Back to Journals » Patient Preference and Adherence » Volume 15
Patient Knowledge of Safe Use of ER/LA Opioid Analgesics Following Implementation of the Class-Wide REMS: A Survey Study
Authors Esposito DB, Desai VC, Stephenson JJ , Cepeda MS , Lyons JG, Holick CN, Wedin GP , Lanes S
Received 30 October 2020
Accepted for publication 30 January 2021
Published 24 February 2021 Volume 2021:15 Pages 431—442
DOI https://doi.org/10.2147/PPA.S286935
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Daina B Esposito,1 Vibha CA Desai,1 Judith J Stephenson,1 M Soledad Cepeda,2 Jennifer G Lyons,1 Crystal N Holick,1 Gregory P Wedin,3 Stephan Lanes1 On behalf of members of the REMS Program Companies Metrics Subteam
1HealthCore, Inc., Wilmington, DE, USA; 2Janssen Research and Development, Titusville, NJ, USA; 3Upsher-Smith Laboratories, LLC, Maple Grove, MN, USA
Correspondence: Judith J Stephenson
HealthCore Inc, 123 Justison Street, Suite 200, Wilmington, DE, 19801, USA
Tel +1 302-547-5770
Email [email protected]
Background/Rationale: The US Food and Drug Administration (FDA) approved a Risk Evaluation and Mitigation Strategy (REMS) for extended release/long-acting (ER/LA) opioids in 2012. The purpose of this study was to assess patient knowledge of the safe use of these products following implementation of the REMS and to determine possible effects of the REMS, including impact on medication access.
Objective: To assess patient knowledge of safe use of ER/LA opioids and use of REMS patient education tools such as the Medication Guide (MG) and Patient Counseling Document (PCD).
Methods: This was a cross-sectional survey of commercially insured (Commercial) and Medicare Advantage-insured (Medicare) adults with ≥ 1 pharmacy claim for an ER/LA opioid (10/01/2015 – 02/28/2017) in the HealthCore Integrated Research Database and Medicaid-insured (Medicaid) adult members of a research panel, about their knowledge of safe use of ER/LA opioids and receipt/comprehension of the MG and PCD.
Results: Survey respondents consisted of 382 Commercial, 43 Medicare and 40 Medicaid adults. While ≥ 95% of respondents received and read the MG, fewer were aware of the PCD (Commercial: 47%, Medicare: 65%, Medicaid: 53%). Almost 75% of the knowledge questions were answered correctly by ≥ 80% of all respondents; fewer respondents recognized that use of opioids as directed can lead to death (Commercial: 73%, Medicare: 56%, Medicaid: 63%), the MG should be read at each dispensing (Commercial: 78%, Medicare: 53%, Medicaid: 75%), opioids should not be stored in the medicine cabinet (Commercial: 77%, Medicare: 79%, Medicaid: 58%), missed doses should not be taken as soon as possible (Commercial: 56%, Medicare: 51%, Medicaid: 50%), and pills should not be crushed (Commercial: 85%, Medicare: 67%, Medicaid: 52%).
Conclusion: Although most respondents reported reading and understanding the MG and exhibited knowledge of safe use of ER/LA opioids, providers’ use of the PCD and increased understanding of safe use core messages need reinforcement.
Keywords: Risk Evaluation and Mitigation Strategy, opioids, patient knowledge
Introduction
Extended release (ER) and long-acting (LA) opioid analgesics are used in the United States (US) for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.1 These medications include buprenorphine, fentanyl, hydromorphone, hydrocodone, methadone, morphine, oxycodone, oxymorphone, and tapentadol. A Risk Evaluation and Mitigation Strategy (REMS) for ER/LA opioid medications was approved by the US Food and Drug Administration (FDA) on 09 July 20122,3 as one element of a national response to increasing rates of opioid overdose and death.4–6 In 2018, this opioid REMS was further expanded to include immediate release opioid analgesics, a program now known as the Opioid Analgesic REMS; however, this study was conducted under the ER/LA Opioid Analgesics REMS. The REMS comprises class-wide safety labeling changes as well as educational efforts that include (1) Medication Guides (MG), FDA-approved printed materials that are provided by pharmacies at the point of medication dispensing with the intent that patients read them prior to taking the dispensed medication that address issues specific to particular drugs and drug classes, and contain information for helping patients avoid serious adverse events; (2) Patient Counseling Documents (PCDs) to be used by ER/LA opioid prescribers and other healthcare providers for facilitating education of and discussions with patients; and (3) voluntary additional prescriber training on all ER/LA opioid analgesics. The educational documents are available online (www.er-la-opioidrems.com).
