Back to Journals » Journal of Blood Medicine » Volume 12
Perceptions About Blood Transfusion Therapy Among the General Public and Healthcare Professionals in the Qassim Region of Saudi Arabia
Authors Alsharidah AS, Alsuhaibani HA, Almansour BS, Alsharidah MS
Received 22 December 2020
Accepted for publication 22 February 2021
Published 11 March 2021 Volume 2021:12 Pages 139—145
DOI https://doi.org/10.2147/JBM.S296036
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Ashwag S Alsharidah,1 Haifa A Alsuhaibani,2 Basma S Almansour,3 Mansour S Alsharidah1
1Department of Physiology, College of Medicine, Qassim University, Buraidah, Qassim, Saudi Arabia; 2Department of Pharmacy Practice, College of Pharmacy, Qassim University, Buraidah, Qassim, Saudi Arabia; 3Department of Medicinal Chemistry, College of Pharmacy, Qassim University, Buraidah, Qassim, Saudi Arabia
Correspondence: Ashwag S Alsharidah
Department of Physiology, College of Medicine, Qassim University, Buraidah, Qassim, 51491, Saudi Arabia
Tel +966 541719777
Email [email protected]
Purpose: Blood transfusion is a conventional therapeutic procedure; however, the perceptions of general public and healthcare professionals (HCPs), especially physicians and nurses, remain unclear, although the insights of HSPs may affect the treatment decision. This study aimed to assess the awareness of HCPs and the public about blood transfusion risks and consent in Qassim region of Saudi Arabia, to uncover the factors that may influence such perceptions.
Patients and Methods: This study used two different closed questionnaires that were distributed electronically between February and March 2018 among the population and HCPs in Qassim region.
Results: A total of 400 general public participants and 135 HCPs completed the survey. Among the surveyed participants, 70% believed that blood transfusion therapy was safe. The perceived risk of human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) was the highest among all complications (74%). Furthermore, 88.2% of respondents were willing to accept a blood transfusion as a therapeutic measure, primarily from a first-degree relative, although the remaining 11.8% rejected the idea of a transfusion due to fear of medical error. From the HCP survey, 80% were previously involved in a blood transfusion therapy consent process. HCPs typically reported explaining the benefits, risks, and alternatives described in the consent form (74.1%, 67.4%, and 53.3%, respectively).
Conclusion: Our results indicated that despite the current high level of acceptance and knowledge regarding blood transfusions, additional educational efforts remain necessary to increase public awareness of blood transfusion therapy.
Keywords: consent, blood therapy, alternative therapy, blood bank, adverse reaction
Introduction
Blood transfusion is a therapeutic intervention often used in the hospital for a variety of purposes and typically saves lives.1 Blood transfusion therapy is a complex treatment that should be performed under the supervision of healthcare professionals (HCPs), particularly physicians and nurses. Blood transfusion therapy may include health risks; therefore, the blood transfusion treatment must be monitored, starting from the beginning of the process, with the selection of a blood donor, and includes immunohematology, serology testing of blood units, and post-process evaluations.
Although blood transfusion is a commonly used therapeutic procedure, it is not a risk-free intervention.2 Many risks may be associated with a blood transfusion, such as transfusion-transmitted infections, transfusing an incorrect blood product, hyperkalemia, hemolytic reactions, transfusion-related circulatory overload (TACO), transfusion-related acute lung injury (TRALI), volume and iron overload, and acute allergic reactions.3,4 The transfusion of incorrect blood components represents the largest cause of adverse incidents associated with blood transfusion therapy. The United States Food and Drug Administration considers TACO to be the second-most common cause of death associated with blood transfusion therapy.4 Bolton-Maggs and Cohen (2013) estimated the risk of infection from transfusion and found that 1 in 1.3 million transfusions was associated with the reciprocal expression of hepatitis B virus (HBV).4 Similarly, 1 in 28 million transfusions are associated with hepatitis C virus (HCV) transmission, and 1 in 6.7 million transfusions have been associated with the transmission of human immunodeficiency virus/acquired immune deficiency syndrome (AIDS/HIV).5
The purposes of blood transfusions, the anticipated benefits, and the expected outcomes should be explained and clarified to the patient before transfusion. Patient consent must be obtained by qualified HCPs who must clarify and be aware of all possible serious hazards of transfusion (SHOTs), in addition to the therapeutic benefits. Subsequently, the consent form should be signed by the patient before the transfusion procedure is initiated.6 All HCPs should be aware of the detailed procedures, the associated risks, and the requirements for performing blood transfusions and be able to explain these concepts to patients prior to obtaining consent form signatures. HCPs should be able to address any doubts expressed by the patient, which will increase patient safety and awareness.
