Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Pemphigoid Gestationis – Case Report and Review of Literature
Authors Ceryn J , Siekierko A, Skibińska M, Doss N, Narbutt J, Lesiak A
Received 7 January 2021
Accepted for publication 18 March 2021
Published 16 June 2021 Volume 2021:14 Pages 665—670
DOI https://doi.org/10.2147/CCID.S297520
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Justyna Ceryn,1 Aleksandra Siekierko,1 Małgorzata Skibińska,1 Nejib Doss,2 Joanna Narbutt,1 Aleksandra Lesiak1
1Department of Dermatology, Pediatric Dermatology and Oncology Clinic, Medical University of Lodz, Lodz, Poland; 2Golden Towers Medical Center, Centre Urbain Nord, Tunis, 1082, Tunisia
Correspondence: Justyna Ceryn
Department of Dermatology, Pediatric Dermatology and Oncology, Medical University of Lodz, gen. Karola Kniaziewicza 1/5, Lodz, 91347, Poland
Email [email protected]
Abstract: Pemphigoid gestationis (PG) is a rare autoimmune bullous skin disorder which usually presents with intense pruritus and urticarial lesions that may evolve into vesicles and tense blisters. In majority of patients, it starts in the second or third trimester of pregnancy and resolves spontaneously after delivery. Lesions appear in the periumbilical area in 90% of patients and rapidly spread centrifugally to other parts of the body. The diagnosis needs to be confirmed by direct immunofluorescence test (DIF) with indirect immunofluorescence test (IIF), ELISA and immunoblot techniques playing role in diagnosis and/or monitoring antibodies level. Mild symptoms of PG can be treated with topical therapy only, but in severe course of the disease the treatment may be escalated to oral corticosteroids. We present an unusual case of PG started 2 weeks after delivery with an updated overview on the epidemiology, pathology, clinical picture, treatment, and complications of the disease.
Keywords: case report, pemphigoid gestationis, gestational pemphigoid, herpes gestationis, pregnancy dermatoses
Introduction
Gestational pemphigoid (pemphigoid gestationis, PG) is a rare, intensely pruritic autoimmune bullous skin disorder occurring during pregnancy, but clinically and pathologically similar to bullous pemphigoid (PB). Historically, PG was named herpes gestationis, because of the vesicular morphology of the lesions. However, it was eventually proven that PG was unrelated to any prior or active herpes virus infection and the name of the disease was changed.4 It usually starts in the second or third trimester of the pregnancy, with single cases reported in the first trimester and postpartum.1,8 Multigravidae, typically with an earlier onset of symptoms, are more susceptible to develop PG than primigravidae. Although PG course is usually self-limiting and most patients go into spontaneous remission after delivery, up to 75% of patients may experience postpartum flares.8 Interestingly, there is an increased risk of Graves' disease, alopecia areata, vitiligo, or ulcerative colitis in patients with PG.16
Case Report
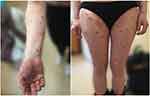
A 30-year-old multigravida was admitted to the Department of Dermatology 2 weeks after delivery with intense pruritus and skin lesions which started gradually 10 days before. There was no personal history of any skin disorders, allergies, taking any medications or having fever. On examination, there were erythematous and edematous lesions with central erosions and single vesicles present mainly on the trunk, limbs, hands and feet (Figure 1A and B). The patient has been treated with topical corticosteroid and with antibiotic (betamethasone dipropionate with gentamicin), loratadine, and oral antibiotic (amoxicillin) without any improvement. Her healthy male neonate (birth weight of 5 kg) was delivered with a 5-minute Apgar score of 10. The baby did not have any skin lesions. The patient’s laboratory tests showed elevated levels of VZV IgG and IgM and HSV IgG, and IgM antibodies. The working diagnosis of chicken pox was established, but treatment with acyclovir at a daily dose of 5 mg/kg iv did not yield satisfactory results. Presence of vesiculobullous lesions suggested a blistering disease, and histopathological examination, direct immunofluorescence test (DIF), indirect immunofluorescence test (IIF), ELISA and salt – split technique test were performed. Histopathological findings revealed large subepidermal blister filled with eosinophils and neutrophils, subepidermal spongiosis with perivascular lymphocytic infiltrate with granulocytes and no acantholysis. DIF demonstrated linear depositions of IgG (++), and C3 (+++) complement along the dermo-epidermal junction (Figure 2). IIF performed on monkey esophagus revealed linear deposits of IgG at the dermal‐epidermal junction. ELISA confirmed presence of circulating IgG antibodies against BP180 in titer 1:160. No test for anti-BP230 titer was performed. Salt-split skin revealed a roof pattern of the immunofluorescence. The final diagnosis of PG was made and 40 mg of oral prednisone (0.5 mg/kg) daily was started. A single intramuscular injection of 4 mg dexamethasone was given by the on-call doctor due to exacerbation of the lesions and the patient’s discomfort, just before the oral prednisone was introduced. The treatment was not causing any side effects and the dose of prednisone was lowered every 2 weeks over 7 months. Patient’s skin lesions gradually improved and finally subsided with no recurrence. As the patient was unable to attend follow-up appointments in person, no further PG monitoring laboratory tests were performed.
