Back to Journals » OncoTargets and Therapy » Volume 8
Percutaneous CT-guided microwave ablation as maintenance after first-line treatment for patients with advanced NSCLC
Authors Ni X, Han J, Ye X, Wei Z
Received 15 June 2015
Accepted for publication 22 September 2015
Published 3 November 2015 Volume 2015:8 Pages 3227—3235
DOI https://doi.org/10.2147/OTT.S90528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Faris Farassati
Xiang Ni, Jun-Qing Han, Xin Ye, Zhi-Gang Wei
Department of Oncology, Shandong Provincial Hospital affiliated to Shandong University, Jinan, People’s Republic of China
Background: Systemic therapy is recommended for advanced non-small-cell lung cancer (NSCLC). However, conventional first-line treatment has generated a plateau in response rate of 25% to 35%. Few studies have shown patients benefit from microwave ablation (MWA) in combination with radiotherapy and chemotherapy. This study aims to evaluate safety and efficacy of percutaneous computed tomography-guided MWA as maintenance after first-line treatment for patients with advanced NSCLC.
Methods: Patients with histologically verified NSCLC stage IIIB or IV between January 2010 and March 2014 were involved. After completion of first-line treatment with partial response or stable disease, 35 patients with 39 tumors underwent 39 MWA procedures. Complications, progression-free survival (PFS), overall survival (OS), and correlated predictors were analyzed.
Results: During a median follow-up of 17.7 months and 10.8 months after initial MWA, local efficacy was 87.2%, median MWA-related local control time was 10.6 months, and tumor size was the only predictor (P=0.002). Median MWA-related PFS, MWA-related OS, PFS, and OS were 5.4, 10.6, 11.8 and 17.7 months, respectively. Local efficacy was significantly correlated with MWA-related PFS (P=0.003), MWA-related OS (P=0.000), and OS (P=0.001). There were no procedure-specific deaths. Total incidence of major complications was 12.8%, including pneumothorax resolved by closed pleural drainage and pneumonia controlled by antibiotics in a short time.
Conclusion: This study concluded two points, including: 1) patients benefited from MWA as maintenance both in local control and survival; 2) as maintenance MWA was superior to conventional maintenance therapy with improved survival and well-tolerated complications. Therefore, MWA was a safe and effective maintenance after first-line treatment in patients with advanced NSCLC.
Keywords: non-small-cell lung cancer, CT-guided microwave ablation, progression-free survival, overall survival
Introduction
Lung cancer is the most common cause of death from cancer worldwide, with an incidence of approximately 1.8 million and mortality of 1.59 million each year.1 Only 16.8% of all lung cancer patients survive more than 5 years after the lung cancer was diagnosed.2 This dismal outcome is mainly because only 15% of patients are initially diagnosed at an early stage without severe comorbidity, thus are eligible for radical surgery.3 Non-small-cell lung cancer (NSCLC) accounts for 89% of lung cancer cases.4 Systemic therapy is recommended for advanced NSCLC according to National Comprehensive Cancer Network guidelines.5 However, conventional first-line treatment has generated a plateau in response rate of 25%–35%, median progression-free survival (PFS) of 3–5 months, and overall survival (OS) of 8–10 months; 1-year and 2-year survival rates are 30%–40% and 10%–15%, respectively.6–8 Considerable progress has been made in the treatment field. Chemotherapy can be optimized by adding bevacizumab to improve PFS and OS in patients with non-squamous carcinoma.9,10 EGFR TKI11–14 and ALK inhibitors15,16 have dramatically improved the response rate, PFS, and OS. However, most patients progress during first-line treatment, and even those responsive or stable to first-line treatment will progress inevitably during the following close observation. Maintenance therapy is another option. Bevacizumab,17 pemetrexed,18,19 gemcitabine,20,21 and erlotinib20,22 administered as maintenance therapy have increased survival. However, maintenance therapy is appropriate for select patients depending on several factors, such as toxicity and intolerance, histologic subtype and genotypes, and limited benefit is not superior to second-line treatment initiated at the time of disease progression.