The purpose of this study was to assess (1) whether patients had received the MG and PCD documents intended to educate them about the safe use of ER/LA opioids; and (2) patients’ knowledge of the safe use of these products following the start of REMS implementation. This study was one of the several studies7 assessing the impact of ER/LA opioid analgesic REMS following its implementation in July 2012.
Methods
Study Design and Patient Population
A cross-sectional survey of adults who had used ER/LA opioid analgesics between October 1, 2015 and February 28, 2017 was conducted. The Commercial and Medicare Advantage patient populations were identified from medical and pharmacy claims in the HealthCore Integrated Research Database (HIRD), a large administrative claims database with longitudinal claims data from members of 14 geographically dispersed US health plans.
The Medicaid population was identified from members of an online survey research panel who indicated they had Medicaid insurance at some time during the past year. Panel members consisted of a pre-screened group of individuals who expressed willingness to participate in surveys, were identified through a recruitment process that complied with standards published by the European Society for Opinion and Marketing Research and Market Research Society and were characterized by a number of attributes, including type of health insurance.8
The claims-based Commercial and Medicare Advantage populations consisted of currently active, survey eligible adults, age 18 years and older, with Commercial or Medicare Advantage health insurance who filled at least one prescription for an ER/LA opioid analgesic, including transdermal patch, transmucosal film, methadone, and oral formulations, within the most recent 12 months of claims data available at the time the sample was identified. Patients with claims for drug or substance abuse were excluded to comply with Title 42 of the Code of Federal Regulations Part 2 (42 CFR Part 2) Rule which protects the privacy of individuals undergoing substance and alcohol abuse treatment.
The Medicaid panel population consisted of adults, age 18 or older, who indicated they were covered by Medicaid insurance at some time during the most recent 12-month period and had used at least one ER/LA opioid analgesic, including transdermal patch, transmucosal film, methadone, and oral formulations, in the most recent 12-months.
Since the Commercial patient population may not be representative of the total patient population using ER/LA opioid analgesics, the Medicaid and Medicare Advantage patient populations were added to supplement the Commercial population. The targeted number of completed surveys from each of these populations was at least 40 in addition to the targeted number of 400 completed Commercial surveys.
Survey Process
Recruitment letters and emails were sent to Commercial (age 18 years and older) and Medicare Advantage (age 65 and older) patients meeting the criteria described above to inform them of the study and solicit their participation. Patients who did not respond to the recruitment letter and/or email and had valid landline telephone numbers were called by interviewers and recruited over the telephone. Survey administration was either via the internet or over the telephone with an interviewer.
Medicaid (age 18 years and older) panel members were recruited by email and completed the survey via the internet. The recruitment email was similar to the letter and email used to recruit Commercial and Medicare Advantage respondents and their survey process was similar to that of the Commercial and Medicare Advantage respondents.
All respondents had to provide electronic or verbal consent and meet study inclusion criteria before starting the 20-minute survey that assessed their level of knowledge about the serious risks associated with the use of their ER/LA opioid analgesic medications and their experiences with the MG and PCD. Screening questions excluded respondents who reported they did not fill a prescription for an ER/LA opioid analgesic within the past 12 months, were employed as a licensed physician, could not speak or understand English, or indicated current or past employment for themselves or their immediate family that posed a conflict of interest (e.g., pharmaceutical company). Once the targeted number of completed surveys was reached (at least 400 Commercial respondents; 40 Medicare respondents and 40 Medicaid respondents), the survey was closed.
The protocol and all survey-related materials were approved by the New England Institutional Review Board (NEIRB) prior to the conduct of the study and all patient data were handled in compliance with the regulations of the US Insurance Portability and Accountability Act of 1996.
Knowledge Assessment Statements
Respondents’ level of knowledge about the serious risks associated with the use of ER/LA opioid analgesics was assessed with 23 questions in which they were asked to identify whether the question was true, false, or do not know. Questions were related to the following key risk messages: (1) The patient understands the serious risks associated with the use of their ER/LA opioid analgesics (7 questions); (2) The patient knows what to do if they take too much drug (2 questions); (3) The patient understands the need to store the drug in a safe place (3 questions); (4) The patient knows they should not share the drug with anyone (2 questions); and (5) The patient understands how to use the drug safely (9 questions).
