 25 June 2018
Published 11 September 2018 Volume 2018:10 Pages 101—111
DOI https://doi.org/10.2147/CPAA.S171044
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Takashi Shigematsu,1 Ryutaro Shimazaki,2 Masafumi Fukagawa,3 Tadao Akizawa4
On behalf of the Evocalcet Study Group
1Department of Nephrology, Wakayama Medical University, Wakayama, Japan; 2R&D Division, Kyowa Hakko Kirin Co., Ltd., Chiyoda-ku, Tokyo, Japan; 3Department of Internal Medicine, Division of Nephrology, Endocrinology and Metabolism, Tokai University School of Medicine, Isehara, Kanagawa, Japan; 4Department of Medicine, Division of Nephrology, Showa University School of Medicine, Minato-ku, Tokyo, Japan
Purpose: Cinacalcet is a positive allosteric modulator of calcium-sensing receptors in the parathyroid gland and an effective treatment for secondary hyperparathyroidism. However, this agent has considerable side effects and dosage limitations, which impair effective treatment. Therefore, we investigated the pharmacokinetics, pharmacodynamics, and safety of the novel calcimimetic, evocalcet.
Patients and methods: This was a multicenter, open-label study of single oral doses of 1, 4, and 12 mg evocalcet using an intrapatient dose escalation protocol in 29 Japanese secondary hyperparathyroidism patients receiving hemodialysis. Pharmacokinetics was assessed by plasma evocalcet concentrations. Pharmacodynamic evaluations consisted of measuring intact parathyroid hormone, serum corrected calcium, and fibroblast growth factor 23 concentrations. Safety and tolerability were evaluated by the analysis of adverse events (AEs).
Results: After a single 1-, 4-, or 12-mg dose, plasma evocalcet levels increased dose proportionally in a linear manner. Pharmacodynamic analyses showed that evocalcet effectively reduced intact parathyroid hormone and serum corrected calcium levels in a dose-dependent manner. AEs occurred in 31.0%, 28.6%, and 38.5% of patients receiving a single dose of 1, 4, or 12 mg, respectively. Most AEs were mild in severity.
Conclusion: Evocalcet is effective in the short term, has linear pharmacokinetics, and is well tolerated as observed by the low incidence of AEs.
Keywords: Calcimimetics, evocalcet, hemodialysis, parathyroid hormone, secondary hyperparathyroidism
Introduction
Secondary hyperparathyroidism (SHPT) develops as a result of chronic kidney disease (CKD). In patients undergoing hemodialysis, the condition progressively worsens as the parathyroid gland produces an excess of parathyroid hormone (PTH).1,2 SHPT causes a loss of calcium from the bones and an increase in calcium levels in the blood, known as chronic kidney disease-mineral and bone disorder (CKD-MBD).3–5 Excess PTH is also associated with arterial calcification, leading to arteriosclerosis, as well as disturbances in the endocrine, immune, and nervous systems.6–8
Current treatments for SHPT patients receiving hemodialysis include an array of vitamin D analogs that suppress PTH secretion; however, these cause a risk of hypercalcemia and hyperphosphatemia.9 The Japanese Society for Dialysis Therapy has published clinical practice guidelines for the management of CKD-MBD, including SHPT, which specify target ranges for serum concentrations of phosphorus, calcium, and PTH. The calcimimetic cinacalcet hydrochloride, a positive allosteric modulator of calcium-sensing receptors, is a therapeutic option that increases the sensitivity of calcium-sensing receptors in the parathyroid gland and effectively lowers PTH levels as well as maintains normal serum calcium and phosphorus concentrations.10
Treatment of SHPT with cinacalcet hydrochloride might reduce arterial calcification and the risk of a cardiovascular event, as well as improve bone metabolism parameters.11,12
However, common adverse events (AEs) related to gastrointestinal (GI) symptoms have been reported in a large proportion of patients treated with cinacalcet hydrochloride.13 A cumulative meta-analysis showed that the occurrence of GI symptoms such as nausea and vomiting after cinacalcet hydrochloride treatment is dose dependent.13 Moreover, the pharmacokinetic behaviors of cinacalcet hydrochloride tend to be nonlinear in SHPT patients.14 Cinacalcet hydrochloride also strongly inhibits the CYP/CYP450 (CYP) isoform CYP2D6, so it may increase the blood concentration of other concurrently prescribed CYP2D6-metabolized drugs, necessitating further dose adjustments.
