Back to Journals » International Journal of Nanomedicine » Volume 14
Pharmacokinetic studies of nanoparticles as a delivery system for conventional drugs and herb-derived compounds for cancer therapy: a systematic review
Authors Abdifetah O , Na-Bangchang K
Received 23 April 2019
Accepted for publication 31 May 2019
Published 23 July 2019 Volume 2019:14 Pages 5659—5677
DOI https://doi.org/10.2147/IJN.S213229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Omar Abdifetah,1,2 Kesara Na-Bangchang1–3
1Graduate Studies, Chulabhorn International College of Medicine, Thammasat University, Pathumthani, Thailand; 2Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, Chulabhorn International College of Medicine, Thammasat University, Pathumthani, Thailand; 3Drug Discovery Center, Thammasat University, Pathumthani, Thailand
Abstract: The poor pharmacokinetic characteristics of most anticancer drugs have limited their clinical effectiveness. The application of nanoparticles as a novel drug delivery system has provided opportunities to tackle the current challenges facing conventional drug delivery systems such as poor pharmacokinetics, lack of specificity to tumor cells, multidrug resistance, and toxicity. This systematic review aims to examine the application of pharmacokinetic studies of nanoparticles loaded in conventional drugs and herb-derived compounds for cancer therapy. The pharmacokinetic parameters of several herbal medicines and chemotherapeutic drugs loaded into nanoparticles were reported. This included area under the curve (AUC) of plasma concentration–time profile, maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), volume of distribution (Vd or Vss), elimination half-life (t½), and clearance (CL). The systematic review was conducted using information available in the PubMed and Science Direct databases up to February 2019. The search terms employed were: pharmacokinetics, pharmacokinetic study, nanoparticles, anticancer, traditional medicine, herbal medicine, herb-derived compounds, natural products, and chemotherapy. Overall, nanoparticle carriers not only significantly improved pharmacokinetics but also further enhanced permeability, solubility, stability, specificity, and selectivity of the carried anticancer drugs/herb-derived compounds to target tumor cells. Additionally, they also limited hepatic first-pass metabolism and P-glycoprotein (P-gp) efflux of the carried anticancer drugs/herb-derived compounds. Based on this systematic review, polymeric nanoparticles were the most commonly used nanocarrier to improve the pharmacokinetic parameters. The use of nanoparticles as a novel drug delivery system has the potential to improve both pharmacokinetics and cytotoxicity activity of the loaded drugs/herb-derived compounds for cancer therapy.
Keywords: anticancer, chemotherapy, herb-derived compounds, nanoparticles, pharmacokinetics, traditional medicines
Introduction
Cancer is the leading cause of death globally.1 Chemotherapy, radiation therapy, and surgery are the main therapeutic approaches for cancer.1,2 The success of chemotherapy has been limited due to the lack of drug specificity to tumor cells, insufficient drug concentration in tumor cells, serious adverse effects, and the emergence of multidrug-resistant tumor cells.1–3 Several strategies have been proposed to tackle these challenges facing conventional chemotherapeutic drugs, which includes the use of traditional or herbal medicines for cancer therapy. The use of herbal medicines for cancer therapy has been rising all over the world due to their biological activity as well as fewer adverse effects as compared to conventional chemotherapeutic drugs. Pharmacokinetics is the study of the relationship between the dose of a drug and its concentrations in the body fluids over time. This relationship is controlled mainly by the rate and extent of drug absorption, distribution, metabolism, and excretion processes, known as ADME. The critical pharmacokinetic parameters used to define these processes include bioavailability (F), elimination half-life (t1/2), volume of distribution (Vd or Vss), and clearance (CL) (Figure 1). Despite the impressive benefits of several herbal medicines, they exhibit some challenges such as poor pharmacokinetic profiles and the requirement of high doses which are commonly associated with toxicity.1 The goal of improving the pharmacokinetic profile of a drug is to obtain the desired therapeutic outcome with minimum toxicity.
 |
Figure 1 Schematic diagram showing the four pharmacokinetic processes: absorption, distribution, metabolism and excretion (ADME) including their pharmacokinetic parameters. |
Recently, the use of novel drug delivery systems such as nanoparticles has paved the way to the development of enhancing the pharmacokinetics of anticancer drugs.5–9 Herb-derived active compounds or conventional chemical synthetic drugs incorporated with nanoparticles offer a solution to overcome their unsuitable pharmacokinetic properties, specificity, efficacy, and toxicity. Encapsulation of these compounds/drugs with nanoparticles would likely impact the pharmacokinetics and stability of the carried compounds. To our knowledge, the impact of nanoparticles on pharmacokinetic properties of the herb-derived active compounds or conventional chemical synthetic drugs has not yet been reviewed thoroughly. This systematic review aims to examine pharmacokinetic studies of nanoparticles loaded with herb-derived compounds and conventional chemotherapeutic drugs for cancer therapy.
