Back to Journals » Journal of Blood Medicine » Volume 13
Peripheral Cytopenia and Its Associated Factors in Type 2 Diabetes Mellitus Patients, Northwest Ethiopia
Authors Aynalem M , Getu F , Adane T
Received 6 April 2022
Accepted for publication 29 June 2022
Published 4 July 2022 Volume 2022:13 Pages 373—383
DOI https://doi.org/10.2147/JBM.S369583
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Melak Aynalem,1 Fasil Getu,2 Tiruneh Adane1
1Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia; 2Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Jigjiga University, Jigjiga, Ethiopia
Correspondence: Melak Aynalem, Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia, Tel +251912692619, Email [email protected]
Background: Hematological abnormalities are linked with diabetes mellitus (DM) and play a major role in diabetes-related micro- and macro-vascular complications. Therefore, this study aimed to investigate the magnitude of peripheral cytopenia and associated factors in type 2 diabetes (T2DM) patients.
Methods: A cross-sectional study was conducted from March to May 2021 at the University of Gondar Comprehensive Specialized Hospital. A total of 357 T2DM participants were selected using a simple random sampling technique. A total of 3 mL of venous blood samples were collected using the vacutainer method for the complete blood count (CBC). A univariate and multivariate regression analysis were used to investigate the association between dependent and independent variables. P-value ˂0.05 was considered statistically significant.
Results: The magnitude of cytopenia, bicytopenia, and pancytopenia were 21% (95% CI: 17.1, 25.53), 1.1% (95% CI: 0.44, 2.85), and 0.56% (95% CI: 0.01, 1.12), respectively. Furthermore, the magnitudes of anemia, leucopenia, and thrombocytopenia were 8.7% (95% CI: 6.18, 12.06), 10.9% (95% CI: 8.09, 14.59), and 5.3% (95% CI: 3.43, 8.16), respectively. Being male (AOR: 3.23; 95% CI: 1.43, 7.56), lack of exercise (AOR: 2.70; 95% CI: 1.137, 6.43), and never married (AOR: 3.90; 95% CI: 1.248, 12.18) were all associated with anemia.
Conclusion: This study showed that T2DM causes disturbances in the hematological parameters and leads to a mild level of cytopenia. It is, therefore, suggested that hematological abnormalities, especially cytopenia, should be monitored and controlled on a regular basis in T2DM patients for better prognosis and quality of life.
Keywords: peripheral cytopenia, type 2 diabetes mellitus, Gondar, Ethiopia
Background
Diabetes mellitus has become one of the leading global diseases of the twenty-first century, with over 422 million diabetics worldwide.1 It is a life-threatening disease that causes nephropathy, retinopathy, and neuropathy, as well as a variety of metabolic diseases.2 DM develops when the body’s cells are unable to utilize glucose for energy. It can be classified as Type 1 diabetes (T1DM), T2DM, and gestational diabetes. T2DM is defined as a combination of low peripheral insulin resistance and insulin production from pancreatic cells.3
Peripheral pancytopenia is defined as a reduction in all three major constituents of the blood to levels below their lower normal range, manifesting as anemia, leucopenia, and thrombocytopenia all occurring at the same time. Cytopenia, on the other hand, is a decline in one of the produced elements.4 DM impacts the erythropoiesis system by inducing a hyperglycemic situation that causes protein glycation. Hence, cytopenia can result in this case. Of the different types of cytopenia, anemia is the most common type of disorder in T2DM. Anemia can be caused by a decrease in erythropoietin production, which is linked to microvascular complications and renal impairment, systemic inflammation that affects iron metabolism, and the release of multiple inflammatory cytokines and free radicals that increase hepcidin (which leads to ferroportin degradation and iron deficiency anemia), and the ferroportin degradation leads to a blockage of duodenal iron transfer.5–9 Besides, red blood cell (RBC) count has been found to be associated with microvascular and macrovascular complications, which negatively affect the circulatory system. All of these changes will have an impact on blood cell count, particularly RBC count.10
White blood cells (WBC) and platelet counts are affected by T2DM and its medications.11 Immune-based pathways are the most common cause of thrombocytopenia, which is linked to nonspecific immune complexes binding to platelets, as seen in autoimmune disorders.12 Besides, the microvascular and macrovascular complications are associated with the dysfunction of platelets.13 The WBC is considered an inflammation marker in different chronic diseases.14 An increased WBC count has been linked to macro and microvascular complications, activation of glycation end products, oxidative stress, and angiotensin II as a result of hyperglycemia, as well as the production of factors like tumor necrosis factor and interleukin 1 that are involved in the pathogenesis of chronic diabetes complications.15,16
In Ethiopia, DM is estimated to affect half a million people.5 Even though previous studies in Ethiopia had primarily focused on the prevalence of anemia and its associated factors, the goal of this study is to determine the prevalence of and associated factors of cytopenia in T2DM, which had not been addressed in previous studies.