Energy-based tumor ablation consists of the direct application of thermal and non-thermal therapies to eradicate or substantially destroy focal tumors.23 Microwave ablation (MWA) induces tumor cells’ coagulation through high-temperature thermal injury using electromagnetic devices with frequencies from 300 MHz to 300 GHz.23 Percutaneous image-guided MWA is a minimally invasive therapeutic modality that has been recently proven to be safe and effective in lung cancer.24–30 Furthermore, its efficacy is not affected by histologic subtype or genotypes theoretically. It was hypothesized that MWA may further eliminate residual tumors and decrease local recurrence after first-line treatment, thus prolonging survival and improving quality of life in a further step. Few studies have shown patients benefit from MWA in combination with radiotherapy31 and chemotherapy.32 For the first time, we retrospectively evaluated the safety and efficacy of percutaneous computed tomography (CT)-guided MWA as maintenance after first-line treatment for patients with advanced NSCLC and attempted to identify correlated predictors.
Materials and methods
Patients
The Institutional Review Board of Shandong Provincial Hospital affiliated to Shandong University granted the ethics approval for this study. A multidisciplinary team (MDT) comprised of surgeons, physicians, interventional oncologists, radiologists, pathologists, pharmacists, and anesthetists provided objective and reliable advice for each patient based on a risk–benefit analysis. Written informed consent was obtained from each patient before treatment.
Inclusion and exclusion criteria
Thirty-five patients with histologically verified advanced (stage IIIB or IV) NSCLC were enrolled between January 2010 and March 2014, including those with recurrence and/or metastasis to advanced stage after initial radical surgery. After completion of four or six cycles of first-line platinum-doublet systemic chemotherapy or targeted therapy (depending on the histologic subtype and genotypes) or concurrent chemo-radiation followed by chemotherapy (merely applied to stage IIIB), patients with partial response or stable disease33 were introduced to MWA based on MDT consultation. Other inclusion criteria were as follows: age ≥18 years, Eastern Cooperative Oncology Group performance status ≤2, at least one measurable lung tumor, well-controlled symptomatic distant metastases in brain or bone by radiotherapy, and adequate hemostatic function. Exclusion criteria were as follows: a life expectancy shorter than 3 months, and the occurrence of other malignancy within the past 5 years (Table 1).
Course of treatment
First-line treatment
1) Chemotherapy regimens were all platinum-doublet regimens that included cisplatin (75 mg/m2 intravenous [IV] on day 1) or carboplatin (with an area under curve of 5–6 on day 1) plus vinorelbine (25 mg/m2 IV on days 1 and 8, n=1), gemcitabine (1,250 mg/m2 IV on days 1 and 8, which is employed as a preferred treatment option for squamous cell carcinoma, n=10), docetaxel (75 mg/m2 IV on day 1, n=12), or pemetrexed (500 mg/m2 IV on day 1, which is employed as a preferred treatment option for non-squamous cell carcinoma, n=8). All chemotherapy regimens were repeated every 3 weeks for four or six cycles. 2) EGFR-TKIs were administered to patients with definite sensitizing mutations (n=5). 3) Concurrent chemo-radiation was administered at a total dose of 60–70 Gy in 1.8–2.0 Gy fractions (n=7), followed by chemotherapy as described earlier in this paragraph.
MWA procedure
Microwave generator (KY-2450B; Kangyou Microwave Institute, Nanjing, People’s Republic of China) with a frequency of 2,450±50 MHz and continuously adjustable output power of 0–100 W was used. The microwave antenna has an effective length of 100–180 mm, an active tip of 15 mm, and an outside diameter of 14–18 gauge, with a dipole design and a water circulation cooling system using normal saline.