Materials and methods
Study selection and inclusion and exclusion criteria
The systematic review was performed until February 2019 using PubMed and Science Direct databases. The search terms used were: “Pharmacokinetics”, AND/OR “Pharmacokinetic study”, AND “Nanoparticles”, AND “Anticancer”, AND/OR “Traditional medicine”, AND/OR “Herbal medicine” AND/OR “Herb-derived compounds”, AND/OR “Natural products”, AND “Chemotherapy”. The articles published from various journals were retrieved and saved in EndNote X8 for further analysis. The inclusion criteria were 1) full-text articles published in English, 2) In vitro or in vivo or clinical studies with application of nanoparticles of herb-derived compounds or conventional drugs for cancer chemotherapy, and 3) articles with in vivo/clinical pharmacokinetic studies reporting at least area under plasma concentration–time curve (AUC). All duplicates, review articles, articles with unclear methodology, or articles related to the application of nanoparticles in diagnostic/imaging, immunotherapy, radiotherapy, photothermal therapy, lipoprotein nanoparticles, gene therapy, hydrogel nanoparticles, conjugated ligands, and targeted therapy, as well as combined anticancer drugs were excluded from the analysis.
Data extraction and collection
The following study characteristics were extracted from each article that fulfilled the inclusion criteria and had none of the exclusion criteria: nanoparticles, drug-loaded, the analytical method applied, type of study, animal or human used, dose and route of loaded drugs given, and pharmacokinetic parameters (at least AUC). Final eligibility check of the full-text articles was performed; only articles that were relevant to the review question and keywords were obtained and processed for final analysis.
Results and discussion
A total of 403 relevant research articles published up to February 2019 were retrieved from PubMed and Science Direct databases. All articles were imported and merged in EndNote reference management software. One hundred sixty-nine duplicate articles and 39 review articles were excluded. Of the remaining 195 articles, 167 articles were available as full-texts for eligibility screening. Finally, 50 articles fulfilling the inclusion criteria were included in the study (Figure 2). The pharmacokinetic studies of the nanoparticles of herb-derived compounds and conventional chemotherapeutic drugs for cancer are summarized in Tables 1 and 2.
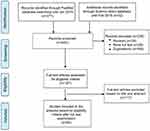 |
Figure 2 Flowchart summarizing inclusion and exclusion of the articles for the study. |
 |
Table 1 (Continued). |
 |
Table 1 (Continued). |
 |
Table 1 (Continued). |
 |
Table 1 (Continued). |
 |
Table 1 (Continued). |
 |
Table 1 (Continued). |
 |
Table 2 (Continued). |
 |
Table 2 (Continued). |
Pharmacokinetic studies for nanoparticle-loaded chemotherapeutic drugs
Taxanes
Paclitaxel (PTX)1,4 and docetaxel (DTX)2,3,5,6 are semisynthetic anticancer drugs derived from the plants of the genus Taxus (yews). The anticancer activity of taxanes involves disruption of the mitotic spindle by binding to microtubules and thereby inhibiting the depolymerization of the microtubules, leading to mitotic arrest at the G2/M phase of the cell cycle.1–4,6,7 PTX and DTX display anticancer activity and have been used in the treatment of various cancers especially breast,2–4,6–11 ovarian,1–4,6–8,11 lung,1–4,6,7,9,11 head and neck,1,2,6,11 colon,1,4 bladder,1,2 prostate,2,7,11 and gastric2,3 cancers, esophageal, endometrium carcinoma,2 hepatocarcinoma,12 acute leukemia,1 and Kaposi’s sarcoma.4 The clinical application of currently available taxanes meets some challenges including poor water solubility, high protein binding, first-pass metabolism, high affinity to P-glycoprotein (P-gp), and some serious side effects. The toxicity such as hypersensitivity, neuropathy, neurotoxicity, and cardiotoxicity is caused by the formulation excipients, ie, Tween 80, cremophor EL, and ethanol to increase its solubility. These excipients not only contribute to toxicity but also alter the pharmacokinetics of both drugs. One of the strategies proposed to address the problem of toxicity and poor pharmacokinetic properties with these drugs is the use of a novel drug delivery system such as nanoparticles. A wide range of biocompatible, biodegradable, and nontoxic polymeric nanoparticles were employed to improve the pharmacokinetic profiles of DTX and PTX to increase the plasma drug concentrations and solubility, and intracellular accumulation to tumors cells via enhanced permeability and retention (EPR) effect.1–4,6–9,11,12
DTX encapsulated with pegylated carboxymethylcellulose (PEG-CMS) was evaluated against tumor-bearing Bagg Albino (BALB/c) mice at 40 mg/kg body weight injected via the tail vein. This nanoparticle provided improved pharmacokinetics of DTX (38-fold increase in AUC, 5.2-fold prolongation of t1/2, 2.5% decrease in CL, and 13.2% decrease in Vd) compared with the free drug.9 This resulted in the increase of drug uptake to tumor cells in EMT-6 tumor-bearing mice with reduced toxicity. Similar findings were observed with poly (2-methyl, 2-carboxytrimethylene carbonate-co-D, L-lactide)-g-poly(ethylene glycol) (TMCCco-LA)-g-PEG nanoparticle delivering DTX, and free DTX. Improved drug plasma concentration in tumor-bearing mice was found with the increase of AUC (2-fold), prolongation of t1/2 (1.6-fold), and decrease of both Vd (2-fold) and CL (3-fold). These changes likely contributed to the favorable pharmacokinetic profile and tumor accumulation of DTX.10 Likewise, poly(lactide-co-glycolide)-monomethoxy-poly-(polyethylene glycol) (PLGA-mPEG)-loaded DTX was shown to provide sustained release of DTX and increase the accumulation of the drug in tumor cells of mice and thus, enhancement of cytotoxic activity.13 The change in the pharmacokinetics of DTX with increase of AUC (2.7-fold), prolongation of t1/2 (3.76-fold), and decrease of CL (2.7-fold) was brought by the addition of PEG to the PLGA nanoparticle which contributed to increased blood circulation, decreased drug-protein binding, and reduced elimination of loaded drug by reticuloendothelial system (RES) organs such as liver and spleen.