Evocalcet is expected to be a novel, oral, positive allosteric modulator of the calcium-sensing receptor for the treatment of SHPT patients receiving hemodialysis or peritoneal dialysis. In a preclinical study, evocalcet showed a similar efficacy to cinacalcet in suppressing intact PTH secretion with fewer AEs related to GI symptoms.15 A recently conducted clinical study in healthy Japanese male adults involving single and multiple oral doses of evocalcet showed that doses up to 20 mg were tolerable.16 Only mild adverse drug reactions (ADRs) were observed in patients receiving 0.3–20 mg evocalcet with no GI events reported for doses up to 12 mg.
A Phase I clinical study in healthy male adults demonstrated that evocalcet is effective, tolerable, and fast acting with a reversible action.16 Based on these findings, the present study was planned with the objectives of examining the pharmacokinetics, pharmacodynamics, and safety of evocalcet administered orally in single and multiple doses in SHPT patients receiving hemodialysis. In this study, evocalcet treatment began with a single dose at 1 mg, followed by intrapatient dose escalation while confirming safety.
Methods
Ethics
The study was approved by the institutional review board of each participating center (Table S1), and all procedures were conducted in accordance with the Declaration of Helsinki and in compliance with the Pharmaceuticals and Medical Devices Act, Ministerial Ordinance on Good Clinical Practice and its partial amendments. All patients provided written informed consent. This clinical trial was registered at ClinicalTrials.gov (NCT01935856).
Patients
Thirty-five SHPT patients receiving hemodialysis gave consent, of whom 29 were eligible for participation in the study. Preliminary screenings were conducted on patients who provided consent. The key inclusion criteria included informed consent given, age 20–75 years, receiving hemodialysis three times weekly for at least 12 weeks, an intact PTH of ≥240 pg/mL, and a serum corrected calcium level of ≥8.4 mg/dL. The full exclusion criteria are described in the Supplementary materials.
From 2 weeks before screening until the end of the study, the concomitant use of the following drugs and therapies was prohibited: cinacalcet hydrochloride, bisphosphonate, denosumab, teriparatide preparations, calcitonin preparations, parathyroidectomy, and parathyroid interventions. For patients receiving vitamin D and its derivatives (calcitriol, maxacalcitol, falecalcitriol, alfacalcidol, and eldecalcitol), phosphate binders or calcium preparations, the dose and mode of administration were prohibited from being changed during the period from 2 weeks before the screening to the end of the study period. Patients requiring changes to dialysis conditions (dialysate calcium level, dialyzer, prescribed dialysis time, and prescribed frequency of dialysis per week) from 2 weeks before the screening to the end of the study period were withdrawn from the study.
Study design
This was a Phase Ib/IIa, multicenter, open-label study that aimed to evaluate the pharmacokinetics, pharmacodynamics, and safety of evocalcet treatment in SHPT patients receiving hemodialysis. Evocalcet was administered orally in a single dose as summarized in Figure 1. A subsequent article will describe the results from a multiple-dose and extension study.17
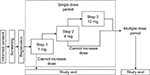  | Figure 1 Study design. |
Evocalcet was administered after breakfast on the next nondialysis day after the maximum interdialytic interval during each step (Step 1, 1 mg; Step 2, 4 mg; Step 3, 12 mg). A detailed overview of the study design is given in the Supplementary section.
For patients to transition from step to step, the following escalation criteria had to be met: having experienced no serious AE that could be causally related to evocalcet, having a serum corrected calcium level of ≥7.5 mg/dL at each time point, and patient safety was not thought by the investigators to be a concern.
Pharmacokinetic and pharmacodynamic analyses
The plasma evocalcet concentration was assessed using a validated liquid chromatography tandem-mass spectrometry (API 5000, AB Sciex Pte. Ltd., Concord, Ontario, Canada) method after extraction from the plasma samples using an OASIS µElution plate (Waters Co., Milford, MA, USA). The pharmacokinetic parameters were evaluated using Phoenix WinNonlin 6.1 software (Pharsight Corporation, Mountain View, CA, USA), including the time to attain maximum plasma concentration (tmax), maximum plasma concentration (Cmax), area under the plasma concentration–time curve (AUC), terminal elimination half-life (t1/2), apparent oral clearance (CL/F), and apparent oral volume of distribution at terminal phase (Vz/F).
