Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 12
Personalized treatment options for thyroid cancer: current perspectives
Authors Khatami F , Larijani B, Nikfar S, Hasanzad M , Fendereski K , Tavangar SM
Received 3 March 2019
Accepted for publication 14 June 2019
Published 13 September 2019 Volume 2019:12 Pages 235—245
DOI https://doi.org/10.2147/PGPM.S181520
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Fatemeh Khatami,1 Bagher Larijani,2,3 Shekoufeh Nikfar,3,4 Mandana Hasanzad,3,5 Kiarad Fendereski,6 Seyed Mohammad Tavangar1,7
1Chronic Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; 2Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; 3Personalized Medicine Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; 4Department of Pharmacoeconomics and Pharmaceutical Administration, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran; 5Medical Genomics Research Center, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran; 6Pediateric Urology and Regenerative Medicine Research Center, Children’s Medical Center, Tehran University of Medical Sciences, Tehran, Iran; 7Department of Pathology, Dr. Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
Correspondence: Seyed Mohammad Tavangar
Department of Pathology, Dr. Shariati Hospital, Tehran University of Medical Sciences, Jalale Ale Ahmad Ave, Tehran, Iran
Tel +98 218 490 2187
Fax +98 218 863 3078
Email [email protected]
Abstract: Thyroid cancer is one of the most common endocrine malignancies, with increasing incidence all over the world. In spite of good prognosis for differentiated thyroid carcinoma, for an unknown reason, about 5–10% of the patients, the cancer will show aggressive behavior, develop metastasis, and be refractory to treatment strategies like radioactive iodine. Regarding the genetic information, each thyroid cancer patient can be considered as an individual unique one, with unique genetic information. Contrary to standard chemotherapy drugs, target therapy components aim at one or more definite molecular pathway on cancer cells, so their selection is underlying patient’s genetic information. Nowadays, several mutations and rearrangements including BRAF, VEGF receptors, RET, and RET/PTC, KDR, KIT, PDGFRA, CD274, and JAK2 are taken into account for the therapeutic components like larotrectinib (TRK inhibitor), vemurafenib, sunitinib, sorafenib, selumetinib, and axitinib. With the new concept of personalized treatment of thyroid cancer diagnoses, planning treatment, finding out how well treatment will work, and estimating a prognosis has changed for the better over the last decade.
Keywords: personalized medicine, target therapy, molecular testing
Introduction
The exact word of “personalized medicine” is defined for dividing people based on their genetic information in order to allocate different subgroups of treatment and predicted response efficacy, disease behavior or risk of the disease.1 Several terms are used for personalized medicine like personalized medicine, precision medicine, stratified medicine, and P4 medicine which are used for particular distinctions but same in principal.2 Personalized medicine emphasizes that clinicians are not facing the disease with an exact definition for all persons but are facing with individual patients with unique genetic information.
Personalized cancer medicine is a subgroup of personalized medicine in which a person’s genetic information support finding more effective strategies for prevention, screening, and treatment.3 The last decade of twentieth century is highlighted as the genomic age because high-throughput molecular technologies like next-generation sequencing (NGS), and gene expression microarrays provide a wide range of cancer genetic information.4 In fact in personalized cancer management approach, several attempts are done to identify the link between patients’ molecular characteristics and their survival or drug response. Genetic testing of cancer cells and normal cells aids clinicians make the treatment strategy more compatible with individual patient needs that may cause less side effects than standard options. Cancer personalized medicine is including of: (a) prediction of the chances to developing cancer and selecting screening strategies to lower the risk, (b) matching people with more effective treatments with less side effects, (c) predicting the risk of recurrence. The development and improvement of chemotherapy have revolutionized the cancer treatment, but there are still some patients fail to respond to the treatment. The secret of several efficacy for the same medication strategy is beneath to the genetic and epigenetic and is needed to be determined for a personalized cancer medicine approach for the “one-treatment-fits-all” mindset.5,6
Thyroid cancer is the most prevalent endocrine malignancy, and papillary thyroid carcinoma (PTC) incidence has increased over the past few decades due to improved diagnosis.7–10 Primary thyroid malignancies are mostly epithelial tumors that initiate from thyroid follicular cells with three main pathological types of carcinomas: PTC, follicular thyroid carcinoma (FTC), and anaplastic thyroid carcinoma (ATC) and poorly differentiated carcinoma (PDC). Another thyroid cancer which originates from thyroid parafollicular (C) cells is medullary thyroid carcinoma (MTC). As a result of well differentiation and indolent tumor growth in PTC and FTC, they are also known as differentiated thyroid cancer (DTC). PTC involves of 85–90%, FTC 5–10%, MTC about 5%, and ATC less than 2% of all thyroid cancer cases and their incidence continues to rise with age.11 Very recently it has been considered that some genetic mutations and polymorphism over DTC should be taken into the account of choosing the high efficacy treatment strategies.12 For example, some of these genetic alterations are BRAF mutation, vascular endothelial growth factor (VEGF) mutation,13 KDR (VEGFR2) mutation,14 KIT/PDGFRA mutation,15 PDGFRA promoter polymorphisms,16 Programmed death-ligand 1 (PD-L1) expression,17 and JAK2.18
Comprehensive data about thyroid cancer-genetics are provided by The Cancer Genome Atlas database which indicated to the mutations in either BRAF (specifically, BRAF-V600E) or RAS as the mitogen-activated protein kinase (MAPK) signaling pathway regulator, and novel driver gene EIF1AX change as the MAPK pathway intermediate.19
Using PM can help physicians to make a better decision for thyroid cancer patients treatment. In the current review, we are focusing on the current benefit of PM in thyroid cancer management and future perspective of PM in thyroid malignancies management.