Formulations of DTX using PLA-TPGS, PS-PDLLA, and PALA polymeric nanoparticles were found to improve the pharmacokinetics of DTX in Sprague–Dawley (SD) rats.5,7,12 The AUC was increased (1.5-2.31-fold), and the t1/2 was prolonged (1.53-13.2-fold) with a significant reduction of drug CL. The improved bioavailability of PLA-TPGS nanoparticle-loaded DTX due to an inhibitory effect on P-gp resulted in enhancing cellular uptake of the drug to cancer cells and overcoming multidrug resistance. The decrease in clearance of the loaded drug was due to sustained release and stability of the drug in the serum. Another study evaluated the pharmacokinetics of PEGylated PLGA nanoparticle-loaded DTX in tumor-bearing mice. The pharmacokinetics of DTX was found to be improved with an increase in AUC (5.4-fold), prolongation of t1/2 (3.7-fold), and decrease of CL (5-fold) and Vd (1.3-fold).2
Lipid-based nanocarriers such as self-nano emulsifying lipidic nanomicellar systems (SNELS) were also used to deliver DTX. Both long-chain and medium-chain glycerides were applied to enhance oral drug bioavailability.11 The nanocarriers of long-chain glycerides enriched with SNELS delivering DTX were shown to markedly increase the AUC and Cmax of DTX compared with the medium-chain glyceride SNELS. The superiority of the long-chain carrier was mainly due to inhibition of P-gp efflux, together with the increase of intestinal lymphatic transport of drug with reduction of the first-pass metabolism. Finally, poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV) delivering DTX was shown to improve the pharmacokinetics of DTX resulting in the increase of AUC (1.6-fold), the prolongation of t1/2 (8.2-fold), the expansion of Vd (2.3-fold), and the reduction of CL (2.3-fold).3 Similarly, DTX incorporated into thiolated chitosan provided higher oral drug bioavailability, sustained release, and longer t1/2 compared to the unloaded drug.6 The 10.6-fold increase of mean AUC of DTX when loaded with thiolated chitosan could be related to its muco-adhesion properties on the gastrointestinal tract.
In contrast to DTX, improvement of drug bioavailability was not demonstrated with PTX when encapsulated with PEG with different molecular weights (2000, 6000, and 10,000)4 and PLGA-TPGS,1 but t1/2 was mostly prolonged. PEGylation of nanoparticles with either PEG6000 or PEG2000 as carriers was more adhesive in the gastrointestinal mucosa than PEGy1000. Both were located at the surface of the absorptive membrane for an extended period, and the loaded drug was slowly released. When PTX was loaded with PCL-TPGS on the other hand, the AUC was increased by 2.7-fold, the t1/2 was significantly increased, while the CL was significantly decreased (3-fold).8
Anthracycline antibiotics
The anthracycline antibiotics doxorubicin (DOX) and daunorubicin (DNR) are broadly used for the treatment of a variety of cancers. They exert their action on cancer cells by intercalation into DNA base pairs resulting in blockage of DNA and RNA synthesis and DNA scission, which suppresses the replication of DNA and induces cell apoptosis.14 The pharmacokinetic study in male SD rats showed that entrapment of DOX in polymersomes resulted in the prolongation of drug t1/2 by 4.54-fold, and the increase in AUC by 5.97-fold compared with free drug. These pharmacokinetic changes significantly enhanced cytotoxicity and reduced resistance of MCF-7/ADR cells to DOX.14 An improved pharmacokinetic profile of DOX and its cytotoxicity were also reported when loaded in chitosan-g-TPGS (CT). This makes CT a suitable carrier in drug-resistant cancer cells.15 In another in vivo study using DOX loaded in PLGA administered to SD, the formulation provided relatively well-sustained plasma concentration, enhanced AUC, and significantly delayed Tmax (36 hrs) when compared to free DTX.16 Furthermore, improved pharmacokinetics and antitumor activity of the mannosylated solid lipid nanoparticles delivering DOX were demonstrated in Balb/c mice compared with free drug. The approximately 9.31-fold increase of t1/2 and the 5-fold increase of AUC suggested that mannosylated solid lipid nanoparticles could selectively deliver DOX to tumor cells.17
A nanodisk formulation of DOX was reported to provide larger AUC, longer t1/2, and higher cytotoxicity in MCF-7/ADR cells compared with free drug.18 Additionally, DOX encapsulated into the chondroitin sulfate A-deoxycholic acid-polyethylene glycol (CSD-PEG) also showed similar improvement in pharmacokinetics and stability of DOX in blood.19 Cholesterol-modified glycol chitosan (CHGC) delivering DOX improved F of about 6.61 times higher than the free drug. The improved pharmacokinetics could be due to the slow release of the drug from the nanoparticle, which contributed to higher uptake by the cancer cells.20 Dextran-conjugated with polypropylene imine (PAD-PPI) delivering DOX was reported to increase AUC and decrease CL by 3.2-fold and 3.12-fold, respectively, compared with free drug. This nanoparticle resulted in the improved cytotoxic effect of DOX to the tumor cells.21
For DNR, the increase in Cmax (1.86-fold), AUC (11.29-fold), Tmax (2-fold), and prolongation of t½ (2.8-fold) compared with free MTX was reported in Wistar rats after oral administration of the drug incorporated with chitosan-poly(lactic-co-glycolic acid) (CS-PLGA) nanoparticle.22 This was due to the potential of the nanoparticle to avoid P-gp-mediated-drug efflux and hepatic first-pass metabolism by cytochrome P450 (CYP450) enzymes. On the other hand, improvement of the pharmacokinetics of DNR conjugated into lecithmer was not well demonstrated in Wistar rats.23 Drug-loaded nanoparticle exhibited an increase in Vd (1.42-fold) and a decrease in AUC (1.3-fold) compared with free drug. The larger Vd of DNR-loaded lecithmer was associated with rapid tissue distribution and uptake of the drug by the RES organs.