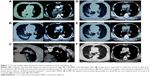
All patients fasted for 4 hours before and after the MWA procedure. The MWA procedure was performed by two experienced interventional oncologists, a radiology technician, and a nurse. Local anesthesia and preemptive analgesia34 were performed with an electrocardiogram monitor. CT (light speed 64V spiral; GE Healthcare, Milwaukee, WI, USA) was used for guidance and assessment. The plan was based on the following: 1) the location, number, size, and shape of the tumors; and 2) the position in relation to nearby critical structures, especially large blood vessels that might be at risk of injury. The antenna was placed into the deepest margin of the tumor with a well-fixed position. Given that MWA with an output power of 60–80 W has a spheroidal ablation zone of 3.5×3×3 cm3, a single antenna’s insertion was used for small tumors (≤3 cm), multiple insertions of a single antenna were applied for intermediate tumors (between 3–5 cm), and multiple antennas inserted simultaneously (at a distance of 10 mm from each other) were used for large tumors (>5 cm); therefore, multiple overlaps achieved a large or conformal ablation zone.35 The antenna was modified and repositioned according to imaging changes until the tumor was covered completely (plus an ablative margin of at least 5 mm, and ideally 10 mm around the tumor) or the planned imaging end point has been reached.23 At the end of each procedure, tract ablation was performed to prevent tumor seeding or tract bleeding before the antenna was removed. A CT scan immediately after the procedure was performed to assess the treatment response as well as to demonstrate immediate complications, such as pneumothorax and hemorrhage (Figure 1A to F).
Follow-up and treatment assessment
The efficacy of chemotherapy was assessed every two cycles. The efficacy of targeted therapy and radiotherapy was assessed every month. Assessment was based on the revised Response Evaluation Criteria in Solid Tumors.33
As a successful ablation zone was usually larger than the target tumor in a short time and with benign enhancement,25 Response Evaluation Criteria in Solid Tumors could not assess ablation correctly; therefore, MWA was assessed by Image-guided Tumor Ablation Standardization of Terminology and Reporting Criteria.23 The 1-month post-ablation contrast-enhanced three-phase CT was used as the new baseline imaging for further assessments,23,36–38 thereafter, CT was performed every 3 months. The response to initial MWA was classified as complete ablation and incomplete ablation.23 Additional MWA was performed for patients with incomplete ablation. The technical efficacy (local efficacy) demonstrated that macroscopic tumors were completely ablated with safe margins or symptoms (mainly pain) were successfully relieved. If the above end point could not be achieved within the window of either four procedures or 3 months, it was classified as a technical failure.23 Then, other therapies were administered instead of additional MWA based on the MDT consultation.
Complications were reported according to the Common Terminology Criteria for Adverse Events v4.03 of the National Cancer Institute.37 As multiple procedures increased the likelihood of complications, complications were analyzed on a per-procedure basis. The procedure-specific death was reported on a per-patient basis. A major complication was defined as an event that led to substantial morbidity and disability that increased the level of care, or resulted in substantially lengthened hospitalization. All the other complications were defined as minor. Side effects were expected and undesired but never resulted in the consequences mentioned earlier.
Statistical analysis
Time interval was calculated from the best overall response to initial MWA procedure. MWA-related local control time was calculated from the complete ablation to local tumor progression. PFS was calculated from diagnosis until progression. OS was calculated from diagnosis to death. As the entire course of treatment may be comprised of more than one MWA procedure, MWA-related PFS and OS were calculated from the initial MWA procedure rather than complete ablation.23 Kaplan–Meier analysis was applied for the assessment of survival. Student’s t-test was performed to identify the predictors for local efficacy. Log-rank analysis was performed to identify the predictors for survival. All analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) two-sided analysis and P<0.05 was considered statistically significant.
Results
Nineteen patients (54.3%) were assessed as having partial response to first-line treatment, while 16 patients (45.7%) were assessed as having stable disease. A total of 35 patients underwent 39 MWA procedures for 39 tumors. Three procedures consisted of two tumors’ ablations simultaneously; three patients underwent additional procedures for three tumors. All 39 procedures were performed according to protocol, and 100% were considered to be technically successful. Twenty-six procedures were performed with single antenna, while 13 procedures were performed with double antennas. The median power and duration of MWA was 70 W (range, 60–75 W) and 7 minutes (range, 3–15 minutes). The median time interval between best overall responses and initial MWA procedure was 1 month (range, 7 days to 22 months).