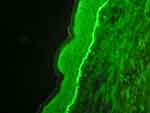 |
Figure 2 DIF demonstrated linear depositions of IgA (+), IgG (++), and C3 (+++) complement along the dermo-epidermal junction. |
Discussion
Epidemiology
Depending on the literature, the incidence of PG is difficult to establish and has been estimated at 0.5 to 2 cases per 1 million women in France, Kuwait, Iran, and Germany. PG occurs in 1 of 60,000 pregnancies worldwide.2,8,14
Jenkins et al10 summarized the data from 142 pregnancies complicated by PG and reported that the time of onset of the disease ranged from 5 weeks gestation to 35 days postpartum. Of the 117 pregnancies, 21 (17.9%) presented in the first trimester, 40 (34.2%) in the second trimester, and a further 40 (34.2%) presented in the third trimester. In 16 out of 117 (13.7%), the eruption began postpartum. Ambros-Rudolph et al1 described 21 patients with PG, ten of whom (48%) were primigravidae and all had a single gestation pregnancy. One out of the eleven multiparous women (9%) had a previously affected pregnancy. The rash started in 15 patients (71%) during the third trimester, in 6 (29%) during the second trimester.3
PG usually resolves within 6 months after delivery.8 Nonetheless, it may persist or deteriorate due to a sudden increase in the level of antibodies, natural fluctuations in female sex hormones, and later can be triggered by subsequent pregnancies, menstruation, or treatment with estrogens and progesterone-containing oral contraceptives.15 Single cases of PG have been described in association with trophoblastic tumors, such as choriocarcinoma, and hydatidiform mole.4,12,13,16 According to Jenkins et al,10 the median duration of symptoms was 16 weeks, and most patients became symptom-free 6 months after the delivery. The duration of postnatal manifestations varied between 2 weeks and 12 years. The recurrences of PG in subsequent pregnancies were reported in 33% to 50% of patients with usually an earlier onset and more severe course than the first episode of the disease.8
Pathogenesis
The pathogenesis of PG is similar to the pathogenesis of bullous pemphigoid as PG is considered to be a variant of BP. Both BP and PG, are associated with the presence of IgG autoreactive antibodies directed against BP180 (also known as BPAG1 or collagen XVII) and partially against BP230 which constitute the hemidesmosomal proteins within the dermo-epidermal junction.5,6,8 This results in loss of connection between dermis and epidermis, and the formation of bullae, erosions, and subsequent inflammation. BP180 is expressed in the skin but also in the first trimester of pregnancy in the placental tissue – trophoblastic cells, and in the amniochorionic stromal cells.5 It starts when the abnormal expression of major histocompatibility complex (MHC) class II antigens in placental stromal cells leads to the presentation of BP180 protein to the maternal immune system which recognized it as foreign.8 This leads to the production of anti-placental IgG antibodies that cross-react with the same BP180 proteins in the skin, causing inflammation and subepidermal blister formation. Moreover, immunologically PG is significantly associated with the maternal MHC class II antigens haplotypes HLA-DR3 and HLA-DR4, which have been reported in majority of patients with PG.8
Clinical Presentation
Initially, the disease presents with pruritic urticarial papules and annular plaques, followed by formation of vesicles and finally large, tense bullae on an erythematous base. It usually starts from the periumbilical area and may spread to the rest of the trunk, upper and lower extremities and even palms and feet. Typically, the face and mucous membranes are spared.4 In some patients severe pruritus could be the only symptom, which makes the diagnosis difficult to establish.14
Diagnosis