Other anticancer drugs
5-Fluorouracil (5-FU), a pyrimidine analog for various types of solid tumors, is a prodrug which covalently binds to thymidylate synthase and interferes with thymidylate synthesis, resulting in inhibition of synthesis of both DNA and RNA. In rabbits, a dose of 30 mg/kg of 5-FU (30 mg/kg body weight) loaded into poly-ethylene glycol and poly (γ-benzyl-L-glutamate) (PEG-PBLG) was reported to result in lower F with slightly favorable t1/2 compared with the non-conjugated drug. The PEG-PBLG nanoparticle significantly improved antitumor activity against human colon and oral cancer cells.24
The bioavailability of the platinum-based oxaliplatin (OLP) containing Fat Employing Supercritical Nano System (FESNS) as nanoparticle was shown to be improved compared with free OLP formulation.25 Furthermore, improvement in the pharmacokinetic profile of methotrexate (MTX) was reported when loaded in glycine-PLGA nanoparticle with an increase in AUC (4-fold), the prolongation of t1/2 (1.74-fold), and the reduction of Vd (1-fold).26 The nanoparticle substantially increased the cytotoxicity of MTX against MDA-MB-231 cancer cells. Different nanoparticles were shown to improve pharmacokinetics, particularly oral bioavailability of mifepristone, gemcitabine, anastrozole, and estrone. Improved bioavailability and cytotoxicity against cancer cells of mifepristone were shown when encapsulated with chitosan.27 Improved bioavailability, reduced CL, and prolonged t1/2 were reported with PLGA nanoparticle-loaded gemcitabine, resulting in enhanced cytotoxicity against MiaPaCa-2 and MCF-7 cells.28 The pharmacokinetic profiles and cytotoxic activity of anastrozole, the aromatase inhibitor used in postmenopausal breast cancer, were shown to be significantly improved when encapsulated in PLGA, PLA, and PCL polymers.29 Estrone was formulated with solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and liposomes. Mean AUC values of the drug were greatly increased with SLN (17,728 μg/mL*h2) and NLC (16,047 μg/mL*h2), but slightly decreased with liposomes (8991 μg/mL*h2) compared with free estrone (12,357 μg/mL*h2). Both CL and Vd were decreased. SLN emerges as a promising carrier of estrone to cancer cells.30 Noscapine, a derivative of alkaloid opium with anti-tubulin action, is currently under investigation for cancer. Poly(ethylene glycol)-co-poly(ε-caprolactone) (PCL-PEG) nanoparticle delivering noscapine was evaluated in Wistar rats, and drug bioavailability and t1/2 were shown to be increased along with the improvement of cytotoxicity against breast cancer cells.31 The pharmacokinetic profile of polymeric nanoparticle containing polyethylene glycol-poly-l-lactic acid (mPEG–PLA) delivering sirolimus, an mTOR inhibitor, was significantly improved with the increase of AUC (3-fold) and Cmax (3.91-fold) compared with free sirolimus.32 The nanoparticle improved the cytotoxic activity of sirolimus against human cancer cell lines. In another study of polyamidoamine-chitosan (PCT) carrying temozolomide, promising pharmacokinetic profiles were reported with improved AUC (1.2-fold) and prolonged t1/2 (1.5-fold) compared with free drug.33
Pharmacokinetic studies for nanoparticles loaded herb-derived compounds in traditional or herbal medicines
Various nanoparticles were developed for the selective delivery of active compounds from traditional or herbal medicines to cancer cells. Curcumin, a molecule found in various spices, notably turmeric, has been used for the treatment of various types of cancer particularly breast, stomach, intestine, colorectal, prostate, ovarian, and melanoma.34,35 Curcumin exerts its anticancer activity by regulating signaling pathways such as Akt/mTOR, NF-κB, and HIF-1α which are important for cell proliferation and apoptosis.34,36 Compared with free drug, the AUC and t1/2 of curcumin-loaded in phospholipid complex in self-nano-emulsifying drug delivery system (SNEDDS) were increased by 52-fold and 5-fold, respectively. The Vd and CL were decreased by 38.8-fold and 50-fold, respectively. The improved oral bioavailability of curcumin resulted in improved luminal solubility. The higher release rate in the intestinal fluids improved absorption by enhancing compound permeability and avoiding hepatic first-pass metabolism.34 The significant improvement in the oral bioavailability of curcumin was reported when encapsulated into cationic polymer EE100 with increased cytotoxic activity against colorectal cancer.35