A study performed in Pakistan found that 56 (16%), out of the 350 surveyed patients, were aware of the possibility of HIV, HBV, and HCV infection associated with transfusion. However, only 2% of the patients in the study reported being asked for their consent, whereas the remaining 98% percent were neither asked nor provided with information regarding consent. In contrast, 236 (67%) of the patients believed that receiving a transfusion from a close friend was associated with lower infection risk than receiving blood from a blood bank.7
Locally, Al-Drees conducted a similar study in Riyadh, Saudi Arabia, and found that infection is the primary reason given by the 20% of 609 participants who stated that they would reject a blood transfusion even if they need one. Furthermore, 84.5% of participants stated a preference for receiving blood from a relative or a friend. Moreover, 11.5% believed that blood transfusion was a serious medical procedure, could be harmful, and carried risks of infection, whereas only 55.1% agreed that blood banks provide contamination-free blood.8
The current study aimed to assess the perception and awareness among the general public and among HCPs regarding the risks, associated diseases, and necessity of blood transfusions in Qassim region of Saudi Arabia and to identify the factors that influence these perceptions. This knowledge could enable HCPs to better understand their patients and allow them to better guide their patients through the decision-making process when faced with the decision to accept blood transfusion therapy, in addition to preventing the risks associated with the procedure.
Patients and Methods
Study Design, Sample Size, and Inclusion Data
A descriptive, exploratory, cross-sectional study with a quantitative, comparative design was conducted in Qassim region of Saudi Arabia. The study was conducted over four weeks, from 25 February to 25 March 2018. The minimum sample size for the study was calculated as 384, based on the population of Qassim region in 2016.9 We collected 400 responses to account for the possibility of missing data from some of the survey responses. We recruited both males and females with ages ranging from 20 to 60 years. Informed consent was obtained from all participants. We limited study participation to residents of Qassim region of Saudi Arabia. We received 135 HCPs (physicians and nurses) responses after informed consent was obtained. This study was conducted in accordance with the Declaration of Helsinki. Ethical approval for the study was obtained from the subcommittee of Health Research Ethics in Qassim University, Buraidah, Saudi Arabia.
Data Collection Tool
Two structured surveys were designed based on an extensive literature review. The surveys were distributed online to the public and to HCPs (physicians and nurses).2,3,10 The questionnaires were originally written in English, but we translated them into Arabic prior to distribution to ensure better understanding by the respondents. The questionnaires were distributed electronically and containing close-ended questions. Questionnaires have many advantages over other data collection tools, such as being inexpensive, easy to perform, and able to collect many findings and outcomes for analysis in a single format.
The general public survey included 24 questions on demographic data, educational level, and their willingness to accept a blood transfusion from a blood bank, expected risks, information regarding transfusion alternatives, and awareness of consent.
Another survey assessed the HCPs’ impressions with 15 questions about the importance of the transfusion consent process, blood transfusion risks, the importance of the patient’s acceptance of blood transfusion therapy, and the benefits and alternatives to blood transfusion.
Statistical Analysis
The questionnaire responses were analyzed using the Statistical Package for the Social Science (SPSS Inc. Chicago, IL, USA), version 23. Categorical variables are described as frequencies and percentages. Descriptive analyses were performed using the Chi-square test to test the significance of associations between categorical variables. The level of significance was set to P < 0.05.
Results
Participants Characteristics
This study aimed to identify community perceptions, awareness, and knowledge regarding blood transfusion therapy. Our community-aimed survey obtained a total of 400 responses, and the population characteristics are described in Table 1.
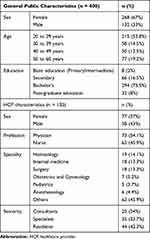 |
Table 1 Demographic Characteristics of Participants |
Participants’ Knowledge of Blood Transfusion Therapy Safety
The majority of participants thought that blood transfusion was safe [283 (70.8%)], although 117 (29.2%) participants expressed the perception that transfusions were not safe. More than half of the participants [237 (59.2%)] thought that the blood groups of the donor and recipient were required to be matched for blood transfusion, whereas the remaining participants either thought that type-matching was not mandatory [135 (33.8%)] or indicated a lack of knowledge [28 (7%)]. Nearly half of the participants did not know whether alternatives to blood transfusion existed [202 (50.5%)], and nearly a third of them thought that no alternatives to blood transfusion were available [129 (32.3%)]; only a few participants stated that they believed alternatives to blood transfusion existed [69 (17.2%)].