Materials and Methods
Study Setting, Population, and Period
A cross-sectional study was conducted from March to May 2021 on a total of 357 T2DM patients attending at the University of Gondar Comprehensive Specialized Hospital chronic illness clinic. All adult T2DM study participants that are on follow-up in the chronic illness clinic of the hospital were considered as a source of the population. All adult T2DM study participants who were on follow-up at the hospital chronic illness clinic during the study period and volunteered to participate in the study were included in the study population. The study participants were recruited by using a simple random sampling technique.
Inclusion and Exclusion Criteria
All adult study participants who were diagnosed with T2DM and who were willing to participate were conveniently included in the study. Participants with severe bleeding, patients undergoing current surgery, cancer, hyperthyroidism, and severely ill patients unable to consent were excluded from this study.
Study Variables
The magnitude of hematological abnormalities was considered as an outcome variable and socio-demographic characteristics (gender, age, residence, educational level, occupation, marital status, religion) and clinical and behavioral characteristics (duration of DM, duration of antidiabetic drug use, malaria infection, history of chronic illness, estrogen-containing oral contraceptives like choice and style (for female patients), hypertension, body mass index (BMI), physical exercise habit, habitual cigarette smoking, history of alcohol consumption, and using traditional medicine were considered as the independent variables.
Operational Definitions
Cytopenia: was defined as a reduction in either RBCs, WBCs, or platelets.17
Bi-cytopenia was defined as two of the three lineage cell counts falling below the levels.18
BMI: was calculated as weight in kg divided by height in m2 and the result is categorized as the following: severely underweight, underweight, normal, overweight, and obese were considered when the BMI is < 16.5, < 18.5, 18.5–24.9, 25–29.9, and ≥ 30, respectively.19
Alcohol consumption: was defined as the act of ingesting (typically orally) a drink containing ethanol or ethyl alcohol.20
Data Collection Procedure
Sociodemographic Data Collection
After obtaining an informed written consent, a structured and pretested questionnaire was used to collect sociodemographic and behavioral data from eligible study participants. The questionnaire was used to conduct face-to-face interviews with the participants. The questionnaire has two parts. The first part of the questionnaire consists of socio-demographic characteristics of study participants, and the second part consists of questions regarding the behavioral information of study participants. The sociodemographic data were gathered through the use of questionnaires and face-to-face interviews conducted by trained nurses working in the chronic illness clinic of the hospital.
Clinical and Behavioral Data Collection
Clinical data including duration of DM, duration of antidiabetic drug intake, blood pressure, and other related data were collected by trained nurses from the patient’s medical chart using data collection sheets. The weight and height of the study participants were measured and were used for the calculation of BMI. The BMI is calculated after careful measurement of the weight and height of the study participants.
Blood Sample Collection
A total of 3 milliliters of venous blood samples were collected using the vacutainer method. The blood was dispensed into a tube containing tri-potassium Ethylene Diamine Tetraacetic Acid (EDTA). The blood sample was used for the CBC examination. The blood sample was collected aseptically by strictly following standard operating procedures.
Complete Blood Cell Count Determination
The Beckman Coulter UniCel DxH 800 fully automated hematology analyzer was used to determine the results. The coulter principle is used in the DxH 800 CBC analysis, which is based on a suspension of blood cells being passed through a small orifice simultaneously with an electric current. Individual blood cells passing through the orifice introduce an impedance change in the orifice determined by the size of the cell. The system counts the individual cells and provides a cell size distribution. The coulter volume, conductivity, and scatter established WBC differential technology using three measurements: individual cell volume, high-frequency conductivity, and laser-light scatter.