The mean AUC values of chitosan-loaded curcumin, chitosan-loaded rutin, free curcumin, and free rutin were 4322, 7621, 1219, and 1146 ng/mL, respectively. Chitosan nanoparticle loading resulted in the improvement of bioavailability of curcumin and rutin by 3.5-fold and 6.65-fold, respectively, compared with free compounds.37 Similarly, another study in rats showed that lipid nanocapsules (LNC) efficiently delivered curcumin into tumor cells and improved its pharmacokinetics.36 The AUC and Cmax were increased by 3.9-fold and 5.3-fold, respectively, while the CL was decreased by 3-fold. These results suggested that LNC could be used for the delivery of traditional medicines to cancer cells. The methoxy poly(ethylene glycol)-b-poly(ε-caprolactone) (mPEG-PCL) micelles could extend the plasma concentration and delay the clearance of curcumin.38 The bioavailability and t1/2 of curcumin were greatly improved when loaded with Poly (D, L-lactic acid)-glycerol (PDLLA-G) when compared with free curcumin.39
Camptothecins constitute a class of anticancer alkaloid traditionally from Chinese medicine that possesses potential anticancer activity against colon, breast, colorectal, stomach, liver, leukemia, and ovarian cancers. Their mechanism of action involves inhibition of tumor-specific topoisomerase I, a critical enzyme responsible for cutting and re-ligating single DNA strands, which results in DNA damage.40–43 The pharmacokinetic profile and anticancer activity of nanocrystals and PEG-PBLG loaded into hydroxycamptothecin (HCPT) were evaluated in rats and rabbits.40,41 Nanocrystals improved the pharmacokinetics of HCPT with an increase of AUC (2.98-fold), the prolongation of t1/2 (2.81-fold), and the decrease of Vd (45.5-fold) compared with free HCPT. The nanocrystals emerged as an effective drug delivery system for HCPT.40 On the other hand, another study in rabbits reported a decrease in Cmax and bioavailability of HCPT when loaded in PEG-PBLG compared with free drug. The nanoparticle provided sustained release and prolongation of t1/2.41 Topotecan, a topoisomerase inhibitor which is a synthetic analog of camptothecin, was shown to enhance intracellular uptake and result in a 13-fold increase in bioavailability of topotecan-loaded PLGA compared with free topotecan.42 The increased bioavailability was associated with enhanced cytotoxic activity against SKOV3 cancer cells. Similarly, the bioavailability of the solid lipid nanoparticles (SLN) delivering camptothecin (CPT) was shown to be improved by 2.38-fold.43
Amoitone B is a cytosporone B (Csn-B) analog obtained from Dothiorella sp. HTF3. It exhibits anticancer activity by tightly binding to a nuclear orphan receptor (Nur77) and induces mitochondrial apoptosis. When Amoitone B was loaded into nanocrystals (NC-Am), the bioavailability was increased by 1.4-fold and the t1/2 was prolonged by 2.8-fold. The nanocrystals improved solubility and reduced toxicity of Amoitone B.44 Further, cubic nanoparticles improved oral bioavailability and anticancer activity of protopanaxadiol (PPD).45 PPD which contains ginsenosides derived from the Araliaceae family was proved to exhibit potential anticancer activity. In another study, biochanin A (BCA), the compound from a phytoestrogenic plant of red clover, was found to exhibit estrogenic activity and cytotoxic activity against human breast cancer cells. The bioavailability of PEG-NLC-loaded BCA was increased by about 2.9-fold after oral administration to rats compared with BCA suspension. This subsequently improved the cytotoxic activity of BCA against MCF-7 cells.46 The bioavailability of celastrol was increased by 4.11-fold, and the t1/2 was prolonged by 10-fold when loaded with phytosomes compared with celastrol suspension.47 This suggested that the phospholipid component of phytosomes enhanced the fluidity of cell membrane, solubility, and intestinal absorption.
Cationic nanoemulsions (CN) containing chitosan delivering Brucea javanicaoil (BJO) resulted in higher cytotoxicity and antitumor activity in a xenografted mouse model (A549 cells). The AUC of BJO-CN was increased by 1.6-fold, and the t1/2 was prolonged by 1.3-fold compared with BJO emulsion. CN has been demonstrated as a promising drug delivery system and has the potential to reduce the required frequency of BJO dosing.48 Genistein (Gen), an active compound found in soybeans, exerted its anticancer activity by inducing apoptosis through inhibiting tyrosine kinases and NF-κB. Micellar emulsions containing methoxy poly (ethylene glycol)-block-(ε-caprolactone) and medium-chain triglycerides (mPEG -PCL/MCTs) as nanocarriers delivering Gen prolonged t1/2 (2.86-fold) reduced CL (10.13-fold) and improved AUC (4.6-fold).49 Similarly, α-tocopherol succinate-loaded in nano-emulsion increased AUC (2.17-fold) and prolonged t1/2 (2.17-fold) compared with free α –TOS.50 The nano-emulsion proved to be a promising drug delivery system for cancer cells.