Awareness Regarding Blood Transfusion Complications
Participants were more aware of the risks of chronic post-transfusion complications than the risks of acute complications. Nearly three-fourths of participants [296 (74%)] were aware of the possibility of transmission of or infection by HIV/AIDS, and nearly two-thirds [249 (62.3%)] were aware of the risks of transmission of or infection by HBV and HCV. However, knowledge of acute post-transfusion complications was poor, and only one-third of participants knew that allergic reactions, fever, and hemolysis were potential acute post-transfusion complications and only one-fifth of them [85 (21.3%)] knew that electrolyte imbalance was a potential acute post-transfusion complication.
Acceptance and Awareness of Participants Towards Blood Transfusion Therapy Consent Forms
The majority of participants believed that the provision of consent prior to blood transfusion therapy was mandatory [335 (83.8%)], whereas only a few indicated that consent was not mandatory [65 (16.2%)]. The aspects that the participants emphasized that HCPs should discuss during the written consent process, represented in terms of the frequency and percentage of responses and multiple answer was allowed, were as follows: possible side effects [262 (65.5%)], medical benefits [257 (64.2%)], possible risks [244 (61%)], and alternative treatments [185 (46.2%)].
Acceptance of Participants Toward Blood Transfusion Therapy
The majority of participants stated that they would accept a blood transfusion if their doctor recommended a blood transfusion or informed them that they required a transfusion [353 (88.2%)], whereas the remaining respondents [47 (11.8%)] stated that they would refuse a transfusion, even if told a transfusion was necessary. Among those who were willing to accept a transfusion (n = 353), when asked who they would prefer to be the donors, their preferred donors, represented by frequencies and percentages, were as follows: first-degree relatives [206 (58.4%)]; any person, it does not matter [130 (36.8%)]; and husband/wife [92 (26.1%)]. Among those who stated that they would refuse a transfusion (n = 47), the following barriers were noted: fear of medical errors during the transfusion process [24 (51.1%)]; fear of transmission of or infection by HBV, HCV, and HIV [14 (29.8%)]; and insufficient alternatives [9 (19.1%)] (Table 2).
 |
Table 2 Acceptance Among Participants of Blood Transfusion Therapy (n = 400) |
Participants’ Education Levels and Awareness of Post-Transfusion Complications
We asked the participants about their awareness of various complications that may occur after blood transfusion therapy (allergic reactions, such as itching, erythema, skin rash, shortness of breath, and anaphylaxis; fever and hemolysis (red blood cell destruction); disturbance and imbalance of electrolytes in the body). Most participants had a low level of awareness regarding the potential complications following blood transfusion therapy, which did not differ significantly across different education levels (Table 3).
 |
Table 3 Relationship Between Education Level and the Potential for Acute Allergic Reaction to Blood Transfusion Therapy (n = 400) |
Consent Perception and Experiences of HCPs
Among the surveyed HCPs, 108 (80%) were involved in or attended a blood transfusion consent process, whereas 27 (20%) had not. Among the 135 HCPs, 129 (95.6%) agreed that the consent process for blood transfusion was necessary, whereas the remaining 6 (4.4%) thought that consent was not necessary. A total of 120 (88.9%) HCPs thought that obtaining the patient’s consent before performing a blood transfusion should be mandatory, whereas 15 (11.1%) thought that consent should not be mandatory. In addition, 119 (88.1%) HCPs thought that all hospitals should adopt and apply a transfusion consent policy, whereas 7 (5.2%) thought that such policies should not be adopted, and 9 (6.7%) did not know. More than three-fourths of HCPs [102 (78.5%)] were satisfied with the transfusion consent process used for blood transfusion therapy at the hospital, whereas the remaining respondents [28 (21.5%)] were unsatisfied.
HCPs who believed that the transfusion consent procedure form adds knowledge to the patients were among the majority [119 (88.1%)], and only a few expressed the opinion that the consent process did not provide knowledge to the patients [16 (11.9%)]. A total of 100 (74.1%) of the HCPs who reported being involved in or attending a transfusion consent process stated that they explained the benefits of blood transfusion when they obtained the transfusion consent, whereas the remaining [8 (5.9%)] HCPs involved in or attending a transfusion consent process and did not fully explain the benefits of blood transfusions when they obtained transfusion consent, and 27 (20%) were not involved in or never attended a transfusion consent process. Among HCPs who were involved in or attended a transfusion consent process, the majority stated that they explained the risks of blood transfusion when they obtained transfusion consent [90 (66.6%)], whereas the 18 (13.3%) did not explain the risks of blood transfusion, and 27 (20%) were not involved in the transfusion consent process. In addition, 72 (53.3%) of HCPs who were involved in or attended a transfusion consent process reported explaining the alternatives to blood transfusions, whereas 36 (26.7%) of those involved in the transfusion consent process did not explain alternatives to blood transfusion, and 27 (20%) were not involved in the transfusion consent process (Table 4).
