Back to Journals » International Medical Case Reports Journal » Volume 12
Peripheral hypertrophic subepithelial corneal degeneration: clinical aspects related to in vivo confocal microscopy and optical coherence tomography
Authors Gunzinger JM , Voulgari N, Petrovic A, Hashemi K, Kymionis G
Received 11 March 2019
Accepted for publication 24 June 2019
Published 26 July 2019 Volume 2019:12 Pages 237—241
DOI https://doi.org/10.2147/IMCRJ.S208297
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jeanne Martine Gunzinger, Nafsika Voulgari, Aleksandra Petrovic, Kattayoon Hashemi, Georgios Kymionis
Lausanne University, Cornea and Refractive Surgery Department, Jules-Gonin Eye Hospital, Lausanne, Switzerland
Purpose: To report the findings of anterior segment optical coherence tomography (AS-OCT) and in vivo confocal microscopy (IVCM) in two patients with peripheral hypertrophic subepithelial corneal degeneration (PHSD).
Methods: Case series by retrospective chart review and imaging analysis of AS-OCT and IVCM.
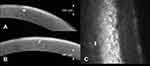
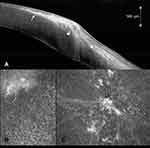
Results: Slit lamp examination of the two patients revealed a bilateral subepithelial-elevated fibrous tissue of the superior-nasal quadrant, as well as inferior-nasal in one of the patients. Best corrected visual acuity ranged from 20/25 to 20/15. AS-OCT showed continuous, homogenous, well-demarked hyperreflective subepithelial band associated with hyperreflectivity in the anterior stroma. IVCM demonstrated normal epithelial cell morphology and arrangement and a fibrous structure subepithelial and in the anterior stroma.
Conclusion: AS-OCT and IVCM can facilitate the diagnosis of PHSD and differentiate it from other corneal entities that present peripheral opacifications.
Keywords: peripheral hypertrophic subepithelial corneal degeneration, anterior segment optical coherence tomography, in vivo confocal microscopy, peripheral corneal opacification
Introduction
Peripheral hypertrophic subepithelial corneal degeneration (PHSD) is a clinical entity of unknown etiology, first reported in 2003,1 that more commonly needs to be differentiated from Salzmann’s nodular degeneration (SND). It is characterized by predominantly bilateral, gradually progressing, perilimbal, subepithelial corneal fibrosis with adjacent superficial neovascularization in the absence of concurrent or preceding clinically visible ocular surface inflammation.1–3 Terrien marginal degeneration and corneal intraepithelial neoplasia (CIN) can equally present with peripheral corneal opacification sharing many similarities with PHSD but otherwise exhibiting distinct clinicopathological properties. We herein present two cases of PHSD that manifested as Terrien marginal degeneration and CIN, respectively. The distinguishable features that allow identification of this uncommon entity are highlighted. To our knowledge, this is the first report of anterior segment optical coherence tomography (AS-OCT) and in vivo confocal microscopy (IVCM) characteristics of PHSD.
Case series
Case 1
An 88-year-old man was referred to the cornea department of the University of Lausanne, Jules Gonin Eye Hospital for suspicion of a CIN or carcinoma in situ due to a superficial corneal lesion in the right eye. According to the referring ophthalmologist, this lesion was first described 10 years ago, showing gradual progression to the paracentral region.
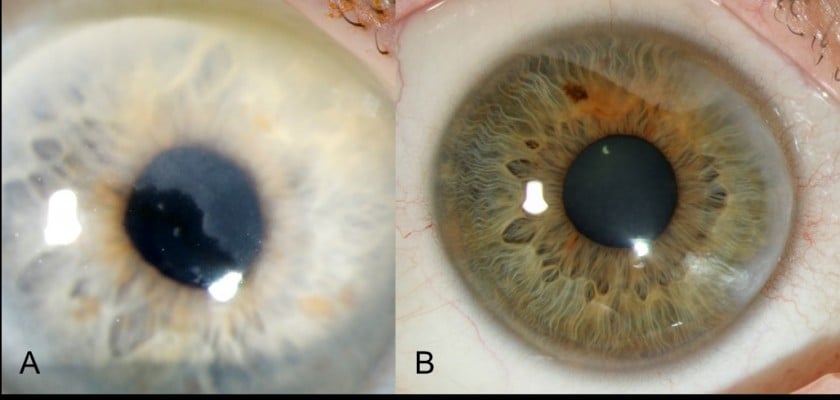
The patient complained of slightly blurred vision in his right eye for a few months. Best corrected visual acuity (BCVA) was 20/25 in the right eye and 20/20 in the left eye. Slit lamp biomicroscopy revealed subepithelial-elevated opacities and fibrosis of the superior-nasal quadrant of the right eye, with otherwise no other ocular surface lesion or scar (Figure 1A). Topography (Pentacam®, Oculus, Wetzlar, Germany) showed a topographic astigmatism of 0.1 diopters at 167° in the right eye. AS-OCT (Avanti-Optovue, Fermont, California, 70,000 scans/second, scan beam wavelength 840 nm) showed a mostly continuous, homogenous, well-demarcated hyperreflective (qualitatively, compared to surrounding structures as the epithelium or the stroma) subepithelial band respecting the Bowman layer while rarely breaking through and expanding slightly into the anterior stroma (Figure 2A and B). IVCM (Rostock Cornea Module for Heidelberg Retina Tomograph, Heidelberg Engineering Inc., Heidelberg, Germany, 670 nm diode laser, axial resolution of approximately 1 µm) showed normally structured epithelium with underlying hyperreflective, fibrotic appearing structures comprising of oval-shaped cells (Figure 2C). Bowman layer was mostly smooth and continuous but showed a reduction of nerve axons. Few images showed breakthrough of fibrosis and slight expansion into the anterior stroma.