Conclusion
It is evident that the pharmacokinetic profiles of conventional chemotherapeutic drugs as well as traditional/herbal medicines used for cancer treatment are significantly improved when loaded with nanoparticles. The main benefits of using nanoparticles for drug delivery are enhancement of vascular and gastrointestinal permeability and selectivity of drugs/compound to tumor cells. The improved permeability and selectivity resulted in the improvement of cellular drug uptake, the inhibition of drug hepatic first-pass metabolism and P-gp efflux, the increase in drug solubility and stability, and the decrease in the rate of drug elimination by the RES organs. Subsequent reduction of dose frequency further contributes to the improvement of patient compliance and minimizes toxicity. It is noted however that the physicochemical properties of the chemotherapeutic drugs or herb-derived compounds play a crucial rule in designing effective and appropriate nanocarriers. The use of nanoparticles as a novel drug delivery system for cancer therapy has the potential to dramatically improve both pharmacokinetics and cytotoxicity activity of the loaded drugs/herb-derived compounds for cancer therapy.
Abbreviations list
MCF-7/ADR, Michigan Cancer Foundation-7-Adriamycin resistant; MDA-MB-231, M.D. Anderson-Metastasis Breast cancer; Akt/mTOR, protein kinase B/mammalian target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; HIF-1α, Hypoxia-inducible factor 1-alpha.
Acknowledgments
The authors would like to thank the Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, Chulabhorn International College of Medicine, and the Drug Discovery Center of Thammasat University (Rangsit Campus), for providing the necessary support for conducting this systematic review. Omar Abdifetah receives financial support for his M.Sc. degree program in Bioclinical Sciences from Chulabhorn International College of Medicine and Thammasat University. Kesara Na-Bangchang receives financial support for research and management from Thammasat University Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Feng -S-S, Zhao L, Zhang Z, et al. Chemotherapeutic engineering: vitamin E TPGS-emulsified nanoparticles of biodegradable polymers realized sustainable paclitaxel chemotherapy for 168h in vivo. Chem Eng Sci. 2007;62(23):6641–6648. doi:10.1016/j.ces.2007.08.006
2. Rafiei P, Haddadi A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile. Int J Nanomedicine. 2017;12:935–947. doi:10.2147/IJN.S121881
3. Vardhan H, Mittal P, Adena SKR, Upadhyay M, Mishra B. Development of long-circulating docetaxel loaded poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanoparticles: optimization, pharmacokinetic, cytotoxicity and in vivo assessments. Int J Biol Macromol. 2017;103:791–801. doi:10.1016/j.ijbiomac.2017.05.125
4. Zabaleta V, Ponchel G, Salman H, Agueros M, Vauthier C, Irache JM. Oral administration of paclitaxel with pegylated poly(anhydride) nanoparticles: permeability and pharmacokinetic study. Eur J Pharm Biopharm. 2012;81(3):514–523. doi:10.1016/j.ejpb.2012.04.001
5. Qiao H, Li J, Wang Y, Ping Q, Wang G, Gu X. Synthesis and characterization of multi-functional linear-dendritic block copolymer for intracellular delivery of antitumor drugs. Int J Pharm. 2013;452(1–2):363–373. doi:10.1016/j.ijpharm.2013.05.003
6. Saremi S, Dinarvand R, Kebriaeezadeh A, Ostad SN, Atyabi F. Enhanced oral delivery of docetaxel using thiolated chitosan nanoparticles: preparation, in vitro and in vivo studies. Biomed Res Int. 2013;2013:150478. doi:10.1155/2013/150478
7. Lee JY, Kim JS, Cho HJ, Kim DD. Poly(styrene)-b-poly(DL-lactide) copolymer-based nanoparticles for anticancer drug delivery. Int J Nanomedicine. 2014;9:2803–2813. doi:10.2147/IJN.S62806
8. Bernabeu E, Helguera G, Legaspi MJ, et al. Paclitaxel-loaded PCL-TPGS nanoparticles: in vitro and in vivo performance compared with Abraxane(R). Colloids Surf B Biointerfaces. 2014;113:43–50. doi:10.1016/j.colsurfb.2013.07.036
9. Ernsting MJ, Tang WL, MacCallum NW, Li SD. Preclinical pharmacokinetic, biodistribution, and anti-cancer efficacy studies of a docetaxel-carboxymethylcellulose nanoparticle in mouse models. Biomaterials. 2012;33(5):1445–1454. doi:10.1016/j.biomaterials.2011.10.061
10. Ho KS, Aman AM, Al-awar RS, Shoichet MS. Amphiphilic micelles of poly(2-methyl-2-carboxytrimethylene carbonate-co-D,L-lactide)-graft-poly(ethylene glycol) for anti-cancer drug delivery to solid tumours. Biomaterials. 2012;33(7):2223–2229. doi:10.1016/j.biomaterials.2011.11.072