 |
Table 4 HCPs Practice of Blood Transfusion Therapy Consent (n = 135) |
Discussion
Over recent years, identifying the variables that affect the awareness and perceptions of Saudis regarding blood transfusion therapy has become crucial, as World Health organization, stated an increase in voluntary blood donations from 2013 to 2018.11 The present study strengthens our understanding and knowledge of the perceptions and knowledge of both the general public and HCPs in terms of blood transfusion therapy.
The demand for blood transfusion therapy is increasing due to the growing population of Saudi Arabia and the increasing number of healthcare facilities; therefore, maintaining the safety of blood transfusion therapy has become essential. HIV/AIDS and HBV/HCV were the highest perceived complications in our study, with 74% and 62.3% of respondents indicating that they perceived the transmission of these viruses to be risks of blood transfusion, respectively. Locally, a study was conducted to measure the prevalence of HBV markers among blood donors between 2013 and 2015 in Qassim regional hospitals, which found identified 0.42% positive units.12
Blood transfusion therapy has many known risks and side effects, including allergic reactions.3 Post-transfusion allergies are the result of several plasma proteins, which can cause an anaphylactic reaction.13
Hemolytic transfusion reactions can cause the lysis of transfused red blood cells due to immunological incompatibility.14 Approximately one-third of our participants believed that blood transfusion therapy might cause fever and hemolysis.
Hyperkalemia related to transfusion depends not only on the potassium concentration in the red blood cell unit but also on the volume and rate of red blood cell administration.15 However, only 21.3% of our participants were aware of the possibility of electrolyte disturbance caused by blood transfusion therapy. Raza et al, in their study, reported that 4% of 125 patients suffered from hyperkalemia after receiving blood transfusion therapy.16
After the donation process, donated blood goes through an extensive screening procedure to identify a variety of viruses and parasites at the blood banks in Saudi Arabia. In 2008, Aldrees’s results stated that 55.1% of patients believed that blood transfusion therapy was a safe procedure.8 Furthermore, Vetter et al, in 2014, found that only 15% of surgical patients rated blood transfusion therapy as a very risky procedure.3 In our study, only 17.2% of our participants were aware of blood transfusion therapy alternatives. Similarly, 18.4% of 294 surgical patients in the study reported by Vetter et al were aware of blood transfusion therapy alternatives.3
HCPs’ opinions and attitudes towards blood transfusion are vital for assessing the credibility of the obtained consent. Our study highlighted their perceptions of transfusion consent and their consent-associated practices. The majority of the HCPs were previously involved in a blood transfusion therapy consent process (80%). In Oman, 77% of the physicians declared their previous involvement in blood transfusion consent.6 Locally, at Buraidah Central Hospital, emergency blood transfusion therapy consent is mandatory prior to blood component release from the blood bank.
A study by Cheung et al found that all surveyed patients reported having a conversation regarding the reason for receiving blood transfusion therapy. Although 85% of participants agreed that the benefits of the procedure were discussed, only one-third of the patients stated that the risks of infection transmission were discussed. Only 24% stated that HCPs discussed alternatives prior to blood transfusion therapy procedures.17
The consent form contains information regarding blood transfusion therapy benefits, risks, and alternatives. Similar results to ours were found in a study by Al-Riyami et al, in which 80% of the surveyed physicians thought that the process of obtaining blood transfusion consent provides the patient with knowledge prior to the blood transfusion therapy.6 However, in Davis et al, the majority of HCPs (83 out of 123) felt that the information provided during the consent process regarding blood transfusion therapy procedures was insufficient.18
A study by Freidman et al showed that all surveyed residents primarily discuss the improvement of anemia during the consent process.2 In contrast, in Ohio, United States, patients interviewed after blood transfusion therapy reported that pre-transfusion written consent was not sufficient to explain the benefits and risks of the procedure, and they reported concerns about the safety of the blood supply.19
Conclusion
Most of the surveyed general public thought that blood transfusion was safe, and we found that the general awareness of allergic reactions as potential blood transfusion complications increased with education level. The major reason stated for refusing blood transfusion therapy was fear of blood-borne infections. The survey responses indicated that the participants were aware of the possible risks associated with the blood transfusion process. HCPs’ responses suggested the need to modify the current consent process by including more information about the benefits and risks to ensure that patients are provided with a full understanding of the procedure and to minimize the chances that HCPs will omit any information.
