Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 9
Patient considerations in type 2 diabetes – role of combination dapagliflozin–metformin XR
Received 23 September 2015
Accepted for publication 21 January 2016
Published 23 February 2016 Volume 2016:9 Pages 25—35
DOI https://doi.org/10.2147/DMSO.S81565
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Catherine M Kuecker, Eva M Vivian
Pharmacy Practice Division, School of Pharmacy, University of Wisconsin-Madison, Madison, WI, USA
Purpose: The purpose of this review article is to provide guidance to health care providers regarding the use of dapagliflozin–metformin XR (extended release) as a therapeutic option for the treatment of patients with type 2 diabetes mellitus (T2DM).
Methods: The PubMed database was searched through August 2015 to identify clinical trials and meta-analyses evaluating the use of the sodium–glucose cotransporter type 2 inhibitor dapagliflozin administered as monotherapy or in combination with metformin.
Results: Fourteen studies were included for this review, six of which evaluated dapagliflozin in combination with metformin, one of which evaluated dapagliflozin monotherapy, and four of which evaluated dapagliflozin as an add-on therapy to other antidiabetic agents. The combination of dapagliflozin and metformin resulted in an A1C decrease of up to 2%, weight loss of 2–3 kg, and modest systolic blood pressure decrease of 3–5 mmHg. However, long-term effects on cancer and cardiovascular health are still being investigated. Providing patients with a fixed-dose combination therapy such as dapagliflozin-metformin XR can increase medication adherence and patient satisfaction, and improve glycemic control. Dapagliflozin–metformin XR is ideal because it can be administered orally once a day, is associated with a low risk of hypoglycemia, and provides the added benefit of weight reduction and modest blood pressure lowering.
Conclusion: The unique combined mechanism of action and favorable efficacy and safety profile of dapagliflozin–metformin XR support consideration of this fixed-dose combination as a treatment option for patients with T2DM.
Keywords: fixed-dose combination therapy, metformin, SGLT2 inhibitor
Introduction
The diabetes epidemic has become a major health threat in the US. While an alarming 14% of the adult US population have diabetes, one-third still remain undiagnosed.1 The prevalence of diabetes is expected to increase, as 20% of the 86 million people with prediabetes are expected to develop the disease within 5 years.1,2 Type 2 diabetes mellitus (T2DM) accounts for 90%–95% of all cases in adults and is associated with factors including older age, obesity, sedentary lifestyle, family history, gestational diabetes, and ethnicity.3 Patients with T2DM are at an increased risk of developing serious health complications such as heart disease, stroke, nephropathy, hypertension, blindness, and neuropathy.3
Patients with diabetes face multiple challenges while managing this complex disease, which involve extensive lifestyle changes, including increased physical activity, healthy nutrition, blood glucose monitoring, medication management, and problem solving.3–5 Managing complex daily care activities can overwhelm patients and result in nonadherence in one or several areas of self-care management.5–7
The medical cost of diabetes in the US was reported at $245 billion in 2012 and is expected to rise as the prevalence of the disease increases.8 Medication nonadherence contributes significantly to the medical cost of diabetes and compromises patient health by increasing the risk of diabetic complications.9 Lack of understanding about the disease, poor self-care management skills, and medical illiteracy are contributing factors that lead to medication nonadherence.6–10
Pharmacologic management of diabetes and associated conditions is necessary to obtain glycemic control and prevent diabetic complications. Each patient with T2DM requires individualized treatment that takes into account comorbid conditions, cardiovascular disease risk factors, macrovascular and microvascular complications, and the risk of severe hypoglycemia.11 There are currently several antidiabetic agents that target one or more of the pathophysiological pathways that contribute to hyperglycemia.11 In most cases, patients are prescribed at least two medications in order to target multiple pathways and obtain adequate glycemic control. Many patients will also need to take additional medications to manage the comorbid conditions that often accompany diabetes, such as hypertension and dyslipidemia, thus increasing the pill burden.10,11 One study conducted in 875 adults with diabetes above the age of 50 years reported that 50% of the participants were prescribed seven or more medications, including at least two antidiabetic agents.12 Therefore, providing patients with a simpler medication regimen can increase medication adherence and improve glycemic control.9
This paper examines the efficacy and safety of the fixed-dose combination (FDC) of dapagliflozin and metformin XR (extended release) and its place in therapy for the management of T2DM. The patient’s perspective will also be presented to provide guidance to health care clinicians regarding the use of dapagliflozin in combination with metformin.
