Back to Journals » Clinical Ophthalmology » Volume 13
Perceived difficulties and barriers to uptake of Descemet’s membrane endothelial keratoplasty among surgeons
Authors Zafar S, Parker JS, de Kort C, Melles G, Sikder S
Received 19 April 2019
Accepted for publication 13 May 2019
Published 21 June 2019 Volume 2019:13 Pages 1055—1061
DOI https://doi.org/10.2147/OPTH.S212871
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sidra Zafar,1 Jack S Parker,2,3 Christa de Kort,3,4 Gerrit Melles,3,4 Shameema Sikder1
1Department of Ophthalmology, Wilmer Eye Institute, Johns Hopkins Hospital, Baltimore, MD, USA; 2Parker Cornea, Birmingham, AL, USA; 3Netherlands Institute for Innovative Ocular Surgery-United States of America, San Diego, CA, USA; 4Melles Cornea Clinic, Rotterdam, Netherlands
Purpose: To determine barriers related to implementation of Descemet’s membrane endothelial keratoplasty (DMEK) among corneal surgeons.
Methods: This was a multicenter survey study of all corneal surgeons who participated in a DMEK wet lab organized by the Netherlands Institute for Innovative Ocular Surgery. Data related to barriers limiting uptake of DMEK surgery, self-perceived levels of competence, and difficulty with different steps of DMEK surgery were analyzed.
Results: The survey response rate was 31% (22 of 72). The most common barrier to uptake of DMEK surgery identified was anxiety related to incorrect insertion of the tissue and the need to regraft (64%, 14 of 22), followed by anxiety related to tissue preparation (50%, eleven of 22). Surgeons also felt anxious regarding the possibility of rebubbling with initial DMEK (41%, nine of 22). Steps related to DMEK graft (76%) preparation, tissue insertion (41%), and graft unfolding (72%) were identified as the most difficult steps to learn by the respondents.
Conclusion: The DMEK learning curve, especially for the novice surgeon, may be shortened by seeking educational resources, including wet labs and surgical videos. Eye banks may facilitate adoption of DMEK by making validated DMEK tissue more accessible to surgeons globally.
Keywords: DMEK, uptake, barriers, difficulties
Introduction
Endothelial keratoplasty (EK) techniques have evolved rapidly in recent years. They have largely replaced penetrating keratoplasty as the standard of care for treatment of endothelial disease. Descemet's membrane EK (DMEK), which involves replacing only the diseased Descemet's membrane and endothelium, is the latest anatomical iteration of EK.1 However, despite being associated with better postoperative visual outcomes, faster visual recovery, and lower rates of endothelial rejection,2–4 widespread implementation of DMEK has been limited.
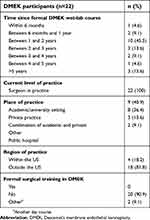 | Table 1 Characteristics of corneal surgeons who participated in the survey |
 | Table 2 Surgical case volume of participants |
 | Table 3 Barriers limiting surgeon adoption of DMEK |
 | Table 4 Self-perceived difficulty with learning different steps of DMEK surgery |
 | Table 5 Self-reported surgeon competence regarding different steps of DMEK surgery |
 | Table 6 Utility of various educational resources in improving surgeons' skills |
Furthermore, a wide variation currently exists in DMEK-uptake rates among countries. For instance, German surgeons were performing DMEK 12 times as often as Descemet's stripping EK (DSEK) in 2016.5 In contrast, DMEK accounted for only 11% of the EKs performed in the US in 2015, while DSEK accounted for approximately 50% of all corneal transplants during the same period.6 The purpose of this study was thus to identify barriers associated with physician uptake of DMEK.
Methods
This was a global, survey-based, cross-sectional study performed between March 27, 2018 and September 28, 2018. A 17-item questionnaire (Qualtrics. Provo, UT, USA) developed at the Wilmer Eye Institute was sent to all corneal surgeons who participated in a DMEK wet lab organized by the Netherlands Institute for Innovative Ocular Surgery (https://www.niios.com/niios-academy/dmek-wetlab-courses-for-beginners-and-early-starters/course-description).
Data collected from the survey included information related to the surgeons' practice (place and region) and their surgical volume for different procedures (penetrating keratoplasty, DSEK/Descemet's stripping automated (DSAEK), DMEK, and cataract). Participants were also asked whether they had received any formal DMEK training, potential barriers to uptake of DMEK in their practice, self-perceived levels of preparedness and competence, and difficulty with the different steps of DMEK surgery. Since no identifying data were requested through the survey, the study was reviewed and deemed exempt by the Johns Hopkins institutional review board. A consent script was included with the surveys to inform respondents of their participation being voluntary. All statistical analysis was performed using SPSS version 23 (IBM, Armonk, NY, USA). Means with SD were computed to describe continuous data. Frequencies and percentages were calculated to describe categorical data.