Additional endpoints also included blood pharmacodynamics analysis of intact PTH, whole PTH, ionized calcium, serum corrected calcium, serum phosphorus, intact fibroblast growth factor 23 (FGF23), and calcitonin. Intact PTH was measured using electrochemiluminescence immunoassay (ECLusys PTH; Roche Diagnostics K. K., Tokyo, Japan). The intact PTH assay detected both 1-84PTH (active) and 7-84PTH (inactive), whereas the whole PTH assay detected only 1-84PTH. Intact FGF23 was measured using ELISA (FGF-23 ELISA Kit; KAINOS Laboratories, Inc., Tokyo, Japan).
During the treatment period, blood sampling was carried out on the following days: Day 1 (from waking to just before administration, at 0.5 and 1 hours after administration, and at 2, 4, 8, and 12 hours after administration); Day 2 (24 hours after administration [before the start of dialysis]); Day 3 (48 hours after administration); Day 4 (72 hours after administration [before the start of dialysis]); and at the time of discontinuation.
Safety and tolerability evaluations
The primary endpoints of this study were the safety and tolerability evaluations and included the analysis of AEs, laboratory values (hematology and blood biochemistry), vital signs, 12-lead electrocardiogram (ECG) measurements, and ophthalmologic examinations. The primary endpoint assessments were carried out on Day 1 (a nondialysis day and before administration of evocalcet on that day), and again on a subsequent examination day (before dialysis if on a dialysis day).
Statistical analysis
The target sample size was 20 to ensure an adequate number of patients to assess the pharmacokinetics, pharmacodynamics, and safety of evocalcet administered once.
For intact PTH, whole PTH, ionized calcium, serum corrected calcium, serum phosphorus, intact FGF23, and calcitonin levels, time profiles were prepared at each assay time point during each treatment period by dose.
For all AEs that occurred after the start of study treatment, occurrence and incidence of AEs and ADRs were summarized by each treatment period and dose. Incidence of AEs and ADRs was summarized by MedDRA/J System Organ Classes (SOC), preferred term, for each treatment period and dose.
Results
Patients
The demographics of the enrolled patients are summarized in Table 1. Twenty-nine patients were enrolled in this study and all patients started from Step 1. Of these, 28 patients proceeded to Step 2 and 26 patients proceeded to Step 3. One patient was ineligible after Step 1 and a further two patients were ineligible after Step 2.
Pharmacokinetic analysis
Plasma evocalcet concentrations after each single dose of evocalcet (1, 4, and 12 mg) reached Cmax approximately 4 hours after administration (Figure 2A). The pharmacokinetic data of plasma evocalcet are shown in Table 2. During the study period, the t1/2 was 20.86–22.52 hours, and the oral clearance (CL/F) was 1.2–1.4 L/hour. Cmax, AUC0-t, and AUC0-∞ values increased in proportion to the dose (Table 2). Evocalcet concentrations in plasma samples collected from the arterial and venous sides of the dialyzer were similar.17 Therefore, evocalcet was unlikely to be cleared by hemodialysis. There was also no difference in plasma evocalcet concentration levels when patients were stratified by sex (Figure 2B).
  | Figure 2 Change in plasma evocalcet concentration (A) and in males and females (B) over time after a single dose. |
Pharmacodynamic analysis
During the treatment period, intact PTH levels showed a dose-dependent decrease after administration of evocalcet (Figure 3A). The changes from baseline at 1, 4, and 12 mg were −13.45%, −48.49%, and −65.92%, respectively, at 4 hours after administration. By 72 hours after administration, intact PTH levels returned almost to baseline levels for all doses. Whole PTH levels also showed a dose-dependent decrease (Figure 3B).
Serum corrected calcium and ionized calcium levels decreased dose dependently after administration of evocalcet (Figure 3C, D). Specifically, lower serum corrected calcium and ionized calcium levels tended to persist with increasing dose. The changes from baseline to 24 hours at 1, 4, and 12 mg were −2.64%, −4.23%, and −6.55%, respectively.