Rationale for the use of FDC therapy in diabetes
Medication nonadherence is a major cause of poor glycemic control in many patients with T2DM and is associated with further disease progression, and increased hospitalizations and deaths in the US.9 Although patients who adhere to their prescribed medication regimen may incur higher drug costs, several studies report that overall medical costs and hospitalizations are lower for these patients.12,13
Benner et al14 evaluated medication adherence in 6,000 patients in a managed care setting and found an inverse relationship between medication adherence and the number of medications taken. These findings are supported by several clinical trials which reported that patients who were prescribed FDC had higher adherence rates compared to patients who were prescribed free-dose combination regimens.15–17 Long-acting medications requiring once-daily dosing are usually preferred over complex medication regimens requiring multiple doses per day.15,17 Further simplifying the regimen by using a pill that contains more than one drug can also improve patient satisfaction and medication adherence.18,19 Simplifying a medication regimen for a patient with T2DM by using a once-daily FDC such as dapagliflozin–metformin XR can improve patient satisfaction and increase adherence.
Side effects also influence medication adherence. Hypoglycemia, a common side effect of many antidiabetic agents, can negatively impact many aspects of a patient’s life such as work productivity, social activities, and quality of life.19 Hypoglycemia is a common cause of nonadherence with antidiabetic agents.20,21 One study conducted in 6,065 patients reported that only 39% of patients who reported experiencing recent hypoglycemia had a high Morisky scale adherence score.21 There are several antidiabetic agents that promote weight gain and may be negatively received by many patients with T2DM who have cormorbid obesity. One study reported that patients with diabetes who lost weight after initiation of antidiabetic drug therapy had significantly better medication adherence and were less likely to be on medications that promote weight gain than patients who experienced weight gain during treatment.21
Strategies that promote weight loss, which include increased physical activity, healthy nutrition, and the use of antidiabetic medications associated with weight loss, may improve medication adherence. The low incidence of hypoglycemia associated with the use of metformin–dapagliflozin in addition to the weight loss and blood-pressure-lowering effects can be very attractive from a patient’s perspective, which could lead to increased adherence.
Role of SGLT2 inhibitors and metformin for T2DM
T2DM involves multiple organ dysfunction that results in insulin resistance and insulin deficiency.22 During the early stages of T2DM, the ability of insulin to facilitate the transport of glucose into the cell is impaired. Defects in insulin receptor function, insulin receptor signal transduction pathway, glucose transport and phosphorylation, and glycogen synthesis and oxidation contribute to muscle insulin resistance.22 In addition, hepatic insulin resistance results in inappropriate hepatic glucose production in the fed state.22 In the early stages of disease, hypersecretion of insulin by the pancreatic β cells compensates for the pathophysiologic effects of insulin resistance in order to maintain normal glucose concentrations. However, β cell loss occurs over time as a result of increased apoptosis and reduced replication, which results in reduced β cell mass, decreased insulin secretion, and hyperglycemia.22 In addition to β cell dysfunction, pancreatic α cells also play an important role in T2DM pathophysiology. Abnormally high secretion of glucagon by the α cells stimulates excessive hepatic glucose production and leads to hyperglycemia in patients with diabetes.