Complications and side effects of MWA
No death occurred during the procedure or within 30 days. Major complications were infrequent with a total incidence of 12.8%, these included symptomatic pneumothorax, bronchial fistula and pneumonia requiring intervention. Pain graded 3 was due to tumor invasion of the thoracic wall during palliative procedures, as pain did not worsen after these procedures, they should be considered as uncontrolled diseases rather than major complications.
Minor complications were common with a total incidence of 38.5%, these included asymptomatic or mild pneumothorax, pleural effusion, and hemorrhage. Side effects were common with a total incidence of 59%, these included pain graded 1–2 and post-ablation syndrome. Both minor complications and side effects were self-limited, well-tolerated or easily resolved (Table 2).
Local efficacy
At 1-month follow-up after initial MWA, 32 tumors (82.1%) were assessed to be completely ablated, and seven tumors (17.9%) were incompletely ablated. Of the incompletely ablated tumors, six were treated with palliative intent (pain relief), two were treated with additional MWA, however, only one was successfully treated finally; the other incompletely ablated tumor was treated with additional MWA and was complete ablated finally. Therefore, the total local efficacy was 87.2% (34/39 tumors).
Tumor size (maximum diameter at initial MWA, cut off =5 cm) was found to be significantly correlated with local efficacy (P=0.002).
Survival
The median follow-up was 17.7 months (range, 6–45 months), while the median follow-up after the initial MWA was 10.8 months (range, 3–36 months). At the last follow-up, among the 34 “local efficacy” tumors, five (14.7%) were considered as local progression, and the median MWA-related local control time was 10.6 months (range, 2.4–35.3 months). No significant prognostic factors were found to be correlated with MWA-related local control time, and there was no significant difference between subgroup sizes small (≤3 cm) and intermediate (3–5 cm) (P=0.902).
Among the total 35 patients, 25 of them (71.4%) experienced progression due to local tumor progression (n=5) and distant metastases (n=20); the median MWA-related PFS and PFS were 5.4 months (range, 0.7–35.3 months) and 11.8 months (range, 3.2–44.7 months), respectively. Local efficacy was significantly correlated with MWA-related PFS (P=0.003, Figure 2A). Time interval between best overall response and initial MWA was significantly correlated with PFS (P=0.011, Figure 2B). Response to first-line treatment (P=0.064) and time interval (P=0.099) also showed relevant tendencies with MWA-related PFS. There were also relevant tendencies between PFS and response to first-line treatment (P=0.050), local efficacy (P=0.120).
Fourteen patients (40.0%) died, among the 21 patients (60.0%) still alive, nine patients (25.7%) are living currently without progression. The causes of death were intrapulmonary progression (n=5), distant metastasis (n=8), and respiratory causes, such as acute exacerbation of chronic obstructive cardiopulmonary disease (n=1). The median MWA-related OS and OS were 10.6 months (range, 3.1–36.2 months) and 17.7 months (range, 5–45 months), respectively. Local efficacy was a predictor of both MWA-related OS (P=0.000, Figure 2C) and OS (P=0.001, Figure 2D). There was also a relevant tendency between OS and time interval (P=0.099) (Figure 2A to D).
Discussion
Advanced NSCLC is still an incurable disease with a poor prognosis.2 Survival benefits from conventional first-line treatment generally reach a plateau.6–8 Maintenance therapy is applied as a systemic therapy to delay progression, and it usually involves either a non-platinum cytotoxic drug or a targeted agent. A previous study17 reported that bevacizumab as continuation maintenance therapy extended PFS (10.3 vs 6.5 months) and OS (20.9 vs 10.2 months) significantly in patients with non-squamous carcinoma. The PARAMOUNT trial found that pemetrexed as continuation maintenance therapy slightly increased PFS (4.1 vs 2.8 months)18 and OS (13.9 vs 11.0 months)19 in the same subgroup of patients. Data from a Phase III randomized trial showed a slight increase in PFS (6.6 vs 5 months) with gemcitabine as continuation maintenance therapy.21 Data from another recent Phase III randomized trial showed a slight increase in PFS (3.8 and 2.9 months, respectively vs 1.9 months) with gemcitabine as continuation maintenance therapy and with erlotinib as switch maintenance therapy. However, neither showed significant improvement on OS.20,21 The SATURN trial showed that erlotinib as maintenance therapy significantly prolonged PFS (12.3 weeks vs 11.1 weeks) in patients with sensitizing EGFR mutations.22 The following issues have been raised concerning the maintenance strategy:39,40 1) its application was limited by residual toxicity of first-line treatment and performance status, which led to the intolerance of long-term maintenance; 2) its efficacy was affected by response to first-line treatment, histologic subtype,17–19 and genotypes;22 3) it has not been evidently proven to improve OS,20–22 although it has been shown to improve PFS; and 4) it has not been demonstrated as superior to second-line therapy initiated at disease progression.