After administration of evocalcet, phosphorus tended to decrease (Figure 3E). The low intact FGF23 values persisted as the dose increased (Figure 3F). Additionally, a dose-dependent increase in calcitonin levels was observed, which dropped almost to baseline levels 48 hours after administration (Figure 3G).
Safety and tolerability
Adverse events
In this study, no AEs led to death, and there were no AEs related to vital signs or body weight. Serious AEs were limited to shunt occlusion in one patient (3.4%) receiving 1 mg evocalcet. This resolved within 24 hours by percutaneous transluminal angioplasty, and a causal relationship with evocalcet was ruled out.
During the treatment period, AEs occurred in 31.0% (9/29) of patients at Step 1, 28.6% (8/28) of patients at Step 2, and 38.5% (10/26) of patients at Step 3 (Table 3). AEs that occurred in ≥2 patients were contact dermatitis in four patients (13.8%) and anemia in two patients (6.9%), both at the 1-mg evocalcet dose (Table 3).
  | Table 3 Adverse events and drug-related adverse events |
GI-related adverse events
GI-related AEs during the treatment period included vomiting in one patient (3.6% and 3.8%) after a single 4-mg evocalcet dose and after a single 12-mg evocalcet dose, and nausea in one patient (3.8%) after a single 12-mg evocalcet dose. Of these, vomiting after a single 4-mg dose and nausea after a single 12-mg dose were judged to be causally related to evocalcet administration.
12-lead ECG adverse events
Two patients had Fridericia’s corrected QT (calculated as QT/3√RR; QTcF) intervals that exceeded 500 ms as measured by 12-lead ECG. However, there were no patients with changes in QTcF intervals from the time-matched baseline values that exceeded 60 ms, for either QTcF interval or Bazett’s corrected QT (calculated as QT/√RR; QTcB) interval, during the treatment period (Figure 4). After administration of evocalcet, a negative correlation was observed between changes in QTcF interval from baseline and serum corrected calcium levels.
  | Figure 4 Time course of change in Fridericia’s corrected QT interval after a single dose. |
Discussion
This study was a multicenter, open-label study examining the pharmacokinetics, pharmacodynamics, and safety of evocalcet after oral administration as a single dose using an intrapatient dose escalation method in 29 SHPT patients receiving hemodialysis. This study shows that evocalcet offers good short-term tolerability in terms of upper GI symptoms while still providing therapeutic efficacy levels similar to cinacalcet hydrochloride.14,18 Additionally, this study shows that after a single dose of evocalcet (1, 4, or 12 mg), Cmax, AUC0-t, and AUC0-∞ values increased in proportion to dose. The dose-proportional increases indicate pharmacokinetic linearity.
During dialysis, drug clearance rates can vary. However, on days with and without hemodialysis, plasma PTH and serum calcium concentrations were shown to decrease in a dose-dependent manner following treatment with the calcimimetic agent cinacalcet.14 Similarly, in this study, evocalcet treatment at 1 and 4 mg showed that hemodialysis is unlikely to have an effect on the evocalcet clearance rate.
The safety of oral evocalcet treatment was confirmed by the low incidence of GI-related AEs even at the maximum dose of 12 mg. The low incidence of GI AEs such as nausea and vomiting suggests that there is a possibility that adherence rates might be improved while maintaining sufficient and effective dosages.
QTc interval prolongation associated with decreased blood calcium concentrations has been reported with cinacalcet administration.19,20 Evocalcet has also been reported to cause QTc interval prolongation in nonclinical studies, which involved a 4-week, repeated-dose, oral toxicity study in monkeys.21 In this study, there was a weak correlation between QTcF prolongation and decreases in blood calcium levels. This may be due to the variable factors of the QT interval prolongation including the background of hemodialysis patients. However, QT interval prolongation can be successfully managed after administration of evocalcet.
Treatment with evocalcet was shown to have an effective and persistent effect, even at a low dose of 1 mg, with dose-dependent decreases observed in both intact PTH and serum corrected calcium levels. Given that evocalcet has fewer GI-related AEs, evocalcet therefore represents a potential alternative to existing calcimimetics in the management of SHPT.
The potential and perceived limitations of this study include the small sample size, the absence of a placebo group, and the open-label nature. However, this was a Phase Ib/IIa clinical trial that aimed to determine the safety and tolerability of a novel, oral compound, which is to be further tested in a larger patient cohort.