T2DM also affects the renal system, as the kidneys play a major role in maintaining glucose homeostasis. The majority of glucose is reabsorbed in the proximal tubule of the kidney by sodium–glucose cotransporter type 2 (SGLT2) in order to maintain normal fasting plasma glucose levels.23 In patients without diabetes, when plasma glucose levels exceed the maximal reabsorptive capacity of the kidney, excess glucose begins to spill into the urine.24 However, in the context of T2DM, the expression and activity of SGLT2 is increased;23 therefore, the kidneys have an increased capacity to reabsorb glucose into the bloodstream, which further worsens hyperglycemia.25
There are several classes of antidiabetic medications that target a specific pathophysiologic pathway (Figure 1). For the purposes of this review, SGLT2 inhibitors and metformin are the focus.27–29 A novel mechanism to treat T2DM is through the SGLT2 inhibitors, which act to inhibit SGLT2 in the proximal nephron of the kidney and block glucose reabsorption, causing glucosuria.4 Because SGLT2 inhibitors are independent of insulin action, there is less risk for hypoglycemia,3 and they may be used at any stage in the progression of the disease.26 Other advantages include weight loss and blood-pressure-lowering effects.26
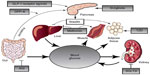  | Figure 1 The organ systems involved in the pathogenesis of T2DM. |
Metformin is a biguanide that suppresses hepatic glucose production via inhibition of gluconeogenesis. Metformin also increases insulin sensitivity and peripheral glucose uptake, and decreases absorption of glucose from the gastrointestinal tract, which results in decreased fasting glucose levels and a decrease in the hemoglobin A1C.27–29 The American Diabetes Association (ADA), American Association of Clinical Endocrinologists (AACE), and the American College of Endocrinology recommend metformin as first-line therapy for the management of T2DM.5,10,11,29–31 Metformin is comparable to the sulfonylureas in terms of efficacy, with a similar reduction in A1C of 1%–2% and in fasting plasma glucose of 60–70 mg/dL.27,31,32 Metformin is generally well tolerated and does not cause hypoglycemia, but it is associated with mainly gastrointestinal side effects, including anorexia, nausea, diarrhea, and abdominal discomfort.28,32 Metformin has also been shown to have beneficial effects on cardiovascular disease outcomes.33
Current FDC therapies for the treatment of T2DM
FDC medications used to treat diabetes employ mechanisms of action that address different aspects of the disease pathophysiology. This is especially crucial due to the progressive nature of T2DM.34,35 According to the ADA, combination medication therapy should be initiated if the target hemoglobin A1C is not reached within 3 months.4 FDCs are proven to have benefit in increasing adherence and satisfaction as well as decreasing medical costs.34,36
For most FDCs, the bioavailability of each included medication is equivalent to the amount given separately and simultaneously. Many of the available FDCs contain metformin as one of the two medications,34,37–47 which follows the ADA guidelines of using metformin as the cornerstone of T2DM pharmacotherapy.4 Other FDCs consist of a combination of a thiazolidinedione and a sulfonylurea.44,45 The most recently US Food and Drug Administration (FDA)-approved FDCs are combinations that contain an SGLT2 inhibitor, Invokamet46 (canagliflozin and metformin; Janssen Pharmaceuticals, Inc, Titusville, NJ, USA), and Xigduo XR (dapagliflozin and metformin XR; AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA).47
Since SGLT2 inhibitors act via a different mechanism of action than metformin, the combination therapy of dapagliflozin and metformin XR is beneficial to treat T2DM, especially for patients with severe uncontrolled hyperglycemia. Multiple trials have shown that SGLT2 inhibitors are efficacious in reducing A1C in an insulin-dependent manner and are well tolerated by most patients.
Efficacy
For treatment-naïve patients, an appropriate starting dose of dapagliflozin–metformin XR is 5 mg/1,000 mg, which is dosed once daily in the morning with food. The medication is titrated slowly up to a maximum of 10 mg dapagliflozin and 2,000 mg metformin to reduce gastrointestinal side effects. In other cases, the dose should be individualized based on the patient’s current antidiabetic medication regimen.47 For example, if a patient currently taking metformin 1,000 mg twice daily does not reach glycemic goals, the provider has the option to switch the patient to dapagliflozin–metformin XR 10 mg/2,000 mg. Patients previously taking an evening dose of metformin XR should skip their last dose before starting dapagliflozin–metformin the following morning.