The diagnosis of PG is based on a characteristic clinical picture, histopathological findings, and the results of the direct immunofluorescence (DIF) test. Histopathological examination of the affected skin depends on the stage of the disease. In the early urticarial stage, the histopathologic is characterized by edema of the superficial and deep dermis with perivascular infiltrate consists of lymphocytes, histiocytes and eosinophils. In the bullous stage, there are subepidermal blisters formations filled with eosinophils and mixed perivascular infiltrate.16 Those histopathological findings are nonspecific and could be seen in other dermatoses eg polymorphic eruption of pregnancy (PEP). Thus, the gold standard for diagnosis of PG is DIF of perilesional skin. DIF test reveals a linear deposition of C3 complement and less frequently IgG along the basement membrane zone. Deposition of C3 is present in 100% cases, whereas deposition of IgG in 25–50% of cases. Interestingly, DIF may remain positive from 6 months to 4 years after clinical remission was achieved.8,16 Indirect immunofluorescence (IIF) test shows circulating IgG antibodies in the patient’s serum in 30–100% of cases. ELISA typically reveals circulating IgG antibodies against BP180, particularly against the NC16A domain, with specificity of 94–98% and sensitivity of 86%–97%.8 ELISA is also suitable for monitoring disease activity as serum levels of anti-BP180/NC16A correlate with disease severity. Immunoblotting can be also used for monitoring purposes.
Differential Diagnosis
In the initial phase PG cannot be distinguished from other dermatoses of pregnancy including not only polymorphic eruption of pregnancy, which is the main differential diagnosis, but also from atopic eruption of pregnancy (AEP) and intrahepatic cholestasis of pregnancy (ICP).
PEP shares many clinical features with PG such us: onset of symptoms, localization, intense pruritus, and urticarial morphology. PEP typically presents late in pregnancy, usually during the third trimester. Patients with PEP typically develop urticarial papules and plaques. Lesions are initially localized on the abdomen, arising within the striae. In the pre-bullous stage of PG, the differentiation between these two diseases is difficult, both in clinical picture and histopathology. However, in polymorphic eruption of pregnancy DIF is negative.9,13
AEP is the most common dermatosis in pregnancy, accounting for up to 50% of cases. It has an earlier onset, in the first and second trimester. Eczematous or papular skin lesions are located mostly on the trunk and limbs. The histopathological findings of AEP are nonspecific, DIF and IIF are negative, whereas the serum level of total IgE may be elevated.1 Our patient had positive DIF and IIF, which excludes the diagnosis of AEP.9
Intrahepatic cholestasis of pregnancy is characterized by intense pruritus. The only skin lesions, secondary to scratching, are excoriations or prurigo-like nodules, usually localized on the extremities. Diagnosis is confirmed by elevated levels of bile acids (>10 µmol) and abnormal liver function tests. Our patient did not have any of those laboratory abnormalities.9
Bullous pemphigoid (BP) and gestational pemphigoid share similar clinical, histopathological, and immunological features.8 Genetically PG shows an association with HLA – DR3 and HLA – DR4, while BP is associated with HLA – DQ3.14 Timing of onset is a differentiating factor, as BP usually starts later in life and PG is exclusively connected with pregnancy. Occurrence of autoimmune blistering skin diseases have been reported with trauma as a triggering factor.28 Other than delivery, our patient did not have any history of injury and the typical clinical picture with laboratory tests results confirmed the diagnosis of PG.
Treatment
The main goal of the treatment is to reduce pruritus and prevent the formation of new blisters. The treatment strategy depends on the severity of the affected skin lesions and whether our patient is pregnant or postpartum.
As for pregnant patients, in mild cases, the use of topical corticosteroids is sufficient while in more severe cases, treatment with oral corticosteroids may be necessary. Genovese et al29 systematically reviewed 109 articles including 140 PG patients published between 1970 and 2020 and analyzed treatments options and clinical outcomes. They reported that systemic corticosteroids ± topical corticosteroids and/or antihistamines were the most frequently prescribed treatment modality (n = 74/137; 54%), with complete remission achieved in 114/136 (83.8%) patients.