Data Quality Control Measures
The sociodemographic, behavioral, and clinical data quality was maintained by preparing the questionnaire in English and translating it to the local language (Amharic), then converting it back to English to examine the uniformity of the data collection tools. The pretest of the questionnaire was performed among 5% of the total participants. All study participants were notified about the objective and importance of the study before the data collection. The gathered data was inspected daily for consistency and accuracy. Further, the laboratory data quality control measures were kept by maintaining the sample’s quality by removing hemolysis, clotting, blood to anticoagulant proportion, and delaying the laboratory analysis. Besides, to remove the hemolysis, the blood was dispensed to the wall of the EDTA test tube. Furthermore, any reagents that are used for CBC, peripheral morphology, and blood film were checked for expiration dates and prepared in compliance with the manufacturer’s guidelines. The commercial Beckman Coulter UniCel® DxH 800 hematology cell controls (low, normal, and high) were run on a daily basis before the samples were examined. The quality of the smear and Giemsa stain were checked using known malaria positive and negative slides.
Data Processing and Analyzing
The information was coded, entered into Epi Info version 7.2.4.0, and then transferred to SPSS version 25 for analysis. Descriptive statistics were used to summarize the characteristics of the study participants. The normality of the data was checked by the Shapiro–Wilk test. Univariate and multivariate logistic regression analyses were used to determine factors associated with cytopenia in T2DM. The odds ratio, with its 95% confidence interval, was used to determine the strength of the association between the independent variable and the outcome variable. Variables with a p-value of less than 0.25 on the univariate analysis were entered into a multivariate logistic regression analysis. A p-value of less than 0.05 was considered statistically significant.
Ethical Considerations
The current study was carried out in accordance with the Helsinki Declaration. Besides, an ethical clearance was delivered by the Ethical Review Committee of the School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, the University of Gondar (Ref. No. SBLS/2746/2021). A permission letter was obtained from the university of Gondar comprehensive specialized hospital chief executive clinical director. An informed written consent was obtained from each patient, and the findings were kept confidentially. The confidentiality of the data was protected by only using codes for specimens and results, and no personal identification was used. In the event of abnormal results, their medical doctors were notified so that they could receive appropriate treatment.
Results
Sociodemographic Characteristics
A total of 357 T2DM patients were enrolled in the current study. Of those, 186 (52.1%) and 288 (80.7%) were females and urban residents, respectively. The mean age of the study participants was 54±12 years old and ranged from 23 to 87 years old (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Participants in the 2020 University of Gondar Comprehensive and Specialized Hospital Chronic Illness Clinic |
Clinical and Behavioral Characteristics
About 43.7% (156/357) of the study participants had a history of chronic disease. Besides, 99.7% (356/357) of the study participants were taking antidiabetic drugs. Furthermore, the number of patients with hypertension was 216 (60.5%). About 42.0% (150/357), 20.7% (74/357), and 44.5% (159/357) have a physical exercise habit, are alcohol drinkers, and have abnormal BMI, respectively (Table 2).
 |
Table 2 Clinical and Behavioral Characteristics of Participants in 2020 the University of Gondar Comprehensive and Specialized Hospital Chronic Illness Clinic |
Magnitude of Hematological Abnormalities
The magnitudes of anemia, leucopenia, and thrombocytopenia were 8.7% (95% CI: 6.18, 12.06), 10.9% (95% CI: 8.09, 14.59), and 5.3% (95% CI: 3.43, 8.16), respectively. Instead, leukocytosis, thrombocytosis, neutrophilia, and lymphocytosis were 4.8% (95% CI: 2.99, 7.49), 1.1% (95% CI: 0.44, 2.85), 4.8% (95% CI: 2.99, 7.49), and 5.3% (95% CI: 3.43, 8.16), respectively (Table 3). The magnitude of cytopenia, bicytopenia, and pancytopenia were 21% (95% CI: 17.1, 25.53), 1.1% (95% CI: 0.44, 2.85), and 0.56% (95% CI: 0.01, 1.12), respectively (Table 3 and Figure 1).
 |
Table 3 The Magnitude of Hematological Abnormality Among Participants in the 2020 University of Gondar Comprehensive and Specialized Hospital Chronic Illness Clinic |
 |
Figure 1 The magnitude of cytopenia among participants in the 2020 University of Gondar comprehensive and specialized hospital chronic illness clinic. |
Factors Associated with Cytopenia and Anemia
Bivariate and multivariate analysis were performed to identify the association of the independent variable with the occurrence of cytopenia. In bivariate analysis, gender, age in years, residence, educational level, occupation, physical exercise, and alcohol consumption habits were associated with cytopenia, but in multivariate analysis, none of the variables was associated with cytopenia. However, the multivariate analysis of anemia demonstrated that being male (AOR: 3.23, 95% CI: 1.43, 7.56), reducing physical exercise (AOR: 2.70, 95% CI: 1.137, 6.43), and never married (AOR: 3.90, 95% CI: 1.248, 12.18) were significantly associated with anemia (Tables 4 and 5).