Disease progression or treatment failure is attributable either to local recurrence or distant metastases. Recently, energy-based tumor ablation has been applied to decrease local recurrence. Several studies have suggested the potential benefits range from the combination of local ablation and conventional therapies ranging from systemic chemotherapy,32,41,42 to targeted therapy,43 and to radiotherapy.31,44,45 A previous study41 reported that the combination of radiofrequency ablation (RFA) with chemotherapy improved OS compared with chemotherapy alone (42 vs 29 months) in stage III to IV. Another study42 reported a PFS of 16 weeks through treatment of chemotherapy followed by RFA in patients with advanced NSCLC. However, these studies were mainly focused on RFA, and none of the investigators reported potential benefits from MWA as maintenance after conventional first-line treatments.
Three major conclusions were drawn from our study: 1) patients benefited from MWA as maintenance with definite local control. As shown in our study, tumors incompletely ablated and local progression were 17.1% and 14.7%, respectively, median MWA-related local control time was as long as 10.6 months, and tumor size was the only and strong predictor of local efficacy. Similar data were obtained from presented studies:25,27,46–48 incomplete ablation: 6%–30.8%, local progression: 3.8%–33%, average MWA-related local control time was 8.3–16.2 months, 95% of initial ablations were successful for tumors smaller than 5 cm,25 and tumor size greater than 1.5–3 cm was a risk factor that predicted local progression.25,48 In addition, local efficacy was not affected by technical factors such as power and duration of MWA in our study. These data indicated our mature and high-level technique of MWA and assured reliable results in this study. 2) Patients benefited from MWA as maintenance with improved survival. Both PFS (11.8 vs 4–6 months) and OS (17.7 vs 8–10 months) were dramatically prolonged compared with chemotherapy in previous studies.6–8 Meanwhile, MWA-related local efficacy was significantly correlated with survival. Therefore, we considered MWA as an effective systemic maintenance therapy rather than a local therapy. 3) MWA was superior to conventional maintenance therapy. Median MWA-related PFS of 5.4 months and MWA-related OS of 10.6 months, were significantly improved in comparison with patients treated with both chemotherapy and targeted therapy.17–22 Furthermore, local efficacy was not affected by histologic subtype, genotypes, and response to first-line treatment in our study as is the case in conventional maintenance therapy; it was also observed in previous studies focused on RFA.41–43 Based on the above three aspects, we considered MWA as more effective maintenance after first-line treatment in patients with advanced NSCLC.
Additionally, smaller tumor size and shorter time interval (between best overall response and initial MWA) predicted better survival, these findings caused patients to undergo early-staged MWA intervention after first-line treatment. Nevertheless, sufficient attention must be paid to the heterogeneity of follow-up treatment after MWA.
Considering that both response to first-line treatment and MWA-related local efficacy were significantly correlated with survival in our study, it made us realize the importance of combining MWA with conventional therapies, thus presuming that there were some synergic mechanisms between them both in theory and practice: 1) residual clonal variants of tumor cells resistant to chemotherapy were considered to be responsible for recurrence and progression. MWA caused irreversible hyperthermic injury to these heterogeneous tumor cells regardless of histologic subtype and genotypes;41,42 2) the centrally located hypoxic tumor cells resistant to radiotherapy were considered as a major source of recurrence. MWA caused irreversible hyperthermic injury to these tumor cells. 3) Residual tumor cells close to large vessels resulted in recurrence after hyperthermia-based ablation, chemotherapy49 and anti-angiogenic targeted agents50 helped to overcome the heat-sink effect by inducing necrosis and thrombosis of tumor blood vessels and inhibition of tumor endothelial cells; 4) local ablation may have potential systemic effects by modulating anti-tumor immunity, thus it may strengthen the efficacy of chemotherapy and targeted therapies. However, mature experimental and clinical data obtained from further research in these fields are still needed.