The major limitation of this study was the paucity of time. As we distributed the survey to approximately 600 HCPs but only received 135 HCP, which is less than the one-third that was expected. The response rate of the study survey was 22.5%. This low response rate is considered a result of the short data collection time and is a significant limitation and may contribute to non-response bias.
The present study reflects a positive attitude among general public towards the safety of blood transfusion. Our results also indicated the need for additional educational efforts to increase public awareness of blood transfusion therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Allard S. Blood transfusion. Medicine. 2013;41(4):242–247. doi:10.1016/j.mpmed.2013.01.010
2. Friedman M, Arja W, Batra R, et al. Informed consent for blood transfusion. Am J Clin Pathol. 2012;138(4):559–565. doi:10.1309/AJCP2TN5ODJLYGQR
3. Vetter T, Adhami L, Porterfield J, Marques M. Perceptions about blood transfusion. Anesth Analg. 2014;118(6):1301–1308. doi:10.1213/ANE.0000000000000131
4. Bolton‐Maggs P, Cohen H. Serious Hazards of Transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol. 2013;163(3):303–314. doi:10.1111/bjh.12547
5. Loor G, Koch C, Sabik J, Li L, Blackstone E. Implications and management of anemia in cardiac surgery: current state of knowledge. J Thorac Cardiovasc Surg. 2012;144(3):538–546. doi:10.1016/j.jtcvs.2012.04.014
6. Al-Riyami A, Al-Marshoodi I, Zia F, et al. Transfusion consent in Oman: physicians’ perception at a tertiary care university hospital. Oman Med J. 2016;31(4):253–257. doi:10.5001/omj.2016.50
7. Habibullah S, Dahar S, Ashraf J, Shakeel Aamir M. To determine patients’ perceptions and attitudes for blood transfusion. Pak J Med Res. 2016;55(2):48–50.
8. Al-Drees A. Attitude, belief and knowledge about blood donation and transfusion in Saudi population. Pak J Med Sci. 2008;24(1):74–79.
9. Demographic survey [Internet]. 2016 [cited March 23, 2018]. Available from: https://www.stats.gov.sa/sites/default/files/ar-demographic-research-2016_5.pdf.
10. Abdul-Aziz B, Lorencatto F, Stanworth S, Francis J. Patients’ and health care professionals’ perceptions of blood transfusion: a systematic review. Transfusion. 2017;58(2):446–455. doi:10.1111/trf.14404
11. World Health Organization. Blood safety and availability[Internet]. 2020 [Cited February 3, 2021] Available from: https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability.
12. Alharbi A, Alouffi S, Alcantara J, et al. Prevalence of hepatitis B virus markers among blood donors in Qassim Region, Saudi Arabia. Int J Pharm Res Allied Sci. 2017;6(1):99–106.
13. Matsuyama N, Yasui K, Amakishi E, et al. The IgE-dependent pathway in allergic transfusion reactions: involvement of donor blood allergens other than plasma proteins. Int J Hematol. 2015;102(1):93–100. doi:10.1007/s12185-015-1794-0
14. Davenport R, Bluth M. Hemolytic transfusion reactions. In: Simon T, editor. Rossi’s Principles of Transfusion Medicine.
15. Smith H, Farrow S, Ackerman J, Stubbs J, Sprung J. Cardiac arrests associated with hyperkalemia during red blood cell transfusion: a case series. Anesth Analg. 2008;106(4):1062–1069. doi:10.1213/ane.0b013e318164f03d
16. Raza S, Ali Baig M, Chang C, et al. A prospective study on red blood cell transfusion related hyperkalemia in critically Ill patients. J Clin Med Res. 2015;7(6):417–421. doi:10.14740/jocmr2123w
17. Cheung D, Lieberman L, Lin Y, Callum J. Consent for blood transfusion: do patients understand the risks and benefits? Transfus Med. 2014;24(5):269–273. doi:10.1111/tme.12141
18. Davis R, Vincent C, Sud A, et al. Consent to transfusion: patients’ and healthcare professionals’ attitudes towards the provision of blood transfusion information. Transfus Med. 2012;22(3):167–172. doi:10.1111/j.1365-3148.2012.01148.x
19. Adams KW, Tolish D. Blood transfusion: the patient’s experience. Am J Nurs. 2011;111(9):24–30. doi:10.1097/01.NAJ.0000405057.23324.a8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