In addition, there were 5 additional questions that were tailored to the type of ER/LA opioid analgesic used. For example, only patients using a transdermal delivery system were presented with the statement “it is okay to cut your patch in half if you want to use less medicine.”
Table 1 presents the knowledge assessment questions and their correct responses by risk message.
 |
Table 1 Knowledge Assessment Questions by REMS Risk Message |
Receipt and Usage of MG and PCD and Healthcare Provider Behaviors
Respondents answered questions about their most recent use of ER/LA opioids and their understanding of the MG and PCD. They were also asked about how often their prescribing health care providers provided counseling on a variety of topics (i.e., risks, discontinuation, side effects, safe disposal, and medication handling storage) in the past 12 months.
Analysis
Descriptive analyses were conducted that determined the proportion of respondents who reported receiving, reading and understanding the MG, and whether providers referred to and/or the extent that patients understood the PCD at visits where opioids were prescribed. We calculated a Knowledge Assessment Score (KAS) from the knowledge assessment questions that was defined as the proportion of the 23 questions that respondents answered correctly using unweighted addition and converted the proportion to a percentage after dichotomizing responses as correct or incorrect. Respondents received a score of 1 for each correct response; an incorrect response was defined as any response other than the correct response and received a score of 0. A threshold of at least 80% correct was considered “acceptable knowledge.”9 We assessed the distribution of KAS scores and determined the proportion of respondents correctly answering each individual question. The results were stratified by insurance type (Commercial, Medicare Advantage, Medicaid).
All analyses were conducted using SAS® Enterprise Guide version 7.12 (SAS Institute Inc., Cary, NC, USA).
Results
Participation
The survey sample list consisted of the contact information of 21,293 Commercial and Medicare Advantage patients who met the study inclusion/exclusion criteria. Recruitment letters or emails were sent to 14,242 of these patients, and 2615 (18%) responded to the letter or email request. Of the patients who responded, 1939 (74%) refused to participate, 676 (26%) provided verbal or electronic consent to participate of which 197 (29%) did not meet survey screening criteria. Of the 479 patients who qualified, 54 (11%) started the survey but did not complete it and 425 (89%) completed the survey (Figure 1). Patients who completed the survey were compensated for their time.
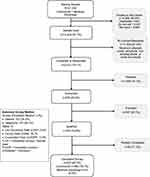 |
Figure 1 Survey sample disposition. |
An additional 40 patients with Medicaid insurance were separately recruited through an online survey research panel and completed the survey online.
Patient Characteristics
The mean ages of the Commercial, Medicare Advantage and Medicaid respondents were 53.1, 72.5 and 47.5 years, respectively (Table 2). A large proportion of respondents were female (Commercial: 64%, Medicare Advantage: 70% and Medicaid: 58%) and white (Commercial: 87%, Medicare Advantage: 86% and Medicaid: 93%). In terms of marital status, 62% among Commercial versus 35% among Medicare Advantage and 43% among Medicaid respondents were married/living with a partner. Only 25% of the Commercial versus 51% and 43% of Medicare Advantage and Medicaid respondents, respectively, had an annual income under $25,000. Thirty five percent of Commercial, 19% of Medicare Advantage, and 48% of Medicaid respondents had an education level of college graduate or higher. Oral ER/LA opioid use was more common among Commercial (68%) and Medicaid (63%) respondents whereas only 49% of Medicare Advantage respondents were oral ER/LA opioid users. Slightly over half of all respondents had their most recent prescription dispensed within one month prior to the survey (Table 2).
 |
Table 2 Demographic and Clinical Characteristics of Survey Respondents |
Receipt and Usage of MG and PCD
Nearly all respondents reported receiving and/or reading the MG (98% Commercial, 100% Medicare Advantage, 95% Medicaid). A majority in all groups reported that they understood most of the information (Table 3).
 |
Table 3 Patient Recall of Medication Guide, Patient Counseling Document, and Specific Counseling Topics |
Far fewer respondents indicated an awareness of the PCD. It was reported as received from and/or referenced by their healthcare provider by 47% of Commercial, 65% of Medicare Advantage, and 53% of Medicaid respondents. Fifty-seven percent of Commercial, 50% of Medicare Advantage and 76% of Medicaid respondents reported that their healthcare provider referred to or discussed the PCD within the last 12 months when prescribing their current ER/LA opioid analgesic (Table 3).