During pregnancy, topical corticosteroids of mild or moderate potency are preferred to potent or very potent ones because of the risk of fetal growth restriction associated with the latter.5 The preferred oral corticosteroids are prednisone and prednisolone as they are inactivated by the 11-β-hydroxysteroid dehydrogenase enzyme of the placenta, resulting in a lower concentration of the corticosteroid reaching the fetus. Fluorinated corticosteroids (betamethasone and dexamethasone) are not metabolized by placental dehydrogenase enzyme; therefore, they are less suitable in PG treatment.5 Among systemic corticosteroids, Genovese et al29 described prednisone (n = 47/109;43.1%) as the most frequently administered corticosteroid. It was followed by prednisolone, betamethasone, methylprednisolone, dexamethasone, and fluocortolone, regardless of pregnancy status. The treatment with prednisolone usually starts from a dose of 0.5 mg/kg/day, which is lowered gradually depending on the achieved clinical improvement and may be increased with the appearance of new blisters.8 The mean initial prednisone-equivalent dosage of systemic corticosteroids reported by Genovese et al29 was 53 mg/day, with mean maximum dosage of approximately 72 mg/day. Treatment duration postpartum is individualized and depends on the speed of remission achieved, so close follow-up for those patients is necessary. Majority of the patients are free of the symptoms 6 months after delivery. Fortunately, treatment with systemic corticosteroids used in PG does not prevent breastfeeding.5 Second-generation antihistamines are recommended to control pruritus.
For patients who developed PG after delivery, a wider range of therapeutic options is available. To start with, a variety of topical and systemic corticosteroids can be used more safely. Genovese et al in their systematic review presented data on steroid-sparing agents used in the treatment of PG including: IVIG, azathioprine, dapsone, cyclosporine, pyridoxine, plasmapheresis, minocycline, nicotinamide, immunoadsorption, rituximab, ritodrine, doxycycline, erythromycin, cyclophosphamide, and methotrexate. Among the steroid-sparing treatment, the most frequently used was intravenous immunoglobulin therapy (n = 12/54; 22.2%), followed by azathioprine, dapsone, cyclosporine and pyridoxine. Pre- and postnatal treatment with cyclosporine combined with prednisolone has been reported in three cases of non-breastfeeding women with good tolerance and good treatment response,7 and in one case cyclosporine was used with intravenous immunoglobulin (IVIG) in persistent postnatal PG.22 Tourte et al23 described the efficacy of the preventative treatment in recurrent case of PG with two infusions of 1 g rituximab received by the patient at week 9 and 11 of her fifth pregnancy. According to Jolles,25 it is more beneficial to treat resistant PG with IVIG therapy combined with standard immunosuppressive therapy than with IVIG alone.24 Garvey et al26 reported chemical oophorectomy with goserelin (a luteinizing hormone) as a promising treatment for severe, chronic gestational pemphigoid. Finally, there are reports that patients with PG refractory to treatment, who may benefit from plasmapheresis or immunoadsorption treatment.27 Recently Genovese et al29 provided an algorithm for PG treatment based on analyzed studies and current knowledge on bullous pemphigoid therapy. In mild, as well as moderate to severe course of the disease they suggest using high potency topical corticosteroids ± oral antihistamines. Prednisone 0.5 mg/kg/day needs to be added if there is inadequate response to the previous modality. If the disease is still not controlled, increasing the dose to 1 mg/kg/day should be considered. Third-line treatment for pregnant females should include IVIG, azathioprine and finally dapsone. For postpartum patients the order should be different, with dapsone followed by azathioprine and rituximab or IVIG therapy.17–21
Our patient responded well to the treatment with oral prednisone in a daily dose of 40 mg (0.5 mg/kg) and the gradual improvement of her skin lesions was achieved. She did not require any additional treatment.