Molecular characteristics of thyroid cancer
Several genetic changes are suggested as the driver genetic alterations with a central role in the triggering of thyrocytes to become malignant.20 The most important one is a specific mutation in the BRAF gene adenine at nucleotide position 1799 (T1799A) in exon 15th iamine trans conversion to, causing in an amino acid substitution at position 600 in BRAF, from a valine (V) to a glutamic acid (E) which is shown as the BRAFV600E.21 BRAF is the intermediate of the MAPK pathway and transcription factors essential for cell growth, differentiation, proliferation, and survival.22 BRAFV600E mutation happens in about 45% of sporadic PTCs, mostly in the aggressive subtypes, such as the tall-cell PTC.23 Another important common mutation in thyroid cancers is RAS mutation. Ras proteins are proto-oncogenes that are often altered in several human malignancies. They are coding by three genes: HRAS, KRAS, and NRAS which are GTPases responsible for proliferation and cell survival pathways. Point mutation at codons 12, 13, or 61 of RAS are linked to the uncontrolled cell proliferation and tumors formation.24 While RAS is the classic activator of the MAPK and PI3K–AKT pathways, RAS modification can specially stimulate the PI3K–AKT pathway in thyrocytes, as proposed by the link of RAS mutations with AKT phosphorylation in thyroid malignancies.25 In fact, the phosphoinositide 3-kinase–protein kinase B/AKT (PI3K-PKB/AKT) pathway is central signaling pathways of the developmental process. Its uncontrolled activation through numerous receptor tyrosine kinases (RTKs) alterations has resulted in aggressive cell proliferation during tumorigenesis, including thyroid carcinomas.26 The phosphatase and tensin homolog (PTEN) genetic and epigenetic alterations are established as the genetic changes that trigger the PI3K–AKT pathway and are the genetic basis for follicular thyroid cell tumor genesis in Cowden’s syndrome.27,28 Moreover, genetic alterations in exon 9 and 20 of phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene can be responsible for coding the p110α catalytic subunit of PI3K and are reported several times in thyroid malignancies like FTC, PDTC, and ATC.29,30 There are a list of other mutated genes in thyroid tumorigenesis like catenin (cadherin-associated protein), Tumor Protein P53 (TP53), isocitrate dehydrogenase 1 (IDH1), anaplastic lymphoma kinase (ALK), epidermal growth factor receptor (EGFR), Zinc and ring finger 3 (ZNRF3), and NADH dehydrogenase (ubiquinone) 1α subcomplex 13 (NDUFA13).31
In addition to point mutations some oncogenic gene amplifications, copy-number changes, and gene translocations are reported as the key driver genetic alterations in thyroid carcinogenesis. Copy-number changes are observed in genes encoding PI3K–AKT pathway members, including PIK3CA, PIK3CB, 3-phosphoinositide-dependent protein kinase 1 (PDPK1), AKT Serine/Threonine Kinase 1/2, IQ-motif-containing GTPase-activating protein 1 (IQGAP1).32–35 Copy-number gaining of oncogenes contributes to tumor formation because of higher protein expression resulting in constitutive activation of their downstream signaling pathway. These copy-number change can be seen alone or simultaneously with BRAFV600E. For example, IQGAP1 copy-number gain and BRAFV600E mutation are linked to the higher risk of recurrent PTC.34,36 Gene translocation more than gene amplification trigger oncogenic rearrangements in thyroid neoplasm. By way of illustration, there are more than ten types of RET–PTC translocation. The RET proto-oncogene with the chromosomal locus of 10q11.2 is responsible for coding a cell membrane RTK. In follicular cells, RET can be stimulated by fusion to other highly expressed genes and make a chimeric oncogene named RET/PTC (recombination of 3′end of RET and the 5′ portion of a recipient gene). By way of illustration, coiled-coil domain-containing gene 6 (CCDC6) in RET–PTC1 and nuclear receptor co-activator 4 (NCOA4) in RET–PTC3.37 Another prominent thyroid cancer-specific gene translocation which is happening in 60% of FTC and follicular variant of PTC, is the paired box 8 (PAX8)–peroxisome proliferator-activated receptor-γ (PPARG) fusion gene (PAX8–PPARG).38,39
Contrary to genetic changes there are some epigenetic alterations like DNA methylation, histone modification, and microRNA that can change chromatin remolding and gene expression pattern of the thyroid epithelial cells (thyrocytes) with no change of the exact DNA sequence. These epigenetic modifications are important to recognize because can be the novel therapeutic targets for personalized thyroid cancer managements.40 Frequent promoter methylation of Ras Association Domain Family Member 1 (RASSF1) has been predominantly reported in thyroid malignancies.41–43 Some other candidate genes with aberrant promoter methylation have been highlighted in thyroid cancers including PTEN, Solute Carrier Family (SLC), DNA methyltransferases (DNMTs), O6-methylguanine DNA methyltransferase (MGMT), Thyroid-stimulating hormone receptor (TSHR), and E-cadherin.44–48
MicroRNAs are small endogenous noncoding RNAs containing about 22 nucleotides that have role in silencing and post-transcriptional regulation of gene expression profile. Recent studies of thyroid cancer have indicated to several microRNAs deregulation as the key tumorgenesis elements with impact on impact cell differentiation, proliferation, and survival.49 Amongst, miR-146b and miR-222 persisted as distinguishing markers of PTC.49,50 MicroRNA-146b stimulates PI3K/AKT pathway and thyroid cancer progression by targeting PTEN.51
Current molecular test of thyroid nodules
Nowadays discrimination of benign thyroid nodules from neoplastic one is done based Bethesda Histological and Immunohistological (IHC) tests and are essential diagnosis of malignancy between the benign and malignant counterpart’s tissues.52–55 System are for Reporting Thyroid Cytopathology (TBSRTC) and through fine needle aspiration biopsy and immunohistochemistry (IHC) reports.56–60 In spite of the fact that several IHC and cytopathological staining are recommended as the discriminative of benign and malignant markers, still some FNA results are not classified exactly as the benign or malignant resulting in an indeterminate diagnosis which are referred as the gray zone.60–62 Several molecular testings are available for supporting the FNA result that is based on genetic and epigenetic profile of the patients.63 These tests are called AFIRMA GENE EXPRESSION CLASSIFIER (GEC), ThyGenX TEST, and ThyroSeq TEST.