Bailey et al48 examined the effects of dapagliflozin in 546 adult patients with T2DM who did not obtain glycemic control with metformin monotherapy (mean dose range: 1,782–1,861 mg/day). Patients were randomized to receive dapagliflozin or placebo in addition to their previous prestudy metformin dose over the 24-week study period. The dapagliflozin-treated groups had significantly greater reductions in mean A1C (P<0.0001) compared to placebo (0.70% and 0.84 in the 5 and 10 mg groups, respectively, versus 0.30% for placebo). Dapagliflozin use also resulted in significant mean weight reductions (3.0 kg, P<0.0001 and 2.9 kg, P<0.0001 in the 5 and 10 mg groups, respectively) compared to a mean 0.9 kg reduction in the placebo group. Glycemic control and weight reduction were sustained in the dapagliflozin treatment groups over an extended, 78-week treatment period.49 At 102 weeks, the dapagliflozin 5 and 10 mg treatment groups had mean reductions from baseline A1C of 0.58% and 0.78%, respectively, compared to an increase of 0.02% for the placebo group (P<0.0001 for both comparisons). Patients receiving both doses of dapagliflozin had mean weight loss of 1.7 kg from baseline weight compared to the placebo group, which experienced a mean gain of 1.4 kg (P<0.001).49
Henry et al50 conducted two randomized, controlled, 24-week studies to evaluate the effects of dapagliflozin in combination with extended-release metformin in treatment-naïve patients with T2DM. The mean A1C value for patients in both studies was 9.2%. In the first study, 598 patients were randomized to receive dapagliflozin 5 mg plus extended-release metformin, dapagliflozin 5 mg alone, or extended-release metformin alone. The second study evaluated the effects of dapagliflozin 10 mg in 638 patients, using a similar study design. Most patients in both studies received extended-release metformin 2,000 mg daily. In both studies, dapagliflozin plus metformin was more effective (P<0.0001) than either drug alone in reducing A1C. In the first study, combination therapy resulted in a mean A1C reduction of 2.1% compared to 1.25% with dapagliflozin monotherapy and 1.4% metformin monotherapy. The reductions in A1C for the combination, dapagliflozin-alone, and metformin-alone treatment arms in the second study were 2.0%, 1.5%, and 1.4%, respectively. The combination treatment groups had greater weight loss than the metformin-alone treatment groups (P<0.0001). Patients receiving combination treatment with dapagliflozin 5 mg plus metformin had a mean weight loss of 2.7 kg compared to 1.3 kg for patients receiving monotherapy with metformin. Combination treatment with dapagliflozin 10 mg plus metformin resulted in a mean weight loss of 3.3 kg compared to 1.4 kg for patients receiving metformin alone.50 Additional add-on studies support the efficacy findings of decreased A1C, weight loss, and reduced blood pressure (Table 1).51–54
Safety and tolerability
In general, most patients tolerated the dapagliflozin–metformin combination well, and few experienced serious side effects that resulted in minimal study withdrawals.48,50–53 Common side effects noted include nasopharyngitis, diarrhea, headache, and back pain.48,50,51,54,55 Other side effects that will be covered include hypoglycemia, effect on lipid levels, genitourinary infections, and effect on cardiovascular health. In addition, rare but serious side effects have been reported, including diabetic ketoacidosis (DKA), bladder and breast cancer, and lactic acidosis.
Several studies have demonstrated that dapagliflozin–metformin combination therapy has a low incidence of hypoglycemia and led to few major hypoglycemic episodes.48,50–55 Multiple studies have found low rates of hypoglycemia when dapagliflozin is used as an add-on therapy to metformin (4% dapagliflozin versus 3% placebo),48 combination therapy (3% dapagliflozin–metformin XR, 0%–1% dapagliflozin alone, and 0%–3% metformin alone),50 and add-on therapy to sitagliptin with or without metformin (3% dapagliflozin versus 2% placebo).53 However, when dapagliflozin is added to insulin therapy, the rates of hypoglycemia greatly increased (54%–56% dapagliflozin versus 52% placebo).55 Nauck et al51 compared dapagliflozin versus glipizide as add-on therapy to metformin and found that the dapagliflozin arm had much lower rates of hypoglycemia (3%) than the glipizide arm (40%).
Dapagliflozin has a slight effect on low-density lipoprotein cholesterol (LDL-C) levels in patients. In a trial with dapagliflozin as add-on therapy to metformin, there was a 10% increase in LDL-C levels with 10 mg dapagliflozin, and 5 mg dapagliflozin was comparable to placebo.48 However, in another add-on therapy trial, there was no statistical difference in LDL-C levels between 10 mg dapaglifozin and placebo.53 In a trial comparing dapagliflozin to glipizide as add-on therapy to metformin, both the dapagliflozin and glipizide groups had a slight decrease in LDL-C levels.51 Overall, it is recommended to monitor LDL-C levels after initiating dapagliflozin–metformin XR and to treat accordingly.47
Both genital and urinary tract infections (UTIs) can occur with dapagliflozin monotherapy and combination therapy. These infections occurred most often in women, and in several trials, few subjects had severe reactions leading to minimal study discontinuations.48,51–53,56–58
UTIs occurred at a similar or higher rate in subjects treated with dapagliflozin. When comparing dapagliflozin to glipizide as add-on therapy to metformin, events suggestive of UTIs occurred at rates of 11% in dapagliflozin group and 6% in glipizide group.51 When comparing dapagliflozin to placebo as add-on therapy to other antidiabetic agents, suggestive UTI rates were similar.48,52,53 A study combined pooled data from 12 trials (n=4,545) for patients treated with dapagliflozin as monotherapy or add-on therapy to other antidiabetic agents.56 There was a statistically significant increase in diagnosed UTIs for 5 mg dapagliflozin (6%), and 10 mg dapagliflozin was comparable to placebo (4%). Diagnosed UTIs were more common in women than in men. Most patients responded to a single course of treatment and did not lead to study discontinuation, but up to 16% in the 10 mg dapagliflozin group needed more than one treatment course.