None of the patients died during the procedure or within 30 days after MWA both in our study and previous ones.25,27,32,46 The most common major complication was pneumothorax requiring closed pleural drainage with an incidence of 5.1% in our study; likewise, its incidence was reported 0%–12.5% in previous studies.25,27,32,46,47 Pneumothorax was mainly encountered in patients with the following characteristics:25,27,32 1) poor pulmonary function associated with smoking history or pulmonary comorbidities, and 2) tumors that were technically difficult to target. Pneumonia is an important major complication with an incidence of 5.1%, similar to 0%–18% reported in previous studies.25,27,32,46,47 Pneumonia was due to the presence of poorly controlled obstructive pneumonia and diabetes before the procedure. Rationally, any kind of infection and diabetes must be controlled before the procedure, prophylactic antibiotics can be used for high-risk patients, such as the elderly with insufficient pulmonary function, inhibited anti-infection immune function, diabetes history or large tumor.32 Obviously, patients suffered severe pain and did not obtain satisfactory pain relief in our studies. Too large tumor size was responsible for the dismal outcome and we need more exploration in palliative MWA.
To the best of our knowledge, this is the first study to demonstrate the following: 1) patients with advanced NSCLC benefited from MWA as maintenance both in local control and survival outcomes after first-line treatment; 2) as maintenance, MWA was superior to conventional maintenance therapy with improved survival and well-tolerated complications; 3) definite local efficacy and earlier MWA intervention predicted better survival. As mentioned earlier, MWA was considered as safe and effective maintenance after first-line treatment in patients with advanced NSCLC.
Despite the encouraging results, data in this area are relatively scarce at present; prospective, randomized clinical trials with large samples and long-term follow-up should be performed to accumulate sufficient and convincing evidence in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
Stewart BW, Wild CP. World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer; 2014. | ||
Surveillance, Epidemiology, and End Results Program [homepage on the Internet]. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014 [updated September 10, 2014]. Available from: http://seer.cancer.gov/csr/1975_2011/. Accessed September 27, 2015. | ||
Edge SB, Byrd DR, Carducci MA, et al. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2009. | ||
Yang P, Allen MS, Aubry MC, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128(1):452–462. | ||
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer, Version 4. 2014. National Comprehensive Cancer Network; 2014. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed May 8, 2014. | ||
Goffin J, Lacchetti C, Ellis PM, et al. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systemic review. J Thorac Oncol. 2010;5(2):260–274. | ||
Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20(21):4285–4291. | ||
Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. | ||
Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(1):20–30. | ||
Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. | ||
Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. | ||
Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. | ||
Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. | ||
Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15(16):5267–5273. | ||
Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. | ||
Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. | ||
Nadler E, Yu E, Ravelo A, Sing A, Forsyth M, Gruschkus S. Bevacizumab treatment to progression after chemotherapy: outcomes from a U.S. community practice network. Oncologist. 2011;16(4):486–496. | ||
Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13(3):247–255. | ||
Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895–2902. | ||
Pérol M, Chouaid C, Pérol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2012;30(28):3516–3524. | ||
Brodowicz T, Krzakowski M, Zwitter M, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52(2):155–163. | ||
Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–529. | ||
Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided Tumor Ablation: Standardization of terminology and reporting criteria. Radiology. 2005;235(3):728–739. | ||