- Afirma gene expression classifier (GEC) (Veracyte Inc, South San Francisco, California) is a microarray-based test for the RNA transcript (gene expression) of 167 genes.64,65 Non-determined samples of FNA or suspicious for Hürthle/follicular neoplasms are candidate of this test. In 2014, Veracyte suggested the Afirma Malignancy Classifiers (AMCs) support Afirma GEC and evaluate assess the risk of malignancy. The AMC tests are done over FNA samples carrying a suspicious diagnosis or a suspicious Afirma GEC result. The AMCs profile is containing BRAFV600E gene more than genes expression of five genes include calcitonin-related polypeptide α (CALCA), carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5), secretogranin III (SCG3), sodium channel voltage-gated type IX α subunit (SCN9A), and synaptotagmin IV (SYT4).63 The negative Afirma GEC result commanded as the significant reduction of thyroid surgical resection rate from 74% to 7.6% on cytological indeterminate nodules.66,67 Afirma XA includes the most common and emerging variants and fusions associated with thyroid carcinoma, such as variations of BRAF, DICER1, EIF1AX, H/K/N RAS, RET, TP53, TG, and ZFHX3 in addition to fusions of ALK,BRAF, NTRK, BAX8, and RET. (https://www.afirma.com/physicians/why-afirma/)
- ThyGenX TEST/ThyraMIR is based on the NGS mutation detection panel of more than 100 genetic changes. ThyGenX TEST is made of point mutations (ALK, BRAF, GNAS, HRAS, KRAS, NRAS, PIK3CA, PTEN, RET, and TERT) and RNA panel and fusion (ALK, BRAF, NTRK, PPARg, RET, THADA).68 Lately, Interspace Diagnostics has represented a new molecular test calling ThyraMIR in which ten different microRNAs (miRNAs) containing miR-29b-1-5p, miR-31-5p, miR-138–1-3p, miR-139-5p, miR-146b-5p, miR-155, miR-204-5p, miR-222-3p, miR-375, and miR-551b-3p are taken into the consideration. Nowadays, it is shown that combination of both ThyGenX and ThyraMIR has the sensitivity and specificity of 89% and 85%, respectively, w dabrafenib eas the NPV and PPV were reported as 94% and 74%, respectively.69,70 (https://www.afirma.com/physicians/why-afirma//thygenext-thyramir.com/combination-testing/)
- ThyroSeq TEST which is based on the high-throughput technique of NGS and fusion platform originally planned to target 12 key cancer genes with 284 mutational hot spots.71 Then in 2014, a new form of the test was presented as ThyroSeq v2 in which more extensive DNA changes (14 genes, including >1,000 mutations) and RNA variations (42 fusions, 16 genes for expression) were included.72 However, more than ThyroSeq, additional formats of NGS-based diagnosis molecular tests for thyroid nodules have been considered including Ion AmpliSeq to assess indeterminate FNA cytology of thyroid nodules.73 ThyroSeq test report is provided in a user-friendly format that states the probability of cancer in the patient’s nodule, suggests potential patient management, and also lists specific genomic alterations that are relevant to the individual patient (https://thyroseq.com/physicians/test-details/test-description).
Treatment strategies for thyroid cancers
Several treatment options are available for thyroid cancer management including surgery as the chief treatment option in almost all thyroid cancer patients, except ATC cases. It can be lobectomy in which a part (one lobe) of the thyroid is removed or thyroidectomy as an operation that involves the surgical removal of all or part of the thyroid gland. However, on the occasion of the entire gland removal, it is named “total thyroidectomy” and on the occasion of almost all thyroid gland removal, it is named “subtotal thyroidectomy”.74 Subsequent treatment plan following thyroidectomy can be radioactive iodine [radioiodine] therapy. Thyroid cancer cases should receive daily thyroid hormone (levothyroxine) pills. Another treatment strategy is Radioactive Iodine (Radioiodine) Therapy in which radioactive iodine (RAI or I-131) is just absorbed by thyrocyte throughout the whole body. Therefore, RAI is delivered into the patient’s body in the form of fluid or tablet and aggregate in thyroid cells.75 The radiation will be able to abolish the malignant thyrocyte, with no harmful effect or any damage to the rest of the body. Following thyroidectomy, patients’ body will not be able to create the essential metabolic regulator hormone, thyroid hormone, so levothyroxine pills should be suggested.
The other treatment strategy for thyroid malignancies is external beam radiation (EBM) therapy in which high-energy emissions (or atoms) are used to remove tumor cells or cease their progress.76,77 Usually, this form of radiation therapy is not suggested in practice for (DTCs) patients’ who are defined as the good responder to RAI iodine. In fact, EBM is commonly practiced as the part of the treatment for MTC and ATC patients. More than that chemotherapy (chemo) is a treatment plan as the anti-cancer drugs that are injected into a vein or muscle, or are taken by mouth. Chemotherapy is a general therapy plan in which chemical components injected into the bloodstream and move throughout the whole body to find and destroy cancer cells. This therapeutic method is rarely beneficial for the most types of thyroid tumors. Therefore, chemotherapy is suggested with EBM therapy for ATC or occasionally is used for further advanced thyroid cancer patients who are no longer responded to other treatment strategies. Recently, scientists have initiated to improve new medications that specially object the critical molecules responsible for tumor formation which is called “target therapy”.78
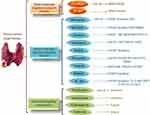
Dissimilar to general chemotherapy drugs, which attack cancer cells as the uncontrolled fast developing cells, target therapy drugs can aim at one or more specific molecular pathway on cancer cells. The list of some target therapy component with their target molecules is represented in Figure 1.