Results
Participant characteristics
The survey response rate was 31% (22 of 72). All respondents were surgeons in practice, and a majority (46%, ten of 22) had participated in the DMEK wet-lab within 1–2 years of this survey. Almost 82% (18 of 22) were from outside the US, and most (91%, 20 of 22) had not received any formal surgical training in DMEK (Table 1). Within the study cohort, 50% (eleven of 22) had performed >50 DSEK/DSAEK procedures. In comparison, 77% (17 of 22) had done <50 DMEK surgeries, with the majority in the 1–10 category (Table 2).
Barriers to DMEK surgery
The most common barrier identified to uptake of DMEK surgery was anxiety related to incorrect insertion of the tissue and having to regraft (64%, 14 of 22), followed by anxiety related to tissue preparation (50%, eleven of 22). Surgeons also felt anxious about the possibility of rebubbling with initial DMEK (41%, nine of 22). Cost (36%, eight of 22) was also identified as a barrier (Table 3).
Steps of DMEK surgery
Steps related to DMEK graft (76%) preparation, tissue insertion (41%), and unfolding (72%) were identified as the most difficult steps to learn by the respondents. More than half (52%) the participants also indicated difficulty managing complications (Table 4). Most respondents reported feeling only moderately prepared during their initial DMEK surgery. We found that 46% of the participants currently felt competent in performing DMEK surgery and did not require input from more experienced surgeons. Approximately a third, however, still required more than minimal input for certain steps of the surgery, including preparing and unfolding the DMEK graft, while 18% required such help for graft insertion (Table 5).
Educational resources
Faculty interaction in either the operating room or the practice lab, watching surgical videos, review of postoperative surgical outcomes, and attending wet-lab training courses were reported as the most helpful resources by participants in helping strengthen their surgical skills (Table 6).
Discussion
The first successful DMEK surgery was performed more than a decade ago,7 and while the number of DMEK procedures being performed rose every year between 2012 and 2016, as per data provided by the Eye Bank Association of America, widespread adoption of the technique by corneal surgeons has been slow, because of the perceived difficulty of the procedure.1,8 In our study, we found steps related to graft preparation, graft insertion, and unfolding as being the most difficult to learn by corneal surgeons. Graft preparation and graft insertion were also identified as important barriers to the uptake of DMEK surgery, in addition to procedure-related complications and cost.
Manual preparation of donor tissue has frequently been cited as a cause of concern by corneal surgeons. Indeed, graft-preparation failure can often result in certain unacceptable risks related to donor-tissue loss, cancellation of the surgery, and associated financial loss.9 Recently, however, several eye banks in the US and abroad have begun prestripping donor tissue. Terry et al9 and Deng et al10 demonstrated successful DMEK surgeries in series of 80 eyes and 40 eyes, respectively, using technician-prepared prestripped donor tissue. None of the donor tissue in either series was damaged by tearing when finishing the final 10% of the stripping maneuver. Moreover, certain eye banks now provide preloaded tissue grafts, thereby further decreasing burden on the surgeon and reducing surgical time and tissue wastage.11 A recent multicenter study involving 55 surgeons, however, found that most surgeons were still preparing grafts themselves.12 The authors suggested that this may have partly resulted from a lack of local eye banks equipped to prepare DMEK grafts surgically.12 As such, this highlights the important role that eye banks may need to play to make validated DMEK donor tissue more widely available, since only by eliminating this tissue-preparation and -insertion risk will DMEK ever become commonplace. Future studies to determine safety of using preloaded DMEK tissue, as well as studies to evaluate methods by which DMEK tissue can be analyzed after preloading, are however needed.
Excessive graft manipulation during preparation and implantation can damage endothelial cells. Therefore, quantifying endothelial cell loss that occurs during graft preparation would be a strong indicator of long-term endothelial cell loss and indirectly of graft-failure rates. In their study involving 31 DMEK grafts, Tran et al reported that tissue preloading incurred more endothelial cell loss than simply prestripping tissue.13 Studies have also found greater cell loss in the S-stamp area in preloaded DMEK grafts, often with complete loss of all cells in the stamped area.14 DMEK presents a steep learning curve and surgical skills that are entirely different from DSEK.8 Experienced DSEK surgeons may not be as compelled to learn DMEK, especially when they might be achieving satisfactory results with their current technique. Furthermore, unlike certain procedures, such as cataract surgery, which has well-defined algorithms for every step of the surgery, a similar “if this, then that” algorithm does not currently exist for DMEK.8 Therefore, the steep learning curve combined with surgeons not knowing the specific “next steps” of surgery and the higher rate of complications experienced initially currently limit the amount of DMEK surgery performed today.15 However, instead of perceiving the learning curve as a barrier, surgeons should focus on methods to optimize the learning curve to ensure progress. Education and surgical practice can play a particularly crucial role in this, while ensuring a smooth transition from DSEK to DMEK.