Conclusion
Oral treatment with evocalcet, which suppresses intact PTH and serum corrected calcium concentrations dose dependently, is a safe and tolerable treatment option for SHPT patients receiving hemodialysis. The linear dose-proportional pharmacokinetic profile of evocalcet, combined with the mild safety concerns, provides evidence that evocalcet might be an alternative to presently available calcimimetics for SHPT patients receiving hemodialysis.
Acknowledgments
This work was sponsored by Kyowa Hakko Kirin Co., Ltd. Kyowa Hakko Kirin Co., Ltd. was involved in the study design, data collection, data analysis, preparation of the manuscript, and the decision to submit the manuscript for publication. The authors wish to thank James Graham, PhD, of Edanz Medical Writing for providing medical writing support, which was funded by Kyowa Hakko Kirin Co., Ltd.
Author contributions
All authors designed the study and contributed to acquisition, analysis, and interpretation of data; TS drafted the paper; TS, RS, MF, and TA revised the paper critically for important intellectual content. All authors approved the final version of the manuscript.
Disclosure
TS has received consulting fees from KHK Co., Ltd., Ono Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., and Fuji Pharma Co., Ltd., and lecture fees from KHK Co., Ltd., Chugai Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Kissei Pharmaceutical Co., Ltd., Torii Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. MF has received consulting fees from KHK Co., Ltd. and Ono Pharmaceutical Co., Ltd., and lecture fees from KHK Co., Ltd., Bayer Yakuhin, Ltd., Torii Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd., and grants from KHK Co., Ltd. and Bayer Yakuhin, Ltd. TA has received consulting fees from KHK Co., Ltd., Astellas Pharma Inc., Bayer Yakuhin, Ltd., Fuso Pharmaceutical Industries, Ltd., Japan Tobacco Inc., Ono Pharmaceutical Co., Ltd., and NIPRO Industry, and lecture fees from KHK Co., Ltd., Chugai Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Kissei Pharmaceutical Co., Ltd., Torii Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. RS is employed by KHK, which funded this research. The authors report no other conflicts of interest in this work.
References
Komaba H, Kakuta T, Fukagawa M. Diseases of the parathyroid gland in chronic kidney disease. Clin Exp Nephrol. 2011;15(6):797–809. | ||
Drüeke TB. Cell biology of parathyroid gland hyperplasia in chronic renal failure. J Am Soc Nephrol. 2000;11(6):1141–1152. | ||
Elder G. Pathophysiology and recent advances in the management of renal osteodystrophy. J Bone Miner Res. 2002;17(12):2094–2105. | ||
Torres A, Lorenzo V, Hernández D, et al. Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of a better bone response to PTH. Kidney Int. 1995;47(5):1434–1442. | ||
Slatopolsky E, Brown A, Dusso A. Pathogenesis of secondary hyperparathyroidism. Kidney Int Suppl. 1999;73:S14–S19. | ||
Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6(4):913–921. | ||
Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. | ||
Martin KJ, González EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol. 2007;18(3):875–885. | ||
Goodman WG. Recent developments in the management of secondary hyperparathyroidism. Kidney Int. 2001;59(3):1187–1201. | ||
Komaba H, Fukagawa M, KRN1493 Study Group. Impact of cinacalcet hydrochloride on the achievement of the Japanese Society for Dialysis Therapy (JSDT) guideline targets: a post-hoc analysis of the KRN1493 study. Ther Apher Dial. 2008;12(Suppl 1):S44–S49. | ||
EVOLVE Trial Investigators, Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. | ||
Akizawa T, Kurita N, Mizobuchi M, et al. PTH-dependence of the effectiveness of cinacalcet in hemodialysis patients with secondary hyperparathyroidism. Sci Rep. 2016;6:19612. | ||
Palmer SC, Nistor I, Craig JC, et al. Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials. PLoS Med. 2013;10(4):e1001436. | ||
Ohashi N, Uematsu T, Nagashima S, et al. The calcimimetic agent KRN 1493 lowers plasma parathyroid hormone and ionized calcium concentrations in patients with chronic renal failure on haemodialysis both on the day of haemodialysis and on the day without haemodialysis. Br J Clin Pharmacol. 2004;57(6):726–734. | ||