Vandetanib (Caprelsa) is for the treatment of aggressive and symptomatic MTC that is a specific drug in the form of 300 mg tablet is an oval-shaped, biconvex. Cabozantinib (Cometriq) is the medication used for MTC and a second line treatment for renal cell carcinoma. It is a small target therapy molecule that inhibits tyrosine kinases c-Met and VEGFR2 more than AXL and RET.79,80 In MTC patients, cabozantinib has been displayed to support break cancers from growing for about seven months longer than a sugar pill. Lenvatinib (Lenvima®) and sorafenib (Nexavar®) are two target therapy drugs for treating radioiodine-refractory differentiated thyroid cancer as the kinase inhibitors.81,82 They help suppress forming new blood vessels in cancer cells and also aim at stopping essential protein expression needed for cancer cell growth. Dabrafenib (Tafinlar®) and Trametinib (Mekinist®) are combined targeted therapy for ATC and advanced melanoma.83,84 Dabrafenib and Trametinib drugs can be used together to treat ATC patients who carry a positive type of BRAF gene mutation and patients with no complete tumor removal by surgery.85
Personalized thyroid cancer treatment
Personalized medicine is the novel strategy of patient care that conducts clinicians to choose treatments with the best efficacy based on a genetic understanding of their disease. NGS and array-based comparative genomic hybridization (array CGH) have assisted scientists to achieve high-throughput mutation screening and genome-wide copy-number analysis. Recent studies based on genetic analysis of thyroid tumors have brought several thinkable personalized treatment options for thyroid cancer. A large-scale analysis was done by N. Pozdeyev and colleagues to define the genetic landscape of advanced differentiated and anaplastic thyroid cancer (ATC) and recognize genetic mutations of potential diagnostic, prognostic, and therapeutic implication 12. It was an updated schematic of thyroid cancer genetic evolution. An innovative aspect of medicine in which particular targeted therapy centered on specific targeted diagnostic tests called “Theranostics”.86 In fact, theranostics will provide a transition from conventional medicine to a contemporary personalized and precision medicine based on the genetic data. Radioiodine has the distinction of being the first theranostic agent and is a distinctive example of personalized medicine that has been used widely for the management of DTC.87,88 DTC has better thyroid cancer prognosis compared to other malignancies which can be the result of the successful treatment of unresectable distant metastasis by a therapeutic dose of I-131 administration. However, about 65% of the patients with distant metastases finally become radioiodine-refractory disease.89,90 This radioiodine-refractory status is related to the sodium/iodide symporter (NIS) which is also identified as SLC NIS as the molecular target.91 Moreover, losing iodine avidity of DTC can be connected to the genetic and epigenetic alterations and MAPK and PI3K-AKT pathways signaling pathways.92,93 Some compounds like retinoic acid, PPARγ agonists, HDAC inhibitors (valproic acid and carbamazepine), PI3K/AKT inhibitors, and MEK/ERK inhibitors, have been recommended for NIS over-expression and have caused improved iodine uptake in both in- vitro and in-vivo studies of thyroid cancers.94 It was shown that Dabrafenib is the selective inhibitor of mutated forms of BRAF and can motivate radioiodine uptake in metastatic PTC BRAFV600E-mutant iodine-refractory patients.95 Higher efficacy of larotrectinib (LOXO-101) as a selective tropomyosin receptor kinase (TRK) inhibitor is suggested in neurotrophin receptors coding gene (NTRK) Fusion-Positive patients.96,97 Some molecular markers are indicators for the aggressive behavior of tumor associated with tumor dedifferentiation are including p53 (25–30%), PIK3CA (10–20%), CTNNB1(10–20%), and AKT1 (5–10%).98 Mutation in the telomerase reverse transcriptase (TERT) promoter can be a marker of malignancy as well. TERT promoter mutations were reported in 7–22% of PTC and about 35% of FTC, were often found in concurrency with BRAF or RAS mutations.99,100
Distant metastatic to lung or bone disease happens in one-fifth of DTC patients and is the main reason for refractory to RAI.101 Innovative personalized based strategies for thyroid cancer treatment are considered as the tyrosine kinase and small molecule inhibitors targeting iodine reuptake pathways.102,103 Amongst different therapeutic agent doxorubicins, motesanib, sunitinib, and sorafenib are non-specific tyrosine kinase inhibitors (TKIs), Vemurafenib is the inhibitor of BRAF V600E mutation and selumetinib is the selective MEK inhibitor and iodine reuptake inducer.104 TKIs non-specifically aim at pro-oncogenic kinases including VEGFR-1, VEGFR-2, EGFR, PDGFR, MET, FGFR, RAF, and RET.105 In a clinical trial Phase II, motesanib was submitted for patients with progressive DTC, 14% had a partial response and 35% had disease stability.102 Axitinib targeted the VEGF receptors and 30% partial response to axitinib was detected in a Phase II trial for patients with advanced or metastatic thyroid cancers.106 In order to evaluate the effectiveness of continuous dosing of sunitinib in patients with fluorodeoxyglucose positron emission tomography)–avid, iodine-refractory well-differentiated thyroid carcinoma (WDTC) and MTC a Phase II clinical trial was done by LL. Carr., and colleagues. One of the VEGF receptors, RET, and RET/PTC 1 and 3 targeting molecule is sunitinib, and the complete responses have been reported for patients with FDG-avid metastatic thyroid cancer.107
Vemurafenib is a small molecular and specific inhibitor of BRAF V600E and the study of 51 papillary thyroid cancer patients between January 2011 and January 2013 showed antitumor activity in patients with progressive, BRAFV600E-positive papillary thyroid cancer refractory to radioactive iodine who had never been treated with a multi-kinase inhibitor.108,109 It also was suggested that specific genetic mutations in ATC, including amplifications of the KDR, KIT, and PDGFRA genes can increase the efficacy of treatment with lenvatinib.12,110 It was shown by Rothschild and his colleagues that lenvatinib has a reasonable clinical activity in unselected patients with RAI-refractory thyroid cancer and about two-third of patients showing clinical benefit and manageable toxicity.111
More than that, some studies identified several genetic alterations as the vital determinant or for the development of personalized therapies for thyroid cancer. By way of illustration, the amplification of CD274, PDCD1LG2, JAK2, and DNA mismatch repair (MMR) deficiencies has been linked to the positive response to immune checkpoint inhibitors such as pembrolizumab and nivolumab.112 The evaluation of serum thyroglobulin (Tg) is currently employed to determine recurrence or persistence of the disease in DTC patients during the follow-up period after thyroidectomy or RAI therapy. Recently, Barbolosi and colleagues proposed a mathematical model to predict Tg kinetics, determine tumor doubling time under RAI treatment, and consequently categorize the patients into RAI-responding and non-responding groups.113 Although further investigations are required to determine the efficacy of these models for extensive clinical application, this novel field of research could eventually provide precise information on each individual patient and guide the physicians into more accurate and sophisticated decision-making. Furthermore, the application of RNAi agents such as small interfering RNA (siRNA) provides novel opportunities for the management of thyroid cancers with specific genetic mutations. Liu et al, introduced a novel near-infrared nanoplatform for systemic delivery of siRNA to treat ATC by targeting BRAF mutations which demonstrated a considerable down-regulation of BRAF protein expression, suppression of tumor growth, and reduced number of lung micrometastases in animal models of ATC with no notable adverse effects in the experimental group.114 In addition, in-vitro and in-vivo studies illustrated that knockdown of zinc-finger transcription factor SLUG by siRNA application could result in growth restraint of SW1736 ATC cells as well as increased sensitivity to doxorubicin administration.115
Very recently liquid biopsy has presented a new non-invasive source for monitoring cancer-genetics in the blood. In several personalized thyroid cancer diagnosis and prognosis strategies, liquid biopsy is taken into consideration.116,117 Recently, in thyroid cancer, circulating nucleic acids and circulating tumor cells), and exosomes have brought new revolutionary insight to thyroid cancer personalized managements.118,119 In PTC patients, circulating microRNA profiles was suggested as potential biomarkers for cancer diagnosis.120
Conclusion
In spite of the fact that thyroid cancers are commonly diagnosed with FNA test, there are some cytological results as the “indeterminate” or “Gray Zone”. Molecular profiling of FNAB cytology not only can improve diagnostic accuracy in the gray zone, but also support the thyroid cancer personalized treatment in a more favorable way. The connection of some genetic mutation and personalized refractory to treatment can improve prognostic consequence with much more optimized precision treatment strategies. Each thyroid cancer patient can be considered with regard to their genetic background to choose the exact therapeutic component.