One such surgical training resource is wet labs. Surgeons can perform and practice essential surgical maneuvers in wet-lab sessions, which are required to confirm the orientation of the endothelium and unfold and position the DMEK graft in a repeatable and reproducible manner. Furthermore, the resources needed to set up DMEK-oriented practice labs are readily available and cheap.16 Sessions supervised by well-experienced trainers may be especially helpful. In fact, one-on-one interactions with experienced faculty were found to be among the most helpful educational resources in our study sample. While formal training has its advantages, the growing platform of video-sharing websites now allows for rapid dissemination of surgical techniques, something not previously possible. Indeed, surgical videos have been shown to be a valuable tool for demonstrating and teaching surgical technique at a minimal cost. In a recent study by McKee et al involving 40 eyes, DMEK surgery was successfully performed in 97% of cases by a corneal surgeon who had learnt the procedure primarily by watching YouTube videos of standardized DMEK techniques.17
Surgeon anxiety regarding postoperative complications was also identified as an important barrier to uptake of DMEK. It is noteworthy that prior studies have shown a decreasing trend in complication rates as the individual surgeon's learning curve is completed.12,18,19 Therefore, it is important that the higher complication rate early on does not deter surgeons. Furthermore, recent advances in instrumentation and techniques, including the development of DSEK and DMEK hybrids, can facilitate the learning process. Other recent refinements in the DMEK technique include the use of SF6 gas instead of air and creation of a larger descemetorhexis, which have been shown successfully to decrease the rebubble rate, as well as marking the graft to avoid incorrect orientation.2,9 Similarly, avoidance of cold storage media, use of glass instead of plastic inserters, and leaving the patient in a supine position with a complete air fill of the anterior chamber for at least 1 hour have been advocated to minimize the risk of graft detachment.20
While the 31% response rate in the present study is within the response-rate range of surveys (10%–51%)21–23 conducted in the field of ophthalmology, the low response rate that we observed may potentially serve to introduce a nonresponse bias in our study. Characteristics of participants who responded may have been different from those who did not respond, which may limit the generalizability of our study findings depending on the extent to which respondents are representative of all corneal surgeons. Therefore, it is important that this limitation be kept in mind when interpreting the results of our study.
In conclusion, while DMEK offers a superior visual advantage and faster recovery, the speed of adoption of this technique by surgeons will additionally be dependent on several other factors, including removing the risk of donor-tissue destruction in the operating room. Future work should emphasize developing techniques that can facilitate the learning curve and help lower complication rates, especially for novice surgeons. The role of educational resources, including supervised wet-lab sessions and surgical videos, should also be highlighted. Eye banks especially have a crucial role to play in making validated, prestripped, and preloaded DMEK tissue more widely available. DSEK was not widely adopted by corneal surgeons when it was first introduced in 2004. That situation changed, however, with the advent of eye bank–prepared donor tissue in 2006, which removed many of the financial and technical obstacles initially associated with the surgery. Finally, future work should attempt to better understand why differences in DMEK uptake exist among regions across the world, factoring in availability of eye bank–prepared tissue and prior trends for adaptation of new surgical techniques. Only when DMEK attains the perceived surgical ease of DSEK will it be widely adopted.