Kawata T, Tokunaga S, Murai M, et al. A novel calcimimetic agent, evocalcet (MT-4580/KHK7580), suppresses the parathyroid cell function with little effect on the gastrointestinal tract or CYP isozymes in vivo and in vitro. PLoS One. 2018;13(4):e0195316. | ||
Akizawa T, Shimazaki R, Shiramoto M, Fukagawa M, and Evocalcet Study Group. Pharmacokinetics, pharmacodynamics, and safety of the novel calcimimetic agent evocalcet in healthy Japanese subjects: first-in-human phase I study. Clin Drug Investig. In press 2018. | ||
Shigematsu T, Shimazaki R, Fukagawa M, Akizawa T, and Evocalcet Study Group. Pharmacodynamics of evocalcet for secondary hyperparathyroidism in Japanese hemodialysis patients. Clin Exp Nephrol. In press 2018. | ||
Akiba T, Akizawa T, Tsukamoto Y, et al. Dose determination of cinacalcet hydrochloride in Japanese hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial. 2008;12(2):117–125. | ||
Borrego-Utiel FJ, Pérez-del Barrio MP, Biechy-Baldan MM, Segura-Torres P. Cinacalcet may prolong the QT interval in patients on haemodialysis with secondary hyperparathyroidism. Nefrologia. 2013;33(2):272–273. | ||
Temiz G, Yalçın AU, Mutluay R, Bozacı I˙, Bal C. Effects of cinacalcet treatment on QT interval in hemodialysis patients. Anatol J Cardiol. 2016;16(7):520–523. | ||
Common technical document for evocalcet. Availabe from: http://www.pmda.go.jp/drugs/2018/P20180413003/index.html. Accessed August 22, 2018. Japanese. |
Supplementary materials
Study design
Patients started with a single dose of 1 mg evocalcet (Step 1), then a single dose of 4 mg evocalcet (Step 2), and then a single dose of 12 mg evocalcet (Step 3). For each step, patients were hospitalized from Day 1 to Day 3. Escalation from Step 1 to Step 2 and from Step 2 to Step 3 was set at a dosing interval of 3 weeks. Patients meeting escalation criteria proceeded to the multiple-dose study described elsewhere (manuscript in preparation).
Exclusion criteria
- Patients who had used cinacalcet hydrochloride within 2 weeks before screening.
- Patients with changes in the dose and mode of administration of active vitamin D or its derivatives, phosphate binders, or calcium preparations within 2 weeks before screening.
- Patients with changes in the dialysis condition (dialysate calcium level, blood purifier, prescribed duration of dialysis, prescribed frequency of dialysis) within 2 weeks before screening.
- Patients who had received bisphosphonates, denosumab, or teriparatide preparations within 24 weeks before screening.
- Patients who had undergone parathyroidectomy or parathyroid intervention in the past.
- Patients with concurrent severe heart disease (eg, Class III or IV on the New York Heart Association Functional Classification).
- Patients whose electrocardiogram (ECG) waveforms were judged unsuitable for the measurement of QT interval on a 12-lead ECG by the investigator at screening.
- Patients who had undergone surgery requiring blood transfusion, or in whom blood collection (including donation or apheresis donation) ≥200 mL had been performed within 12 weeks before screening.
- Patients with severe hepatic function disorder (eg, AST or ALT of ≥100 IU/L at screening).
- Patients with poorly controlled hypertension or diabetes mellitus.
- Pregnant, lactating, possibly pregnant women (women of childbearing potential with positive pregnancy test at screening or with negative pregnancy test but not using any contraceptive methods), or unwilling to use the adequate contraceptive method(s) according to the physician’s instructions. Amenorrhea for ≥12 months after the last menstrual period without an alternative medical cause was considered as a non-childbearing potential.
- History of serious drug allergy. History of drug or alcohol dependence. Current drug or alcohol dependence.
- History of diagnosis and treatment of malignant tumor within 5 years before screening (excluding basal cell carcinoma or surgically resected intraepithelial carcinoma of uterine cervix).
- Infection requiring hospitalization within 8 weeks before screening.
- Treatment with an investigational drug in a clinical study within 12 weeks before screening.
- Exposure to an investigational product in a prior clinical study of evocalcet.
- Primary hyperparathyroidism.
- Other conditions rendering the patient unfit for participation in this study at the discretion of the investigator or sub-investigator.
  | Table S1 List of participating institutions |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