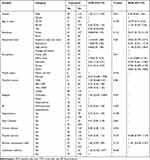 |
Table 4 Factors Associated with Cytopenia Among Participants in the 2020 University of Gondar Comprehensive and Specialized Hospital Chronic Illness Clinic |
 |
Table 5 Factors Associated with Anemia Among Study Participants |
Discussion
Diabetes is associated with peripheral cytopenia, which plays a crucial role in diabetes-related micro and macrovascular complications. As a result, the goal of this study was to assess the degree of cytopenia in T2DM. Anemia was identified in 8.7% (95% CI: 7.44, 9.92) of the T2DM patients in the current study. The current study confirmed that anemia is a mild public health problem among T2DM in the study area, according to the WHO classification of anemia’s public health relevance in populations.21 The reason for the mild anemia prevalence in T2DM patients is that patients with DM have systemic inflammation, inhibition of Epo release, damage to the renal interstitium, severe symptomatic autonomic neuropathy causing efferent sympathetic denervation of the kidney, drugs, altered iron metabolism, and hyperglycemia are some of the factors that lead to the earlier onset of anemia in DM patients.22 Additionally, anemia in DM patients is linked to older age, worsening renal function, peripheral vascular disease, lower weight or BMI, lower diastolic blood pressure, polypharmacy, and longer duration of T2DM, as well as not utilizing an angiotensin converting enzyme inhibitor.23,24
The finding of this study was similar to a previous study in Gondar by Kebede et al where the magnitude of anemia was 8.06%.25 However, it is lower than studies conducted in Debre Berhan (20.1%)26 and Debre Tabor (29.81%)27 that reported a higher magnitude of anemia than the current study. In general, a systematic review and meta-analysis conducted by Adane et al showed that the overall magnitude of anemia was 31.12% (95% CI; 9.66, 52.58) in DM patients.28 The discrepancy might be as a result of variations in the type of DM. The present study included T2DM patients only. This is in line with the fact that T2DM patients are more vulnerable to various forms of both short-term and long-term complications due to the commonness of this type of DM, its insidious onset, and late recognition, especially in developing countries.29 Anemia has a major negative impact on DM patients’ quality of life and is linked to disease progression and the development of comorbidities. It’s linked to a quick deterioration in renal function and a greater requirement for renal replacement therapy, which is frequently unavailable or costly in most underdeveloped nations, including Ethiopia.30 Anemia in DM patients is harmful to their health since it increases the risk of cardiovascular disease and hypoxia-induced end-organ damage such as diabetic retinopathy, nephropathy, and foot ulcers.31
The result of the logistic regression showed that males are 3 times more anemic as compared to females. The findings of the study are similar to those of studies conducted in India32 and Ethiopia.33 This might be explained by the fact that low testosterone levels and hypo-gonadotropic hypogonadism are common in male DM patients. Since testosterone stimulates erythropoiesis, low testosterone levels may contribute to anemia in male patients.34 Moreover, T2DM patients with reduced or lack of physical exercise are 2.70 times more at risk of anemia than their counterparts. Notably, in T2DM subjects, reduced exercise capacity appears to be a predictor of all-cause mortality.35 During exercise, optimal oxygen delivery and utilization imply a delicate interplay of multiple physiological functions, such as pulmonary ventilation, gas exchange, cardiac output, muscle blood distribution and diffusion, skeletal muscle aerobic and force-generating capacity, as well as fatigue perception.36 According to the findings of this study, the never married participants are also 3.9 times more anemic than their counterparts.