Healthcare Provider Behaviors
A large majority of the respondents stated that their healthcare providers always or regularly talked about how much medication to take or use when ER/LA opioid analgesics were prescribed (Commercial: 86%, Medicare Advantage: 81%, Medicaid: 88%), while fewer stated that their healthcare providers always or regularly cautioned them about important risks associated with the use of ER/LA opioid analgesics, including overdose or taking too much (Commercial: 68%, Medicare Advantage: 77%, Medicaid: 65%) and always or regularly instructed them about keeping ER/LA opioid analgesics safe and away from children (Commercial: 65%, Medicare Advantage: 74%, Medicaid: 63%; Table 3). While 79% percent of Commercial and 88% of Medicaid respondents reported that their physicians always or regularly asked about their medical history, only 65% of Medicare Advantage respondents reported the same. Deficits were also observed in several other healthcare provider behaviors across the groups. However, some behaviors were more often reported by Medicaid than Commercial and Medicare Advantage respondents, such as: healthcare provider always/regularly discussed how to safely discontinue ER/LA opioid analgesics if they are no longer needed (Medicaid: 68% vs. Commercial: 50% and Medicare Advantage: 49%); always or regularly instructed about the importance of how to safely dispose any unused ER/LA opioid analgesics (Medicaid: 60% vs. Commercial: 46% and Medicare Advantage: 56%); always or regularly talked about what to do with extra medication when ER/LA opioid analgesics were prescribed (Medicaid: 58% vs Commercial: 47% and Medicare Advantage: 51%). Healthcare provider behaviors such as always or regularly counseling on the most common side effects of ER/LA opioids and always or regularly not sharing ER/LA opioid analgesic with anyone else were consistent across insurance groups (65–68% and 63–67%, respectively; Table 3).
Knowledge of Serious Risks of ER/LA Opioid Analgesics
Eighty-five percent of Commercial, 60% of Medicare Advantage and 53% of Medicaid respondents achieved a KAS score (i.e., proportion of knowledge questions that a respondent answered correctly) of at least 80%, the threshold considered “acceptable knowledge.” The mean KAS was 87 (standard deviation [SD] 9.73) for Commercial, 81 (SD 13.01) for Medicare Advantage, and 78 (SD 15.53) for Medicaid respondents. Overall, there were 17 of 23 knowledge questions that at least 80% of respondents answered correctly including 13 questions that were answered correctly by at least 90% of respondents. Fewer respondents were aware that opioids can cause serious side effects that can lead to death even when taken as recommended (Commercial: 73%, Medicare: 56%, Medicaid: 63%), the MG should be read at each dispensing (Commercial: 78%, Medicare: 53%, Medicaid: 75%), ER/LA opioid analgesics should not be stored in a medicine cabinet with other medications in the household (Commercial: 77%, Medicare: 79%, Medicaid: 58%), missed doses should be taken as soon as possible (Commercial: 56%, Medicare: 51%, Medicaid: 50%), and ER/LA opioid analgesic pills should not be split or crushed if the respondent is having trouble swallowing their medication (Commercial: 85%, Medicare: 67%, Medicaid: 52%) (Table 4).
 |
Table 4 Knowledge Assessment Scores and Statements (KAS) |
Discussion
This study evaluated the use of education tools such as the MG by pharmacists and the PCD by healthcare providers and assessed patient knowledge of the safe use of ER/LA opioid analgesics. Results were obtained from surveying ER/LA opioid analgesic users with Commercial, Medicare Advantage and Medicaid health insurance plans. Although many key messages from the MG and PCD were understood by most respondents, deficits in the knowledge of certain risk messages were more common among Medicare Advantage and/or Medicaid respondents.
Approximately 50% or more of respondents in all groups reported that healthcare providers always or regularly engaged in a variety of counseling behaviors and/or used the PCD. While patients may not be aware that their healthcare providers are using the PCD or that comprehensive screening and discussion may take place with the assistance of other tools or be performed by other members of the healthcare team, promoting improved counseling and use of the PCD by all types of healthcare providers may help to improve patient knowledge in areas where gaps were observed.