Acknowledgment
The authors would like to thank the Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences for providing the location and a good atmosphere to do the research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Flores M, Glusman G, Brogaard K, Price ND, Hood L. P4 medicine: how systems medicine will transform the healthcare sector and society. Per Med. 2013;10(6):565–576. doi:10.2217/pme.13.57
2. Movafagh A. Personalised medicine in modern era. Asian Pac J Cancer Biol. 2016;1:2.
3. Turnbull AK. Personalized medicine in cancer: where are we today? Future Oncol. 2015;11(20):2795–2798. doi:10.2217/fon.15.204
4. Keller EF. The century beyond the gene. J Biosci. 2005;30(1):3–10.
5. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nature Rev Cancer. 2013;13(10):714. doi:10.1038/nrc3599
6. Giaccone G, Pinedo HM. Drug resistance. Oncologist. 1996;1(1 & 2):82–87.
7. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.20107
8. Larijani B, Mohagheghi MA, Bastanhagh MH, et al. Primary thyroid malignancies in Tehran, Iran. Med principles pract. 2005;14(6):396–400. doi:10.1159/000088112
9. Haghpanah V, Soliemanpour B, Heshmat R, et al. Endocrine cancer in Iran: based on cancer registry system. Indian J Cancer. 2006;43(2):80. doi:10.4103/0019-509X.25889
10. Larijani B, Shirzad M, Mohagheghi M, et al. Epidemiologic analysis of the Tehran cancer institute data system registry (TCIDSR). Asian Pac J Cancer Prev. 2004;5(1):36–39.
11. Katoh H, Yamashita K, Enomoto T, Watanabe M. Classification and general considerations of thyroid cancer. Ann Clin Pathol. 2015;3(1):1045.
12. Pozdeyev N, Gay LM, Sokol ES, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res. 2018;24(13):3059–3068. doi:10.1158/1078-0432.CCR-18-0373
13. Lin J-D, Chao T-C. Vascular endothelial growth factor in thyroid cancers. Cancer Biother Radiopharm. 2005;20(6):648–661. doi:10.1089/cbr.2005.20.648
14. Capp C, Wajner SM, Siqueira DR, Brasil BA, Meurer L, Maia AL. Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid. 2010;20(8):863–871. doi:10.1089/thy.2009.0417
15. Agaimy A, Terracciano L, Dirnhofer S, et al. V600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours. J Clin Pathol. 2009;62(7):613–616. doi:10.1136/jcp.2009.064550
16. Kim M-J, Kim SK, Park HJ, et al. PDGFRA promoter polymorphisms are associated with the risk of papillary thyroid cancer. Mol Med Rep. 2012;5(5):1267–1270. doi:10.3892/mmr.2012.784
17. Chowdhury S, Veyhl J, Jessa F, et al. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget. 2016;7(22):32318. doi:10.18632/oncotarget.8698
18. Ameur N, Lacroix L, Motte N, et al. Mutational status of EGFR, BRAF, PI3KCA and JAK2 genes in endocrine tumors. Int J cancer. 2009;124(3):751–753. doi:10.1002/ijc.23999
19. Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn). 2015;19(1A):A68–A77. doi:10.5114/wo.2014.47136
20. Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8(1):83–95. doi:10.1586/14737159.8.1.83
21. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–262. doi:10.1677/erc.1.0978
22. Zuidervaart W, van Nieuwpoort F, Stark M, et al. Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. Br J Cancer. 2005;92(11):2032–2038. doi:10.1038/sj.bjc.6602598
23. Kim WW, Ha TK, Bae SK. Clinical implications of the BRAF mutation in papillary thyroid carcinoma and chronic lymphocytic thyroiditis. J Otolaryngol Head Neck Surg. 2018;47(1):4. doi:10.1186/s40463-017-0247-6
24. Quinlan MP, Settleman J. Isoform-specific ras functions in development and cancer. Future Oncol. 2009;5(1):105–116. doi:10.2217/14796694.5.1.105
25. Xing M. Clinical utility of RAS mutations in thyroid cancer: a blurred picture now emerging clearer. BMC Med. 2016;14(1):12. doi:10.1186/s12916-016-0559-9
26. Nozhat Z, Hedayati M. PI3K/AKT pathway and its mediators in thyroid carcinomas. Mol Diagn Ther. 2016;20(1):13–26. doi:10.1007/s40291-015-0175-y
27. Nagy R, Ganapathi S, Comeras I, et al. Frequency of germline PTEN mutations in differentiated thyroid cancer. Thyroid. 2011;21(5):505–510. doi:10.1089/thy.2010.0365
28. Sarmadi S, Izadi-Mood N, Sotoudeh K, Tavangar SM. Altered PTEN expression; a diagnostic marker for differentiating normal, hyperplastic and neoplastic endometrium. Diagn Pathol. 2009;4(1):41. doi:10.1186/1746-1596-4-41
29. García-Rostán G, Costa AM, Pereira-Castro I, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005;65(22):10199–10207. doi:10.1158/0008-5472.CAN-04-4259
30. Wu G, Mambo E, Guo Z, et al. Uncommon mutation, but common amplifications, of the PIK3CA gene in thyroid tumors. J Clin Endocrinol Metab. 2005;90(8):4688–4693. doi:10.1210/jc.2004-2281
31. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nature Rev Cancer. 2013;13(3):184. doi:10.1038/nrc3431
32. Lin Y, Jiang X, Shen Y, et al. Frequent mutations and amplifications of the PIK3CA gene in pituitary tumors. Endocr Relat Cancer. 2009;16(1):301–310. doi:10.1677/ERC-08-0167
33. Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and PI3K/Akt and MAP kinase pathways in anaplastic and follicular thyroid cancers. Med Baltimore. 2008;1500:21287.