Leukopenia and leukocytosis were detected in 10.9% (95% CI: 8.09, 14.59) and 4.8% (95% CI: 2.99, 7.49) of T2DM patients, respectively. Our study is in consonance with a report by Arkew et al in which 3.7% (95% CI: 2.46, 4.95) of DM patients developed leukocytosis.37 Total peripheral WBC count, a nonspecific marker of inflammation, has been suggested to be associated with diabetes risk. The influence of hyperglycemia and the pathophysiology of T2DM may explain the mechanism driving the increase in total and differential WBC counts in T2DM patients. In T2DM patients, chronic inflammation, as shown by a higher WBC count, is linked to microvascular problems. In DM, impaired WBC function is also reported.38
Thrombocytopenia was found in 5.3% (95% CI: 3.43, 8.16) of T2DM patients. While 1.1% of the participants had thrombocytosis. By mediating the first phase of hemostasis, blood platelets play a critical role in the clotting process. Platelets in diabetic individuals have dysregulated signaling pathways, resulting in an increased tendency to activate and aggregate in response to a stimulus. Increased clotting, poor clot breakdown, and endothelial dysfunction are all linked to platelet abnormalities.39,40 Platelets in patients with diabetes are reported to exhibit hyper-reactivity to sub-threshold stimuli and undergo rapid consumption, resulting in accelerated thrombopoiesis of more reactive platelets.41 The enhanced platelet reactivity seen in diabetic individuals has been linked to a number of processes. These mechanisms include hyperglycemia, insulin deficiency and resistance, associated metabolic conditions (obesity, dyslipidemia, and increased systemic inflammation), and other cellular abnormalities.42 Acute hyperglycemia results in increased platelet activation, as documented by elevated levels of surface adhesion molecules such as P-selectin and soluble markers of platelet activation.43,44
The magnitude of cytopenia, bicytopenia, and pancytopenia were 21% (95% CI: 17.1, 25.53), 1.1% (95% CI: 0.44, 2.85), and 0.56% (95% CI: 0.01, 1.12), respectively. Hyperglycemia in T2DM and/or its treatment may cause a reduction in RBC, WBC, and platelets. However, we did not get enough available literature to evaluate our findings. In addition to this, none of the analyzed variables were associated with cytopenia. Therefore, it paves a path for researchers to investigate the magnitude of cytopenia and its contributing factors in T2DM patients in large cohorts of participants.
Conclusion and Recommendation
This study showed that T2DM causes disturbances in the hematological parameters and leads to a mild level of cytopenia. It is, therefore, suggested that hematological abnormalities, especially cytopenia, should be monitored and controlled on a regular basis in T2DM patients for better prognosis and quality of life.
Abbreviations
AOR, Adjusted Odds Ratio; BMI, Body Mass Index; BP, Blood Pressure; CBC, Complete Blood Count; COR, Crude Odds Ratio; DM, Diabetes Mellitus; EDTA, Ethylene Diamine Tetra acetic Acid; RBC, red blood cell; T2DM, Type II Diabetes Mellitus; WBC, White blood cells.
Data Sharing Statement
All the data supporting these findings is contained within the manuscript.
Acknowledgment
First of all, we acknowledge the University of Gondar comprehensive and specialized hospital administrative office for their willingness during the data collection. Furthermore, we would like to extend our gratitude to the study participants for their voluntary and cooperative participation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflicts of interest regarding the publication of this manuscript.
References
1. Milosevic D, Panin VL. Relationship between hematological parameters and glycemic control in type 2 diabetes mellitus patients. J Med Biochem. 2019;38(2):164. doi:10.2478/jomb-2018-0021
2. Panda AK, Ambade RA. Prevalence of anemia and its correlation with HBA1c of patients in type-II diabetes mellitus: a pilot study. National J Physiol Pharm Pharmacol. 2018;8(10):1409–1413. doi:10.5455/njppp.2018.8.0621511072018
3. Antwi-Baffour S, Kyeremeh R, Boateng SO, Annison L, Seidu MA. Haematological parameters and lipid profile abnormalities among patients with Type-2 diabetes mellitus in Ghana. Lipids Health Dis. 2018;17(1):1–9. doi:10.1186/s12944-018-0926-y
4. Jain A, Naniwadekar M. An etiological reappraisal of pancytopenia-largest series reported to date from a single tertiary care teaching hospital. BMC Blood Disord. 2013;13(1):1–9. doi:10.1186/2052-1839-13-10
5. Atlaw D, Tariku Z. Magnitude and factors associated with anemia among diabetic patients in Ethiopia: a systematic review and meta-analysis. SAGE Open Med. 2021;9:20503121211031126. doi:10.1177/20503121211031126
6. Thomas MC, MacIsaac RJ, Tsalamandris C, Power D, Jerums G. Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care. 2003;26(4):1164–1169. doi:10.2337/diacare.26.4.1164