11. Khurana RK, Beg S, Burrow AJ, et al. Enhancing biopharmaceutical performance of an anticancer drug by long chain PUFA based self-nanoemulsifying lipidic nanomicellar systems. Eur J Pharm Biopharm. 2017;121:42–60. doi:10.1016/j.ejpb.2017.09.001
12. Yu Y, Tan S, Zhao S, et al. Antitumor activity of docetaxel-loaded polymeric nanoparticles fabricated by Shirasu porous glass membrane-emulsification technique. Int J Nanomedicine. 2013;8:2641–2652. doi:10.2147/IJN.S48214
13. Senthilkumar M, Mishra P, Jain NK. Long circulating PEGylated poly(D,L-lactide-co-glycolide) nanoparticulate delivery of Docetaxel to solid tumors. J Drug Target. 2008;16(5):424–435. doi:10.1080/10611860802088598
14. Chao Y, Liang Y, Fang G, et al. Biodegradable polymersomes as nanocarriers for doxorubicin hydrochloride: enhanced cytotoxicity in MCF-7/ADR cells and prolonged blood circulation. Pharm Res. 2017;34(3):610–618. doi:10.1007/s11095-016-2088-9
15. Guo Y, Chu M, Tan S, et al. Chitosan-g-TPGS nanoparticles for anticancer drug delivery and overcoming multidrug resistance. Mol Pharm. 2014;11(1):59–70. doi:10.1021/mp400514t
16. Kalaria DR, Sharma G, Beniwal V, Ravi Kumar MN. Design of biodegradable nanoparticles for oral delivery of doxorubicin: in vivo pharmacokinetics and toxicity studies in rats. Pharm Res. 2009;26(3):492–501. doi:10.1007/s11095-008-9763-4
17. Jain A, Agarwal A, Majumder S, et al. Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J Control Release. 2010;148(3):359–367. doi:10.1016/j.jconrel.2010.09.003
18. Zhang W, Sun J, Liu Y, et al. PEG-stabilized bilayer nanodisks as carriers for doxorubicin delivery. Mol Pharm. 2014;11(10):3279–3290. doi:10.1021/mp400566a
19. Lee JY, Park JH, Lee JJ, et al. Polyethylene glycol-conjugated chondroitin sulfate A derivative nanoparticles for tumor-targeted delivery of anticancer drugs. Carbohydr Polym. 2016;151:68–77. doi:10.1016/j.carbpol.2016.05.043
20. Yu JM, Li YJ, Qiu LY, Jin Y. Polymeric nanoparticles of cholesterol-modified glycol chitosan for doxorubicin delivery: preparation and in-vitro and in-vivo characterization. J Pharm Pharmacol. 2009;61(6):713–719. doi:10.1211/jpp.61.06.0003
21. Agarwal A, Gupta U, Asthana A, Jain NK. Dextran conjugated dendritic nanoconstructs as potential vectors for anti-cancer agent. Biomaterials. 2009;30(21):3588–3596. doi:10.1016/j.biomaterials.2009.03.016
22. Ahmad N, Ahmad R, Alam MA, et al. Daunorubicin oral bioavailability enhancement by surface coated natural biodegradable macromolecule chitosan based polymeric nanoparticles. Int J Biol Macromol. 2019;128:825–838. doi:10.1016/j.ijbiomac.2019.01.142
23. Varghese SE, Fariya MK, Rajawat GS, Steiniger F, Fahr A, Nagarsenker MS. Lecithin and PLGA-based self-assembled nanocomposite, Lecithmer: preparation, characterization, and pharmacokinetic/pharmacodynamic evaluation. Drug Deliv Transl Res. 2016;6(4):342–353. doi:10.1007/s13346-016-0314-y
24. Li S, Wang A, Jiang W, Guan Z. Pharmacokinetic characteristics and anticancer effects of 5-fluorouracil loaded nanoparticles. BMC Cancer. 2008;8:103. doi:10.1186/1471-2407-8-172
25. Kim YH, Lee SJ, Lee SH, Hahn M. Preclinical efficacy and safety assessment of nano-oxaliplatin oral formulation prepared by novel Fat Employing Supercritical Nano System, the FESNS(R). Pharm Dev Technol. 2012;17(6):677–686. doi:10.3109/10837450.2011.565349
26. Kumar R, Kumar P, Singh B, et al. In vivo pharmacokinetic studies and intracellular delivery of methotrexate by means of glycine-tethered PLGA-based polymeric micelles. Int J Pharm. 2017;519(1–2):138–144. doi:10.1016/j.ijpharm.2017.01.021
27. Zhang H, Wu F, Li Y, et al. Chitosan-based nanoparticles for improved anticancer efficacy and bioavailability of mifepristone. Beilstein J Nanotechnol. 2016;7:1861–1870. doi:10.3762/bjnano.7.178
28. Khare V, Singh A, Mahajan G, et al. Long-circulatory nanoparticles for gemcitabine delivery: development and investigation of pharmacokinetics and in-vivo anticancer efficacy. Eur J Pharm Sci. 2016;92:183–193. doi:10.1016/j.ejps.2016.07.007
29. Shavi GV, Nayak UY, Maliyakkal N, et al. Nanomedicine of anastrozole for breast cancer: physicochemical evaluation, in vitro cytotoxicity on BT-549 and MCF-7 cell lines and preclinical study on rat model. Life Sci. 2015;141:143–155. doi:10.1016/j.lfs.2015.09.021
30. Andey T, Sudhakar G, Marepally S, Patel A, Banerjee R, Singh M. Lipid nanocarriers of a lipid-conjugated estrogenic derivative inhibit tumor growth and enhance cisplatin activity against triple-negative breast cancer: pharmacokinetic and efficacy evaluation. Mol Pharm. 2015;12(4):1105–1120. doi:10.1021/mp5008629