It must be remembered that PG itself, as well as the treatment given to the pregnant females, may have an influence on fetus’ health. According to the literature, in 3% to 10% of cases, the neonate is directly affected.26 In the first trimester, the use of prednisolone causes an increased risk of malformations especially orofacial clefts and in the last trimester, it may result in intrauterine growth retardation, eclampsia, and premature delivery.2,10 Fortunately, there is no higher risk of stillbirth and miscarriage.11 Moreover, approximately 10% of all neonates of PG mothers can temporary develop mild urticarial or vesicular skin lesions called neonatal pemphigoid, which are caused by an increased level of circulating pemphigoid IgG antibodies in the baby’s serum because of their passive transfer from the maternal circulation. Neonatal pemphigoid usually spontaneously resolves without treatment within months.2
Comment
The purpose of our article was to present a case of the patient with PG which started 2 weeks after delivery. It was treated with systemic corticosteroids with no significant side effects and gradual improvement of skin lesions. Furthermore, we wanted to provide an updated overview on the pathology, clinical picture, treatment, and complications of this rare autoimmune blistering skin disease. Although PG is described in the literature as self-limiting, treatment with topical or/and systemic corticosteroids is usually required to control the course of the disease. Moreover, due to the PG association with obstetrical and neonatal complications (eg, preterm birth/prematurity, fetal growth restriction, neonatal PG), the course of pregnancy and the neonate’s health need to be closely monitored respectively by an obstetrician and pediatrician. The mother and the baby must be also observed regarding possible side effects connected with topical and systemic corticosteroids treatment, including adrenal suppression. Each patient with a history of PG needs to be informed about a higher risk of PG relapse not only in subsequent pregnancies but also during ovulation, menstruation and while using oral contraceptives. Those patients should also be warned that a further conversion to bullous pemphigoid is a possibility. To sum up, rare as it is, gestational pemphigoid should be considered in every case of pruritus and vesiculobullous rash in a pregnant or postpartum patient.
Consent
The patient had given written informed consent for the publication of her case details and all accompanying images. Institutional approval is not required to publish the case details.
Acknowledgments
This research was funded by statutory activities of Medical University of Lodz 503/5-064-04/503-01.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ambros-Rudolph CM, Müllegger RR, Vaughan-Jones SA, Kerl H, Black MM. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54(3):395–404. doi:10.1016/j.jaad.2005.12.012
2. Ambros-Rudolph CM. Dermatoses of pregnancy - clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23(3):265–275. doi:10.5021/ad.2011.23.3.265
3. Vaughan Jones SA, Hern S, Nelson-Piercy C, Seed PT, Black MM. A prospective study of 200 women with dermatoses of pregnancy correlating clinical findings with hormonal and immunopathological profiles. Br J Dermatol. 1999;141(1):71–81. doi:10.1046/j.1365-2133.1999.02923.x
4. Lipozenčić J, Ljubojevic S, Bukvić-Mokos Z. Pemphigoid gestationis. Clin Dermatol. 2012;30(1):51–55. doi:10.1016/j.clindermatol.2011.03.009
5. Huilaja L, Mäkikallio K, Tasanen K. Gestational pemphigoid. Orphanet J Rare Dis. 2014;9:136. doi:10.1186/s13023-014-0136-2
6. Huilaja L, Mäkikallio K, Sormunen R, Lohi J, Hurskainen T, Tasanen K. Gestational pemphigoid: placental morphology and function. Acta Derm Venereol. 2013;93(1):33–38. doi:10.2340/00015555-1370
7. Huilaja L, Mäkikallio K, Hannula-Jouppi K, Väkevä L, Höök-Nikanne J, Tasanen K. Cyclosporine treatment in severe gestational pemphigoid. Acta Derm Venereol. 2015;95(5):593–595. doi:10.2340/00015555-2032
8. Sävervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441–449. doi:10.2147/CCID.S128144
9. Sävervall C, Sand FL, Thomsen SF. Dermatological diseases associated with pregnancy: pemphigoid gestationis, polymorphic eruption of pregnancy, intrahepatic cholestasis of pregnancy, and atopic eruption of pregnancy. Dermatol Res Pract. 2015;2015:979635. doi:10.1155/2015/979635
10. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol. 1999;24(4):255–259. doi:10.1046/j.1365-2230.1999.00472.x
11. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26(1):63–68. doi:10.1016/0190-9622(92)70008-4
12. Djahansouzi S, Nestle-Kraemling C, Dall P, Bender HG, Hanstein B. Herpes gestationis may present itself as a paraneoplastic syndrome of choriocarcinoma-a case report. Gynecol Oncol. 2003;89(2):334–337. doi:10.1016/s0090-8258(03)00070-2
13. Soutou B, Aractingi S. Skin disease in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29(5):732–740. doi:10.1016/j.bpobgyn.2015.03.005
14. Hallaji Z, Mortazavi H, Ashtari S, Nikoo A, Abdollahi M, Nasimi M. Pemphigoid gestationis: clinical and histologic features of twenty-three patients. Int J Womens Dermatol. 2016;3(2):86–90. doi:10.1016/j.ijwd.2016.11.004
15. Fania L, Guerriero C, Ricci F, Gagliano MF, De Simone C. Herpes gestationis and oral contraceptive: case report and review of the literature. Dermatol Ther. 2017;30(5):e12518. doi:10.1111/dth.12518
16. Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145(2):138–144. doi:10.1016/j.ejogrb.2009.05.012
17. Intong LR, Murrell DF. Pemphigoid gestationis: current management. Dermatol Clin. 2011;29(4):621–628. doi:10.1016/j.det.2011.06.013
18. Takatsuka Y, Komine M, Ohtsuki M. Pemphigoid gestationis with a complete hydatidiform mole. J Dermatol. 2012;39(5):474–476. doi:10.1111/j.1346-8138.2011.01315.x
19. Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2014;124(1):120–133. doi:10.1097/AOG.0000000000000346
20. Chi CC, Wang SH, Wojnarowska F, Kirtschig G, Davies E, Bennett C. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015;10:CD007346. doi:10.1002/14651858.CD007346.pub3
21. Chi CC, Wang SH, Charles-Holmes R, et al. Pemphigoid gestationis: early onset and blister formation are associated with adverse pregnancy outcomes. Br J Dermatol. 2009;160(6):1222–1228. doi:10.1111/j.1365-2133.2009.09086.x
22. Hern S, Harman K, Bhogal BS, Black MM. A severe persistent case of pemphigoid gestationis treated with intravenous immunoglobulins and cyclosporin. Clin Exp Dermatol. 1998;23(4):185–188. doi:10.1046/j.1365-2230.1998.00342.x
23. Tourte M, Brunet-Possenti F, Mignot S, Gavard L, Descamps V. Pemphigoid gestationis: a successful preventive treatment by rituximab. J Eur Acad Dermatol Venereol. 2017;31(4):e206–e207. doi:10.1111/jdv.13962
24. Kreuter A, Harati A, Breuckmann F, Appelhans C, Altmeyer P. Intravenous immune globulin in the treatment of persistent pemphigoid gestationis. J Am Acad Dermatol. 2004;51(6):1027–1028. doi:10.1016/j.jaad.2004.07.052
25. Jolles S. A review of high-dose intravenous immunoglobulin (hdIVIg) in the treatment of the autoimmune blistering disorders. Clin Exp Dermatol. 2001;26(2):127–131. doi:10.1046/j.1365-2230.2001.00779.x
26. Garvey MP, Handfield-Jones SE, Black MM. Pemphigoid gestationis–response to chemical oophorectomy with goserelin. Clin Exp Dermatol. 1992;17(6):443–445. doi:10.1111/j.1365-2230.1992.tb00256.x
27. Patsatsi A, Vavilis D, Tsikeloudi M, Kalabalikis D, Sotiriadis D. Refractory pemphigoid gestationis postpartum. Acta Obstet Gynecol Scand. 2012;91(5):636–637. doi:10.1111/j.1600-0412.2012.01379.x
28. Kim YB, Choi HS, Cho HK, Seo GW. Diagnosis and treatment of bullous pemphigoid that developed twice after total knee replacement arthroplasty: a case report. BMC Musculoskelet Disord. 2021;22(1):118. doi:10.1186/s12891-021-04000-6
29. Genovese G, Derlino F, Cerri A, et al. A systematic review of treatment options and clinical outcomes in pemphigoid gestationis. Front Med. 2020;7:604945. doi:10.3389/fmed.2020.604945
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.