7. Greenburg AG. Pathophysiology of anemia. Am J Med. 1996;101(2):7S–11S. doi:10.1016/S0002-9343(96)00161-1
8. Al-Salman M. Anemia in patients with diabetes mellitus: prevalence and progression. General Med. 2015;1:1–4.
9. Barbieri J, Fontela PC, Winkelmann ER, et al. Anemia in patients with type 2 diabetes mellitus. Anemia. 2015;2015:548.
10. Alamri B, Bahabri A, Aldereihim A, et al. Hyperglycemia effect on red blood cells indices. Eur Rev Med Pharmacol Sci. 2019;23(5):2139–2150. doi:10.26355/eurrev_201903_17259
11. Digman C, Klein AK, Pittas AG. Leukopenia and thrombocytopenia caused by thiazolidinediones. Ann Intern Med. 2005;143(6):465–466. doi:10.7326/0003-4819-143-6-200509200-00016
12. Shimizu H. Thrombocytopenia in insulin-dependent diabetes mellitus. KITAKANTO Med J. 1998;48(4):283–285. doi:10.2974/kmj.48.283
13. Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24(8):1476–1485. doi:10.2337/diacare.24.8.1476
14. Park J-M, Lee HS, Park J-Y, Jung D-H, Lee J-W. White blood cell count as a predictor of incident type 2 diabetes mellitus among non-obese adults: a longitudinal 10-year analysis of the Korean genome and epidemiology study. J Inflamm Res. 2021;14:1235. doi:10.2147/JIR.S300026
15. Moradi S, Kerman SRJ, Rohani F, Salari F. Association between diabetes complications and leukocyte counts in Iranian patients. J Inflamm Res. 2012;5:7. doi:10.2147/JIR.S26917
16. Biadgo B, Melku M, Abebe SM, Abebe M. Hematological indices and their correlation with fasting blood glucose level and anthropometric measurements in type 2 diabetes mellitus patients in Gondar, Northwest Ethiopia. Diabetes Metab Syndrome Obesity. 2016;9:91. doi:10.2147/DMSO.S97563
17. Naseem S, Varma N, Das R, Ahluwalia J, Sachdeva MUS, Marwaha RK. Pediatric patients with bicytopenia/pancytopenia: review of etiologies and clinico-hematological profile at a tertiary center. Indian J Pathol Microbiol. 2011;54(1):75. doi:10.4103/0377-4929.77329
18. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi:10.1016/S2213-2600(20)30079-5
19. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 201-2016. JAMA. 2018;319(16):1723–1725. doi:10.1001/jama.2018.3060
20. Collins SE, Kirouac M. Alcohol Consumption. In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine. New York: Springer New York; 2013:61–65.
21. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(suppl_1):s5–s10.
22. Craig KJ, Williams JD, Riley SG, et al. Anemia and diabetes in the absence of nephropathy. Diabetes Care. 2005;28(5):1118–1123. doi:10.2337/diacare.28.5.1118
23. Thomas MC, MacIsaac RJ, Tsalamandris C, et al. The burden of anaemia in type 2 diabetes and the role of nephropathy: a cross-sectional audit. Nephrol Dialysis Transplantation. 2004;19(7):1792–1797. doi:10.1093/ndt/gfh248
24. Al-Khoury S, Afzali B, Shah N, Covic A, Thomas S, Goldsmith D. Anaemia in diabetic patients with chronic kidney disease—prevalence and predictors. Diabetologia. 2006;49(6):1183–1189. doi:10.1007/s00125-006-0254-z
25. Kebede SA, Tusa BS, Weldesenbet AB. Prevalence of anaemia and its associated factors among type 2 diabetes mellitus patients in University of Gondar comprehensive specialized hospital. Anemia. 2021;2021:154.