On close examination of the left eye an identical, but smaller, only peripheral opacity was also found in the superior-nasal quadrant. Systemic evaluation was normal, there was no sign of paraproteinemia.
Due to the preserved BCVA of 20/25 and the findings of AS-OCT and IVCM, keratectomy was not recommended.
Case 2
A 42-year-old woman was referred for suspicion of Terrien marginal degeneration in both eyes. The patient complained of red eyes for 3 months prior to the consultation.
BCVA was 20/20 in the right eye and 20/15 in the left eye. Slit lamp biomicroscopy revealed symmetric slightly elevated, nodular, peripheral, subepithelial, and anterior stromal fibrosis in the nasal quadrant of both eyes with a non-nodular zone toward the limbus giving the false impression of thinning (Figure 1B). Superficial new vessels were present from to limbus toward the basis of the fibrosis. Topography showed astigmatism of 1.0 diopter at 13° in the right eye and 1.1 diopters at 126° in the left eye. Pachymetry did not show any sign of peripheral corneal thinning. AS-OCT demonstrated a continuous, homogenous, well demarcated, slightly nodular hyperreflective subepithelial band. This band respects the Bowman layer central to the lesion, while showing an inhomogeneous structure breaking through the Bowman layer and expanding into the anterior stroma peripheral to the lesion (Figure 3A). IVCM showed normally structured epithelium with underlying hyperreflective, fibrous structures with rare cells (Figure 3B and C). Bowman layer was smooth and continuous centrally, but not well demarcated peripherally. Expansion of fibrosis into the anterior stroma was visible on peripheral images. Systemic evaluation was normal, there was no sign of paraproteinemia.
The patient was treated with ciclosporin 0.05% eye drops twice daily and fluorometholone 0.1% one drop every second day. Six and nine months follow-ups showed no evolution of the lesion and topical treatment was slowly tapered.
Discussion
We describe herein two cases of PHSD, resembling CIN and Terrien marginal degeneration, respectively.
Recently proposed diagnostic criteria for PHSD include perilimbal subepithelial fibrosis with thickening of the cornea, possible extension to the mid-periphery but no central cornea involvement.2,3 Superficial neovascularization up to the base of the fibrous tissue and two or more diopters of topographic astigmatism represent additional distinguishable features. Typical symptoms consist of ocular surface irritation and decreased visual acuity due to regular and irregular astigmatism. PHSD must be differentiated from other corneal diseases such as SND, auto-immune diseases, paraproteinemic keratopathy due to monoclonal gammopathy,4 CIN or other entities such as Terrien marginal degeneration as noted in one of our cases.
CIN is a squamous cell in situ carcinoma showing dysplastic epithelial cells which can involve less than 25% (Grade I) or up to more than 75% (Grade III) of epithelial thickness.5 Clinical presentation, indistinguishable from PHSD, includes ocular surface irritation, redness, foreign body sensation or decreased vision. Slit lamp examination, similar to PHSD, reveals a mostly well demarcated, slightly elevated superficial corneal lesion. However, in contrast to PHSD, it presents as a translucent corneal clouding rarely accompanied by leukoplakia, lacking subepithelial fibrosis and accompanied by feeder vessels expanding into the lesion.6 The lesion tends to evolve rapidly. As CIN is an epithelial disorder, AS-OCT will show a typical pattern of hyperreflective, thickened epithelium (normal epithelial thickness is 50–52 µm) and an abrupt transition from normal to abnormal epithelium.7 In our first patient, epithelium was clearly normal on AS-OCT and thus rule out the diagnosis of CIN.
If the clinical presentation is in favor of PHSD, AS-OCT is invaluable in identifying a hyperreflective, strictly subepithelial lesion with intact overlying epithelial structures1,8 – and absence of epithelial destruction or large fibrovascular structures, as noted in CIN.9 IVCM can further help to clarify the diagnosis of PHSD by showing regularly structured epithelial cells with no sign of enlargement, irregularity, anisocytosis, or anisonucleosis as seen in CIN.9 If there is doubt about the diagnosis, further investigations must be performed to exclude CIN.