34. Soares P, Celestino R, Melo M, Fonseca E, Sobrinho-Simões M. Prognostic biomarkers in thyroid cancer. Virchows Archiv. 2014;464(3):333–346. doi:10.1007/s00428-013-1521-2
35. Xing MM Genetic amplification of IQGAP1 in cancer. Google Patents; 2015.
36. Liu Z, Xie Liu D, Bojdani E, El-Naggar AK, Vasko V, Xing M. IQGAP1 plays an important role in the invasiveness of thyroid cancer. Clin Cancer Res. 2010. clincanres. 1627.2010. doi:10.1158/1078-0432.CCR-10-1627
37. Nakazawa T, Kondo T, Kobayashi Y, et al. RET gene rearrangements (RET/PTC1 and RET/PTC3) in papillary thyroid carcinomas from an iodine‐rich country (Japan). Cancer. 2005;104(5):943–951. doi:10.1002/cncr.21270
38. Eberhardt NL, Grebe SK, McIver B, Reddi HV. The role of the PAX8/PPARγ fusion oncogene in the pathogenesis of follicular thyroid cancer. Mol Cell Endocrinol. 2010;321(1):50–56. doi:10.1016/j.mce.2009.10.013
39. Castro P, Rebocho A, Soares R, et al. PAX8-PPAR γ rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2006;91(1):213–220. doi:10.1210/jc.2005-1336
40. Russo D, Damante G, Puxeddu E, Durante C, Filetti S. Epigenetics of thyroid cancer and novel therapeutic targets. J Mol Endocrinol. 2011;46(3):R73-81. doi:10.1530/JME-10-0150
41. Schagdarsurengin U, Gimm O, Hoang-Vu C, Dralle H, Pfeifer GP, Dammann R. Frequent epigenetic silencing of the CpG island promoter of RASSF1A in thyroid carcinoma. Cancer Res. 2002;62(13):3698–3701.
42. Mohammadi-asl J, Larijani B, Khorgami Z, et al. Qualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARβ2 genes in papillary thyroid carcinoma. Med Oncol. 2011;28(4):1123–1128. doi:10.1007/s12032-010-9587-z
43. Khatami F, Tavangar SM. Genetic and epigenetic of medullary thyroid cancer. Iran Biomed J. 2018;22(3):142.
44. Xing M, Usadel H, Cohen Y, et al. Methylation of the thyroid-stimulating hormone receptor gene in epithelial thyroid tumors: a marker of malignancy and a cause of gene silencing. Cancer Res. 2003;63(9):2316–2321.
45. Hoque M, Rosenbaum E, Westra W, et al. Quantitative assessment of promoter methylation profiles in thyroid neoplasms. J Clin Endocrinol Metab. 2005;90(7):4011–4018. doi:10.1210/jc.2005-0313
46. Alvarez-Nuñez F, Bussaglia E, Mauricio D, et al. PTEN promoter methylation in sporadic thyroid carcinomas. Thyroid. 2006;16(1):17–23. doi:10.1089/thy.2006.16.17
47. Khatami F, Larijani B, Heshmat R, et al. Meta-analysis of promoter methylation in eight tumor-suppressor genes and its association with the risk of thyroid cancer. PLoS One. 2017;12(9):e0184892. doi:10.1371/journal.pone.0184892
48. Cai -L-L, Liu G-Y, Tzeng C-M. Genome-wide DNA methylation profiling and its involved molecular pathways from one individual with thyroid malignant/benign tumor and hyperplasia: a case report. Medicine. 2016;95:35. doi:10.1097/MD.0000000000004864
49. Pallante P, Visone R, Ferracin M, et al. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer. 2006;13(2):497–508. doi:10.1677/erc.1.01209
50. Yip L, Kelly L, Shuai Y, et al. MicroRNA signature distinguishes the degree of aggressiveness of papillary thyroid carcinoma. Ann Surg Oncol. 2011;18(7):2035–2041. doi:10.1245/s10434-011-1733-0
51. Ramírez-Moya J, Wert-Lamas L, Santisteban P. MicroRNA-146b promotes PI3K/AKT pathway hyperactivation and thyroid cancer progression by targeting PTEN. Oncogene. 2018;37(25):3369–3383. doi:10.1038/s41388-017-0088-9
52. Natanzi MM, Pasalar P, Kamalinejad M, et al. Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta Med Iran. 2012;50(9):589–596.
53. Alimoghaddam K, Shariftabrizi A, Tavangar M, et al. Anti-leukemic and anti-angiogenesis efficacy of arsenic trioxide in new cases of acute promyelocytic leukemia. Leuk Lymphoma. 2006;47(1):81–88. doi:10.1080/10428190500300373
54. Parvizi MR, Parviz M, Tavangar SM, et al. Protective effect of magnesium on renal function in STZ-induced diabetic rats. J Diabetes Metab Disord. 2014;13(1):84. doi:10.1186/s40200-014-0084-3
55. Tavangar SM, Larijani B, Mahta A, Hosseini SMA, Mehrazine M, Bandarian F. Craniopharyngioma: a clinicopathological study of 141 cases. Endocr Pathol. 2004;15(4):339–344.
56. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. 2012;56(4):333–339. doi:10.1159/000339959
57. Omidfar K, Moinfar Z, Sohi AN, et al. Expression of EGFRvIII in thyroid carcinoma: immunohistochemical study by camel antibodies. Immunol Invest. 2009;38(2):165–180. doi:10.1080/08820130902735998
58. Haghpanah V, Shooshtarizadeh P, Heshmat R, Larijani B, Tavangar SM. Immunohistochemical analysis of survivin expression in thyroid follicular adenoma and carcinoma. Appl Immunohistochem Mol Morphol. 2006;14(4):422–425. doi:10.1097/01.pai.0000213100.88074.b8
59. Saffar H, Sanii S, Emami B, et al. Evaluation of MMP2 and Caspase-3 expression in 107 cases of papillary thyroid carcinoma and its association with prognostic factors. Pathol Res Pract. 2013;209(3):195–199. doi:10.1016/j.prp.2012.06.011
60. Haddadi-Nezhad S, Larijani B, Tavangar SM, Nouraei SM. Comparison of fine-needle-nonaspiration with fine-needle-aspiration technique in the cytologic studies of thyroid nodules. Endocr Pathol. 2003;14(4):369–373.