Acknowledgments
The authors would like to acknowledge Xinyi Chen for her help with the study survey and the Mitchell Trust for their continued support of our work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vasquez Perez A, Zarei-Ghanavati M, Liu C. DMEK calling. J Ophthalmic Vis Res. 2016;11(4):343–344. doi:10.4103/2008-322X.194067
2. Price MO, Price FW
3. Droutsas K, Lazaridis A, Papaconstantinou D, et al. Visual outcomes after Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty-comparison of specific matched pairs. Cornea. 2016;35(6):765–771. doi:10.1097/ICO.0000000000000822
4. Anshu A, Price MO, Price FW
5. Flockerzi E, Maier P, Böhringer D, et al. Trends in corneal transplantation from 2001 to 2016 in Germany: a report of the DOG–section cornea and its keratoplasty registry. Am J Ophthalmol. 2018;188:91–98. doi:10.1016/j.ajo.2018.01.018
6. Park CY, Lee JK, Gore PK, Lim CY, Chuck RS. Keratoplasty in the United States: a 10-year review from 2005 through 2014. Ophthalmology. 2015;122(12):2432–2442. doi:10.1016/j.ophtha.2015.08.017
7. Melles GR, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty (DMEK). Cornea. 2006;25(8):987–990. doi:10.1097/01.ico.0000248385.16896.34
8. Sidiqi AM, Navajas EV. Spontaneous improvement of syphilis chorioretinitis: case report and review of the literature. Retin Cases Brief Rep. 2017. doi:10.1097/ICB.0000000000000670
9. Terry MA, Straiko MD, Veldman PB, et al. Standardized DMEK technique: reducing complications using prestripped tissue, novel glass injector, and sulfur hexafluoride (SF6) gas. Cornea. 2015;34(8):845–852. doi:10.1097/ICO.0000000000000479
10. Deng SX, Sanchez PJ, Chen L. Clinical outcomes of Descemet membrane endothelial keratoplasty using eye bank-prepared tissues. Am J Ophthalmol. 2015;159(3):590–596. doi:10.1016/j.ajo.2014.12.007
11. Parekh M, Ruzza A, Ferrari S, Busin M, Ponzin D. Preloaded Tissues for descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2016;166:120–125. doi:10.1016/j.ajo.2016.03.048
12. Oellerich S, Baydoun L, Peraza-Nieves J, et al. Multicenter study of 6-month clinical outcomes after descemet membrane endothelial keratoplasty. Cornea. 2017;36(12):1467–1476. doi:10.1097/ICO.0000000000001374
13. Tran KD, Dye PK, Odell K, et al. Evaluation and quality assessment of prestripped, preloaded Descemet membrane endothelial keratoplasty grafts. Cornea. 2017;36(4):484–490. doi:10.1097/ICO.0000000000001150
14. Wolle MA, DeMill DL, Johnson L, Lentz SI, Woodward MA, Mian SI. Quantitative analysis of endothelial cell loss in preloaded Descemet membrane endothelial keratoplasty grafts. Cornea. 2017;36(11):1295–1301. doi:10.1097/ICO.0000000000001301
15. Terry MA. Endothelial keratoplasty: why aren’t we all doing Descemet membrane endothelial keratoplasty? Cornea. 2012;31(5):469–471. doi:10.1097/ICO.0b013e31823f8ee2
16. Droutsas K, Petrak M, Melles GRJ, Koutsandrea C, Georgalas I, Sekundo W. A simple ex vivo model for teaching Descemet membrane endothelial keratoplasty. Acta Ophthalmol (Copenh). 2014;92(5):e362–e365. doi:10.1111/aos.12371
17. McKee HD, Jhanji V. Learning DMEK from youtube. Cornea. 2017;36(12):1477–1479. doi:10.1097/ICO.0000000000001399
18. Dapena I, Ham L, Droutsas K, van Dijk K, Moutsouris K, Melles GR. Learning curve in Descemet’s Membrane endothelial keratoplasty: first series of 135 consecutive cases. Ophthalmology. 2011;118(11):2147–2154. doi:10.1016/j.ophtha.2011.03.037
19. Dirisamer M, Ham L, Dapena I, et al. Efficacy of descemet membrane endothelial keratoplasty: clinical outcome of 200 consecutive cases after a learning curve of 25 cases. Arch Ophthalmol. 2011;129(11):1435–1443. doi:10.1001/archophthalmol.2011.195
20. Monnereau C, Quilendrino R, Dapena I, et al. Multicenter study of descemet membrane endothelial keratoplasty: first case series of 18 surgeons. JAMA Ophthalmol. 2014;132(10):1192–1198. doi:10.1001/jamaophthalmol.2014.1710
21. Chiang MF, Boland MV, Margolis JW, Lum F, Abramoff MD, Hildebrand PL. Adoption and perceptions of electronic health record systems by ophthalmologists: an American Academy of Ophthalmology survey. Ophthalmology. 2008;115(9):
22. Macaluso DC, Andre M, Caroline PJ, Suhler EB, Rich LF. Assessment of ophthalmology residents’ contact lens training. CLAO J. 2000;26(4):221–224.
23. Gedde SJ, Budenz DL, Haft P, Tielsch JM, Lee Y, Quigley HA. Factors influencing career choices among graduating ophthalmology residents. Ophthalmology. 2005;112(7):1247–1254.e1242. doi:10.1016/j.ophtha.2005.01.038
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