26. Taderegew MM, Gebremariam T, Tareke AA, Woldeamanuel GG. Anemia and its associated factors among type 2 diabetes mellitus patients attending Debre Berhan Referral Hospital, North-East Ethiopia: a cross-sectional study. J Blood Med. 2020;11:47. doi:10.2147/JBM.S243234
27. Engidaw MT, Feyisa MS. Prevalence of anemia and its associated factors among adult diabetes mellitus patients at Debre Tabor General Hospital, Northcentral Ethiopia. Diabetes Metab Syndrome Obesity. 2020;13:5017. doi:10.2147/DMSO.S286365
28. Adane T, Getawa S. Anaemia and its associated factors among diabetes mellitus patients in Ethiopia: a systematic review and meta‐analysis. Endocrinol Diabetes Metab. 2021;4(3):e00260. doi:10.1002/edm2.260
29. Mbanya JCN, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-saharan Africa. lancet. 2010;375(9733):2254–2266. doi:10.1016/S0140-6736(10)60550-8
30. Tong PC, Kong AP, So W-Y, et al. Hematocrit, independent of chronic kidney disease, predicts adverse cardiovascular outcomes in Chinese patients with type 2 diabetes. Diabetes Care. 2006;29(11):2439–2444. doi:10.2337/dc06-0887
31. Zoppini G, Targher G, Chonchol M, et al. Anaemia, independent of chronic kidney disease, predicts all-cause and cardiovascular mortality in type 2 diabetic patients. Atherosclerosis. 2010;210(2):575–580. doi:10.1016/j.atherosclerosis.2009.12.008
32. Bharathi K. Study of hematological profile and its significance in type 2 diabetes mellitus patients. J Diagn Pathol Oncol. 2016;1:14–17.
33. Adane T, Getaneh Z, Asrie F. Red blood cell parameters and their correlation with renal function tests among diabetes mellitus patients: a comparative cross-sectional study. Diabetes Metab Syndrome Obesity. 2020;13:3937. doi:10.2147/DMSO.S275392
34. Grossmann M, Panagiotopolous S, Sharpe K, et al. Low testosterone and anaemia in men with type 2 diabetes. Clin Endocrinol (Oxf). 2009;70(4):547–553. doi:10.1111/j.1365-2265.2008.03357.x
35. Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165(18):2114–2120. doi:10.1001/archinte.165.18.2114
36. Nesti L, Pugliese NR, Sciuto P, Natali A. Type 2 diabetes and reduced exercise tolerance: a review of the literature through an integrated physiology approach. Cardiovasc Diabetol. 2020;19(1):1–17. doi:10.1186/s12933-020-01109-1
37. Arkew M, Yemane T, Mengistu Y, Gemechu K, Tesfaye G. Hematological parameters of type 2 diabetic adult patients at Debre Berhan Referral Hospital, Northeast Ethiopia: a comparative cross-sectional study. PLoS One. 2021;16(6):e0253286. doi:10.1371/journal.pone.0253286
38. Tong PC, Lee K-F, So W-Y, et al. White blood cell count is associated with macro-and microvascular complications in Chinese patients with type 2 diabetes. Diabetes Care. 2004;27(1):216–222. doi:10.2337/diacare.27.1.216
39. Kakouros N, Rade JJ, Kourliouros A, Resar JR. Platelet function in patients with diabetes mellitus: from a theoretical to a practical perspective. Int J Endocrinol. 2011;2011:487.
40. Ferreiro JL, Gómez-Hospital JA, Angiolillo DJ. Platelet abnormalities in diabetes mellitus. Diabetes Vascular Dis Res. 2010;7(4):251–259. doi:10.1177/1479164110383994
41. Santilli F, Simeone P, Liani R. The Role of Platelets in Diabetes Mellitus. Platelets: Elsevier; 2019:469–503.
42. Adane T, Asrie F, Getaneh Z, Getawa S. White blood cells and platelet profiles of diabetic patients at University of Gondar specialized referral hospital: a comparative cross‐sectional study. J Clin Lab Anal. 2021;35(6):e23808. doi:10.1002/jcla.23808
43. Yngen M, Östenson C, Li N, Hjemdahl P, Wallen N. Acute hyperglycemia increases soluble P-selectin in male patients with mild diabetes mellitus. Blood Coagulation Fibrinolysis. 2001;12(2):109–116. doi:10.1097/00001721-200103000-00004
44. Undas A, Wiek I, Stêpien E, Zmudka K, Tracz W. Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes Care. 2008;31(8):1590–1595. doi:10.2337/dc08-0282
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