Terrien marginal degeneration is a slowly progressive degeneration of unclear origin, usually starting in the superonasal quadrant.10 Clinical presentation often includes vision loss due to induced astigmatism. Slit lamp examination typically shows peripheral thinning and superficial vascularization with lipid deposition at the leading edge. Similar to PHSD, it presents as a perilimbal opacity, however, in contrast to PHSD, it is often accompanied by yellow lipid deposits and peripheral thinning. AS-OCT is helpful to differentiate between the two entities. Whereas in Terrien marginal degeneration, findings include corneal thinning (normal peripheral corneal thickness is 612–640 µm11) but no associated reflectivity changes in subepithelial structures12 in contrast to the typical findings in PHSD which include a hyperreflective subepithelial band and might be associated with hyperreflective areas in the anterior stroma, but does not show corneal thinning. To our knowledge there are only two case reports of IVCM findings in Terrien marginal degeneration, both showing lipid deposits, activated keratocytes and needle-like material or honeycomb-like pattern respectively, and in one case inflammatory cells,13,14 whereas in our case IVCM presented fibrous subepithelial structures with expansion into the anterior stroma, but no lipid deposits.
In conclusion, our cases suggest that PHSD might masquerade as Terrien marginal degeneration or CIN and highlight the importance of considering this pathology in the differential diagnosis of peripheral corneal opacifications not fitting the criteria of other broadly known entities. In both cases, the diagnosis of PHSD was made by slit lamp examination and confirmed by AS-OCT and IVCM, demonstrating their value as complementary tools in the diagnosis of PHSD.
Ethics statement
Written informed consent has been provided by both patients for the use of retrospective chart data as well as publication of images. Institutional approval was granted for the publication of the cases.
Acknowledgments
None of the authors have any commercial or propriety interest related to the case series.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maust HA, Raber IM. Peripheral hypertrophic subepithelial corneal degeneration. Eye Contact Lens. 2003;29(4):266–269. doi:10.1097/01.icl.0000087489.61955.82
2. Järventausta PJ, Tervo TMT, Kivelä T, Holopainen JM. Peripheral hypertrophic subepithelial corneal degeneration – clinical and histopathological features. Acta Ophthalmol. 2014;92(8):774–782. doi:10.1111/aos.12394
3. Gore DM, Iovieno A, Connell BJ, Alexander R, Meligonis G, Dart JK. Peripheral hypertrophic subepithelial corneal degeneration: nomenclature, phenotypes, and long-term outcomes. Ophthalmology. 2013;120(5):892–898. doi:10.1016/j.ophtha.2012.10.037
4. Skalicka P, Dudakova L, Palos M, et al. Paraproteinemic keratopathy associated with monoclonal gammopathy of undetermined significance (MGUS): clinical findings in twelve patients including recurrence after keratoplasty. Acta Ophthalmol. 2019:
5. Hamam R, Bhat P, Foster CS. Conjunctival/corneal intraepithelial neoplasia. Int Ophthalmol Clin. 2009;49(1):63–70. doi:10.1097/IIO.0b013e3181924ec3
6. Honavar SG, Manjandavida FP. Tumors of the ocular surface: a review. Indian J Ophthalmol. 2015;63(3):187–203. doi:10.4103/0301-4738.156912
7. Atallah M, Joag M, Galor A, et al. Role of high resolution optical coherence tomography in diagnosing ocular surface squamous neoplasia with coexisting ocular surface diseases. Ocul Surf. 2017;15(4):688–695. doi:10.1016/j.jtos.2017.03.003
8. Rommel F, Grisanti S, Ranjbar M. Peripheral hypertrophic subepithelial corneal degeneration. JAMA Ophthalmol. 2017;135(6):e170664. doi:10.1001/jamaophthalmol.2017.0664
9. Xu Y, Zhou Z, Wang M, Liu F, Qu H, Hong J. The clinical value of in vivo confocal microscopy for diagnosis of ocular surface squamous neoplasia. Eye (Lond). 2012;26(6):781–787. doi:10.1038/eye.2012.15
10. Chan AT, Ulate R, Goldich Y, Rootman DS, Chan CC. Terrien marginal degeneration: clinical characteristics and outcomes. Am J Ophthalmol. 2015;160(5):867–872.e1. doi:10.1016/j.ajo.2015.07.031
11. Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66(2):190–194. doi:10.4103/ijo.IJO_646_17
12. Rodriguez M, Yesilirmak N, Chhadva P, Goldhagen B, Karp C, Galor A. High-resolution optical coherence tomography in the differentiation of inflammatory versus noninflammatory peripheral corneal thinning. Cornea. 2017;36(1):48–52. doi:10.1097/ICO.0000000000001023
13. Ceresara G, Migliavacca L, Orzalesi N, Rossetti L. In vivo confocal microscopy in terrien marginal corneal degeneration: a case report. Cornea. 2011;30(7):820–824. doi:10.1097/ICO.0b013e31820143ed
14. Ferrari G, Tedesco S, Delfini E, Macaluso C. Laser scanning in vivo confocal microscopy in a case of Terrien marginal degeneration. Cornea. 2010;29(4):471–475. doi:10.1097/ICO.0b013e3181b46aa3
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.