Sofocleous CT, May B, Petre EN, et al. Pulmonary thermal ablation in patients with prior pneumonectomy. AJR Am J Roentgenol. 2011;196(5):W606–W612. | ||
Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(3):871–879. | ||
Belfiore G, Ronza F, Belfiore MP, et al. Patients’ survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol. 2013;82(1):177–181. | ||
Carrafiello G, Mangini M, Fontana F, et al. Microwave ablation of lung tumours: single-centre preliminary experience. Radiol Med. 2014;119(1):75–82. | ||
Kwan SW, Mortell KE, Talenfeld AD, Brunner MC. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non–small cell lung cancer. J Vasc Interv Radiol. 2014;25(1):1–9.e1. | ||
Dupuy DE. Treatment of medically inoperable non-small-cell lung cancer with stereotactic body radiation therapy versus image-guided tumor ablation: can interventional radiology compete? J Vasc Interv Radiol. 2013;24(8):1139–1145. | ||
Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. | ||
Grieco CA, Simon CJ, Mayo-Smith WW, DiPetrillo TA, Ready NE, Dupuy DE. Percutaneous image-guided thermal ablation and radiation therapy: outcomes of combined treatment for 41 patients with inoperable stage I/II non-small-cell lung cancer. J Vasc Interv Radiol. 2006;17(7):1117–1124. | ||
Wei Z, Ye X, Yang X, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38(1):135–142. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | ||
Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005;100(3):757–773. | ||
Durick NA, Laeseke PF, Broderick LS, et al. Microwave ablation with triaxial antennas tuned for lung: results in an in vivo porcine model. Radiology. 2008;247(1):80–87. | ||
Pereira PL, Masala S; Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Standards of practice: guidelines for thermal ablation of primary and secondary lung tumors. Cardiovasc Intervent Radiol. 2012;35(2):247–254. | ||
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. U.S. Department Of Health And Human Services; 2010. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed September 27, 2015. | ||
Ye X, Fan W; Minimally Invasive and Comprehensive Treatment of Lung Cancer Branch, Professional Committee of Minimally Invasive Treatment of Cancer, Chinese Anti-Cancer Association. [Expert consensus for thermal ablation of primary and metastatic lung tumors]. Zhongguo Fei Ai Za Zhi. 2014;17(4):294–301. Chinese. | ||
Dearing KR, Sangal A, Weiss GJ. Maintaining clarity: Review of maintenance therapy in non-small cell lung cancer. World J Clin Oncol. 2014;5(2):103–113. | ||
Polo V, Besse B. Maintenance strategies in stage IV non-small-cell lung cancer (NSCLC): in which patients, with which drugs? Ann Oncol. 2014;25(7):1283–1293. | ||
Lee H, Jin GY, Han YM, et al. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol. 2012;35(2):343–350. | ||
Li X, Zhao M, Wang J, et al. Percutaneous CT-Guided Radiofrequency Ablation as Supplemental Therapy After Systemic Chemotherapy for Selected Advanced Non–Small Cell Lung Cancers. AJR Am J Roentgenol. 2013;201(6):1362–1367. | ||
Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8(3):346–351. | ||
Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest. 2006;129(3):738–745. | ||
Chan MD, Dupuy DE, Mayo-Smith WW, Ng T, DiPetrillo TA. Combined radiofrequency ablation and high-dose rate brachytherapy for early-stage non-small-cell lung cancer. Brachytherapy. 2011;10(3):253–259. | ||
Liu H, Steinke K. High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: a preliminary study. J Med Imaging Radiat Oncol. 2013;57(4):466–474. | ||
Lu Q, Cao W, Huang L, et al. CT-guided percutaneous microwave ablation of pulmonary malignancies: Results in 69 cases. World J Surg Oncol. 2012;10:80. | ||
Vogl TJ, Worst TS, Naguib NN, Ackermann H, Gruber-Rouh T, Nour-Eldin NE. Factors influencing local tumor control in patients with neoplastic pulmonary nodules treated with microwaveablation: a risk-factor analysis. AJR Am J Roentgenol. 2013;200(3):665–672. | ||
Hines-Peralta A, Sukhatme V, Regan M, Signoretti S, Liu ZJ, Goldberg SN. Improved tumor destruction with arsenic trioxide and radiofrequency ablation in three animal models. Radiology. 2006;240(1):82–89. | ||
Hakimé A, Hines-Peralta A, Peddi H, et al. Combination of radiofrequency ablation with antiangiogenic therapy for tumor ablation efficacy: study in mice. Radiology. 2007;244(2):464–470. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.