31. Shalaby KS, Soliman ME, Bonacucina G, et al. Nanoparticles based on linear and star-shaped poly(ethylene glycol)-poly(epsilon-caprolactone) copolymers for the delivery of antitubulin drug. Pharm Res. 2016;33(8):2010–2024. doi:10.1007/s11095-016-1939-8
32. Woo HN, Chung HK, Ju EJ, et al. Preclinical evaluation of injectable sirolimus formulated with polymeric nanoparticle for cancer therapy. Int J Nanomedicine. 2012;7:2197–2208. doi:10.2147/IJN.S29480
33. Sharma AK, Gupta L, Sahu H, et al. Chitosan engineered PAMAM dendrimers as nanoconstructs for the enhanced anti-cancer potential and improved in vivo brain pharmacokinetics of temozolomide. Pharm Res. 2018;35(1):9. doi:10.1007/s11095-017-2324-y
34. Shukla M, Jaiswal S, Sharma A, et al. A combination of complexation and self-nanoemulsifying drug delivery system for enhancing oral bioavailability and anticancer efficacy of curcumin. Drug Dev Ind Pharm. 2017;43(5):847–861. doi:10.1080/03639045.2016.1239732
35. Chaurasia S, Chaubey P, Patel RR, Kumar N, Mishra B. Curcumin-polymeric nanoparticles against colon-26 tumor-bearing mice: cytotoxicity, pharmacokinetic and anticancer efficacy studies. Drug Dev Ind Pharm. 2016;42(5):694–700. doi:10.3109/03639045.2015.1064941
36. Lollo G, Ullio-Gamboa G, Fuentes E, Matha K, Lautram N, Benoit JP. In vitro anti-cancer activity and pharmacokinetic evaluation of curcumin-loaded lipid nanocapsules. Mater Sci Eng C Mater Biol Appl. 2018;91:859–867. doi:10.1016/j.msec.2018.06.014
37. Ramaswamy S, Dwarampudi LP, Kadiyala M, et al. Formulation and characterization of chitosan encapsulated phytoconstituents of curcumin and rutin nanoparticles. Int J Biol Macromol. 2017;104:1807–1812. doi:10.1016/j.ijbiomac.2017.06.112
38. Song Z, Zhu W, Song J, et al. Linear-dendrimer type methoxy-poly (ethylene glycol)-b-poly (epsilon-caprolactone) copolymer micelles for the delivery of curcumin. Drug Deliv. 2015;22(1):58–68. doi:10.3109/10717544.2014.901436
39. Yoon IS, Park JH, Kang HJ, et al. Poly(D,L-lactic acid)-glycerol-based nanoparticles for curcumin delivery. Int J Pharm. 2015;488(1–2):70–77. doi:10.1016/j.ijpharm.2015.04.046
40. Yang X, Liu Y, Zhao Y, et al. A stabilizer-free and organic solvent-free method to prepare 10-hydroxycamptothecin nanocrystals: in vitro and in vivo evaluation. Int J Nanomedicine. 2016;11:2979–2994. doi:10.2147/IJN.S102726
41. Wang A, Li S. Hydroxycamptothecin-loaded nanoparticles enhance target drug delivery and anticancer effect. BMC Biotechnol. 2008;8:46. doi:10.1186/1472-6750-8-46
42. Padhi S, Kapoor R, Verma D, Panda AK, Iqbal Z. Formulation and optimization of topotecan nanoparticles: in vitro characterization, cytotoxicity, cellular uptake and pharmacokinetic outcomes. J Photochem Photobiol B. 2018;183:222–232. doi:10.1016/j.jphotobiol.2018.04.022
43. Du Y, Ling L, Ismail M, et al. Redox sensitive lipid-camptothecin conjugate encapsulated solid lipid nanoparticles for oral delivery. Int J Pharm. 2018;549(1–2):352–362. doi:10.1016/j.ijpharm.2018.08.010
44. Hao L, Wang X, Zhang D, et al. Studies on the preparation, characterization and pharmacokinetics of Amoitone B nanocrystals. Int J Pharm. 2012;433(1–2):157–164. doi:10.1016/j.ijpharm.2012.05.002
45. Jin X, Zhang ZH, Sun E, et al. Enhanced oral absorption of 20(S)-protopanaxadiol by self-assembled liquid crystalline nanoparticles containing piperine: in vitro and in vivo studies. Int J Nanomedicine. 2013;8:641–652. doi:10.2147/IJN.S38203
46. Wang L, Luo Q, Lin T, et al. PEGylated nanostructured lipid carriers (PEG-NLC) as a novel drug delivery system for biochanin A. Drug Dev Ind Pharm. 2015;41(7):1204–1212. doi:10.3109/03639045.2014.938082
47. Freag MS, Saleh WM, Abdallah OY. Self-assembled phospholipid-based phytosomal nanocarriers as promising platforms for improving oral bioavailability of the anticancer celastrol. Int J Pharm. 2018;535(1–2):18–26. doi:10.1016/j.ijpharm.2017.10.053
48. Liu TT, Mu LQ, Dai W, Wang CB, Liu XY, Xiang DX. Preparation, characterization, and evaluation of antitumor effect of Brucea javanica oil cationic nanoemulsions. Int J Nanomedicine. 2016;11:2515–2529. doi:10.2147/IJN.S101918
49. Zhang T, Wang H, Ye Y, Zhang X, Wu B. Micellar emulsions composed of mPEG-PCL/MCT as novel nanocarriers for systemic delivery of genistein: a comparative study with micelles. Int J Nanomedicine. 2015;10:6175–6184. doi:10.2147/IJN.S91348
50. Gao Y, Qi X, Zheng Y, et al. Nanoemulsion enhances alpha-tocopherol succinate bioavailability in rats. Int J Pharm. 2016;515(1–2):506–514. doi:10.1016/j.ijpharm.2016.10.026
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