61. Sanii S, Saffar H, Tabriz HM, Qorbani M, Haghpanah V, Tavangar SM. Expression of matrix metalloproteinase-2, but not caspase-3, facilitates distinction between benign and malignant thyroid follicular neoplasms. Asian Pac J Cancer Prev. 2012;13(5):2175–2178. doi:10.7314/apjcp.2012.13.5.2175
62. Tabriz HM, Adabi K, Lashkari A, et al. Immunohistochemical analysis of nm23 protein expression in thyroid papillary carcinoma and follicular neoplasm. Pathol Res Pract. 2009;205(2):83–87. doi:10.1016/j.prp.2008.08.007
63. Zhang M, Lin O. Molecular testing of thyroid nodules: a review of current available tests for fine-needle aspiration specimens. Arch Pathol Lab Med. 2016;140(12):1338–1344. doi:10.5858/arpa.2016-0100-RA
64. Chudova D, Wilde JI, Wang ET, et al. Molecular classification of thyroid nodules using high-dimensionality genomic data. J Clin Endocrinol Metab. 2010;95(12):5296–5304. doi:10.1210/jc.2010-1087
65. Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367(8):705–715. doi:10.1056/NEJMoa1203208
66. Marti JL, Avadhani V, Donatelli LA, et al. Wide inter-institutional variation in performance of a molecular classifier for indeterminate thyroid nodules. Ann Surg Oncol. 2015;22(12):3996–4001. doi:10.1245/s10434-015-4486-3
67. Duick DS, Klopper JP, Diggans JC, et al. The impact of benign gene expression classifier test results on the endocrinologist–patient decision to operate on patients with thyroid nodules with indeterminate fine-needle aspiration cytopathology. Thyroid. 2012;22(10):996–1001. doi:10.1089/thy.2012.0180
68. Saji M, Ringel MD. The PI3K-Akt-mTOR pathway in initiation and progression of thyroid tumors. Mol Cell Endocrinol. 2010;321(1):20–28. doi:10.1016/j.mce.2009.10.016
69. Pallante P, Battista S, Pierantoni GM, Fusco A. Deregulation of microRNA expression in thyroid neoplasias. Nature Rev Endocrinol. 2014;10(2):88. doi:10.1038/nrendo.2013.223
70. Stokowy T, Wojtaś B, Krajewska J, et al. A two miRNA classifier differentiates follicular thyroid carcinomas from follicular thyroid adenomas. Mol Cell Endocrinol. 2015;399:43–49. doi:10.1016/j.mce.2014.09.017
71. Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab. 2013;98(11):E1852–E1860. doi:10.1210/jc.2013-2292
72. Nikiforov YE, Carty SE, Chiosea SI, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next‐generation sequencing assay. Cancer. 2014;120(23):3627–3634. doi:10.1002/cncr.29038
73. Le Mercier M, D’haene N, De Nève N, et al. Next‐generation sequencing improves the diagnosis of thyroid FNA specimens with indeterminate cytology. Histopathology. 2015;66(2):215–224. doi:10.1111/his.12461
74. Singer PA, Cooper DS, Daniels GH, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. Arch Int Med. 1996;156(19):2165–2172.
75. Higashi T, Kudo T, Kinuya S. Radioactive iodine (131 I) therapy for differentiated thyroid cancer in Japan: current issues with historical review and future perspective. Ann Nucl Med. 2012;26(2):99–112. doi:10.1007/s12149-011-0553-4
76. Tubiana M, Haddad E, Schlumberger M, Hill C, Rougier P, Sarrazin D. External radiotherapy in thyroid cancers. Cancer. 1985;55(S9):2062–2071. doi:10.1002/1097-0142(19850501)55:9+<2062::aid-cncr2820551406>3.0.co;2-o
77. Brierley JD, Tsang RW, editors. External‐beam radiation therapy in the treatment of differentiated thyroid cancer. Semin Surg Oncol. 1999. Wiley Online Library. doi:10.1002/(SICI)1098-2388(199901/02)16:1<42::AID-SSU8>3.0.CO;2-4
78. Sherman SI. Targeted therapy of thyroid cancer. Biochem Pharmacol. 2010;80(5):592–601. doi:10.1016/j.bcp.2010.05.003
79. Degrauwe N, Sosa JA, Roman S, Deshpande HA. Vandetanib for the treatment of metastatic medullary thyroid cancer. Clin Med Insights Oncol. 2012;6:
80. Hoy SM. Cabozantinib: a review of its use in patients with medullary thyroid cancer. Drugs. 2014;74(12):1435–1444. doi:10.1007/s40265-014-0265-x
81. Fallahi P, Ferrari SM, Santini F, et al. Sorafenib and thyroid cancer. BioDrugs. 2013;27(6):615–628. doi:10.1007/s40259-013-0049-y
82. Cabanillas ME, Habra MA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev. 2016;42:47–55. doi:10.1016/j.ctrv.2015.11.003
83. Ballantyne AD, Garnock-Jones KP. Dabrafenib: first global approval. Drugs. 2013;73(12):1367–1376. doi:10.1007/s40265-013-0095-2
84. McGETTIGAN S. Dabrafenib: a new therapy for use in BRAF-mutated metastatic melanoma. J Adv Pract Oncol. 2014;5(3):211.
85. Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600–mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36(1):7. doi:10.1200/JCO.2017.73.6785
86. Jeelani S, Reddy RJ, Maheswaran T, Asokan G, Dany A, Anand B. Theranostics: a treasured tailor for tomorrow. J Pharm Bioallied Sci. 2014;6(Suppl 1):S6. doi:10.4103/0975-7406.137249
87. Oh J-R, Ahn B-C, Jeong SY, Lee S-W, Lee J. Radioiodine scan index: a simplified, quantitative treatment response parameter for metastatic thyroid carcinoma. Nucl Med Mol Imaging (2010). 2015;49(3):174–181. doi:10.1007/s13139-015-0335-3
88. Kim D-H, Jung J-H, Son SH, et al. Difference of clinical and radiological characteristics according to radioiodine avidity in pulmonary metastases of differentiated thyroid cancer. Nucl Med Mol Imaging (2010). 2014;48(1):55–62. doi:10.1007/s13139-013-0239-z
89. Hong C, Ahn B-C, Jeong S, Lee S-W, Lee J. Distant metastatic lesions in patients with differentiated thyroid carcinoma. Nuklearmedizin. 2013;52(04):121–129. doi:10.3413/Nukmed-0541-12-11
90. Lee JW, Min HS, Lee SM, Kwon HW, Chung J-K. Relations between pathological markers and radioiodine scan and 18 F-FDG PET/CT findings in papillary thyroid cancer patients with recurrent cervical nodal metastases. Nucl Med Mol Imaging (2010). 2015;49(2):127–134. doi:10.1007/s13139-015-0324-6
91. Spitzweg C, Bible KC, Hofbauer LC, Morris JC. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. lancet Diabetes endocrinol. 2014;2(10):830–842. doi:10.1016/S2213-8587(14)70051-8
92. Micali S, Bulotta S, Puppin C, et al. Sodium iodide symporter (NIS) in extrathyroidal malignancies: focus on breast and urological cancer. BMC Cancer. 2014;14(1):303. doi:10.1186/1471-2407-14-303
93. Plantinga TS, Heinhuis B, Gerrits D, et al. mTOR Inhibition promotes TTF1-dependent redifferentiation and restores iodine uptake in thyroid carcinoma cell lines. J Clin Endocrinol Metab. 2014;99(7):E1368–E1375. doi:10.1210/jc.2014-1171
94. Wong K-P, Lang B-H-H. New molecular targeted therapy and redifferentiation therapy for radioiodine-refractory advanced papillary thyroid carcinoma: literature review. J Thyroid Res. 2012;2012:818204. doi:10.1155/2012/818204
95. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21(5):1028–1035. doi:10.1158/1078-0432.CCR-14-2915
96. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. doi:10.1056/NEJMoa1714448
97. Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705–714. doi:10.1016/S1470-2045(18)30119-0
98. Yip L. Molecular markers for thyroid cancer diagnosis, prognosis, and targeted therapy. J Surg Oncol. 2015;111(1):43–50. doi:10.1002/jso.23768
99. Landa I, Ganly I, Chan TA, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98(9):E1562–E1566. doi:10.1210/jc.2013-2383
100. Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20(4):603–610. doi:10.1530/ERC-13-0210
101. Shoup M, Stojadinovic A, Nissan A, et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg. 2003;197(2):191–197. doi:10.1016/S1072-7515(03)00332-6
102. Sherman SI, Wirth LJ, Droz J-P, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359(1):31–42. doi:10.1056/NEJMoa075853
103. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368(7):623–632. doi:10.1056/NEJMoa1209288
104. Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAFV600E mutation. Thyroid. 2013;23(10):1277–1283. doi:10.1089/thy.2013.0057
105. Sherman SI. Tyrosine kinase inhibitors and the thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23(6):713–722. doi:10.1016/j.beem.2009.08.001
106. Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26(29):4708. doi:10.1200/JCO.2007.15.2777
107. Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG-PET–positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16(21):5260–5268. doi:10.1158/1078-0432.CCR-10-0994
108. Khatami F, Larijani B, Tavangar SM. Circulating tumor BRAF mutation and personalized thyroid cancer treatment. Asian Pacif j cancer prev. 2017;18(2):293. doi:10.22034/APJCP.2017.18.1.23
109. Brose MS, Cabanillas ME, Cohen EE, et al. Vemurafenib in patients with BRAFV600E-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(9):1272–1282. doi:10.1016/S1470-2045(16)30166-8
110. Ferrari SM, Ruffilli I, Centanni M, et al. Lenvatinib in the therapy of aggressive thyroid cancer: state of the art and new perspectives with patents recently applied. Recent Pat Anticancer Drug Discov. 2018;13(2):201–208. doi:10.2174/1574892813666180220110729
111. Balmelli C, Railic N, Siano M, et al. Lenvatinib in advanced radioiodine-refractory thyroid cancer – a retrospective analysis of the Swiss Lenvatinib Named Patient Program. J Cancer. 2018;9(2):250–255. doi:10.7150/jca.22318
112. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi:10.1126/science.aan6733
113. Barbolosi D, Summer I, Meille C, et al. Modeling therapeutic response to radioiodine in metastatic thyroid cancer: a proof-of-concept study for individualized medicine. Oncotarget. 2017;8(24):39167. doi:10.18632/oncotarget.16637
114. Liu Y, Gunda V, Zhu X, et al. Theranostic near-infrared fluorescent nanoplatform for imaging and systemic siRNA delivery to metastatic anaplastic thyroid cancer. Proc National Acad Sci. 2016;113(28):7750–7755. doi:10.1073/pnas.1605841113
115. Pan Y, Liu P, Chen D, Dou L. Small interfering RNA (siRNA) against Slug induces apoptosis and sensitizes human anaplastic thyroid carcinoma cells to doxorubicin. Cancer Biomarkers. 2017;18(4):357–366. doi:10.3233/CBM-160192
116. Fadda G, Rossi ED. Liquid-based cytology in fine-needle aspiration biopsies of the thyroid gland. Acta Cytol. 2011;55(5):389–400. doi:10.1159/000329029
117. Khatami F, Larijani B, Tavangar SM. The presence of tumor extrachomosomal circular DNA (ecDNA) as a component of liquid biopsy in blood. Med Hypotheses. 2018;114:5–7. doi:10.1016/j.mehy.2018.02.018
118. Khatami F, Tavangar SM. Liquid biopsy in thyroid cancer: new Insight. Int J Hematol Oncol Stem Cell Res. 2018;12(3):235.
119. Bardelli A. Medical research: personalized test tracks cancer relapse. Nature. 2017;545(7655):417. doi:10.1038/545417a
120. Yu S, Liu Y, Wang J, et al. Circulating microRNA profiles as potential biomarkers for diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2012;97(6):2084–2092. doi:10.1210/jc.2011-3059
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.