Differences in responses across the insurance groups in the survey results for knowledge of certain risk messages may be at least partially explained by differences in the demographic characteristics of the survey respondents that are typical of Commercial versus Medicare Advantage and Medicaid groups, such as age, marital status, income and education level. Areas of action include promoting the use of the PCD across all provider specialties and ensuring that information about potential serious risks associated with opioids, safe storage, and formulation-specific risks is stressed to patients in a way that is understandable to them.
Commercial respondents made up the largest patient subgroup in this study. The HIRD is demographically representative of the Commercial population in the US in terms of gender and age; however, applicability of results to individuals without medical insurance may be limited. This was the fourth and final year that this FDA-mandated post-marketing survey was conducted, and in this year the population was expanded to include Medicare Advantage and Medicaid patients in addition to the Commercial patients to provide a more comprehensive view. While we were able to identify Medicare Advantage patients from their administrative claims in the HIRD and survey them, we did not have access to Medicaid patients and had to depend on their recruitment from a research panel. We were not able to verify the insurance status and ER/LA opioid analgesic use of the Medicaid patients. Based on the small number of Medicare Advantage and Medicaid respondents, it is possible that these respondents may differ from the broader population of individuals with US government sponsored insurance.
While we evaluated respondents’ abilities to correctly identify key risk messages from the MG and PCD, we did not measure the extent to which this knowledge is applied. For example, while it is assumed that awareness that ER/LA opioid analgesics should not be used with alcohol translates into actual avoidance of this dangerous behavior, the extent to which this is true is not captured by this assessment.
Further, it is possible that responses to some of the KAS component items are informed by common sense and general awareness of opioid risks promoted by various public health campaigns more than by genuine recollection of the education materials received. Although the knowledge questions were directly related to content in the REMS education tools, patients may receive information about the safe use of their medication from many different sources. As such, observed patient knowledge cannot necessarily be attributed solely to the REMS.
Conclusion
The ER/LA Opioid REMS appears to be effective in promoting receipt of the MG by patients using ER/LA opioid analgesics, and although respondents demonstrated knowledge of the safe use of ER/LA opioid analgesics with respect to many of the questions associated with key risk messages included in the MG, there were questions where level of knowledge varied. Overall, the PCD was less widely received and used by healthcare providers to educate patients. Additional efforts appear warranted to reinforce knowledge of the core messages of safe use and promote more consistent provider counseling and PCD use across prescribing specialties and patient demographic groups.
Disclosure
JJ Stephenson, CN Holick and S Lanes are employees of HealthCore, which received funding from the REMS Program Companies (RPC) to conduct the study. DB Esposito, VCA Desai and JG Lyons were employees of HealthCore at the time the study was conducted. MS Cepeda is employed by Janssen Research and Development, and GP Wedin is employed by Upsher-Smith Laboratories, LLC. The authors report no other conflicts of interest in this work.
References
1. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11(2 Suppl):S5–S62.
2. Stanos S. Evolution of opioid risk management and review of the classwide REMS for extended-release/long-acting opioids. Phys Sportsmed. 2012;40(4):12–20. doi:10.3810/psm.2012.11.1975
3. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategy (REMS) for Extended-Release and Long-Acting Opioids, 2013. Available from: https://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf. Accessed February 17, 2021.
4. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths–United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. doi:10.15585/mmwr.mm6450a3
5. Hasegawa K, Espinola JA, Brown DF, Camargo CA
6. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. doi:10.1056/NEJMsa1406143
7. Divino V, Cepeda MS, Coplan P, Maziere JY, Yuan Y, Wade RL. Assessing the impact of the extended-release/long-acting opioid an-algesics risk evaluation and mitigation strategies on opioid prescription volume. J Opioid Manag. 2017;13(3):157–168. doi:10.5055/jom.2017.0383
8. Hays RD, Liu H, Kapteyn A. Use of internet panels to conduct surveys. Behav Res Methods. 2015;47(3):685–690. doi:10.3758/s13428-015-0617-9
9. Knox C, Hampp C, Willy M, Winterstein AG, Pan GD. Patient understanding of drug risks: an evaluation of medication guide assessments. Pharmacoepidemiol Drug Saf. 2015;24(5):518–525. doi:10.1002/pds.3762
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