Events suggestive of genital infections occurred at a higher rate in subjects treated with dapagliflozin. Most patients responded to a single course of treatment.52,53 For add-on therapy to metformin, genital infections occurred at rates of 8%–13% for dapagliflozin compared to 5% for placebo,48 and 12% for dapagliflozin compared to 3% for glipizide.51 In a triple add-on therapy study, the rates of genital infections were 6% and 0% for dapagliflozin and placebo, respectively.52 In the same study with 12 pooled trials as mentioned previously, diagnosed genital infections were higher in the dapagliflozin treatment groups (6% 5 mg and 5% 10 mg) as compared to placebo (1%).56 In all treatment groups, these infections were more common in women than in men. The most commonly diagnosed infections in women were vulvovaginal mycotic infection, vaginal infection, vulvovaginal candidiasis, and genital fungal infection; the most commonly diagnosed infection in men was balanitis.
Dapagliflozin has been shown to be safe and effective in older adults with advanced T2DM, comorbid cardiovascular disease, and with high use of concomitant medications.59 In addition, there is a 20-year simulation study that has estimated the long-term cardiovascular effects of dapagliflozin compared with the standard of care.60 It is projected that there will be a relative reduction in the incidence of myocardial infarction, stroke, cardiovascular mortality, and all-cause mortality of 13.8%, 9.1%, 9.6%, and 5.0%, respectively.60 In another study that pooled data from 19 trials (n=8,282), the point estimate for the Cox proportional hazard ratio for a composite end point of cardiovascular death, myocardial infarction, stroke, or hospitalization for unstable angina was 0.819 (95% confidence interval [CI]: 0.583–1.152).61 Despite these trials that suggest that dapagliflozin has no negative impact on cardiovascular events, there is an ongoing trial, the Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58; ClinicalTrials.gov identifier: NCT01730534), to further evaluate the link between cardiovascular events and dapagliflozin and is expected to be completed in April 2019.62
In May 2015, the FDA released a drug safety communication regarding an association between SGLT2 inhibitor use and the development of DKA with mild-to-moderate glucose levels (euglycemic DKA).63 As of May 19, 2015, there have been 101 cases of DKA in patients with T2DM and 46 cases of DKA in patients with type 1 diabetes reported worldwide.64 The agency indicated that DKA case presentations were atypical and presented with high anion gap metabolic acidosis, elevated blood or urine ketones, and mildly elevated glucose levels at less than 200 mg/dL. Patients displayed symptoms such as difficulty breathing, nausea, vomiting, abdominal pain, confusion, unusual fatigue, and sleepiness. Potential triggers identified in some cases included infection, acute illness (UTI, influenza, or trauma), reduced caloric or fluid intake, acute renal failure, reduced insulin dose, and alcohol consumption.63
There have been concerns with an association between the use of dapagliflozin and bladder and breast cancers. According to the FDA Advisory Committee, there had been a total of ten cases of bladder cancer (n=6,045) in patients taking dapagliflozin compared to one case of bladder cancer (n=3,512) in the control group.65 The FDA has concluded that the risk between dapagliflozin and bladder cancer cannot be ruled out. Therefore, it has proposed to continue surveillance of this potential association through the DECLARE-TIMI58 trial.62 In the meantime, dapagliflozin–metformin XR should not be used in patients who have active bladder cancer and should be used with caution in patients with a history of bladder cancer.47
The FDA Advisory Committee also discussed the link between dapagliflozin and breast cancer.65 There have been 12 reported cases of breast cancer (n=2,693 females) in patients taking dapagliflozin compared to three cases (n=1,429 females) in the comparator group. The incidence rate ratio for breast cancer was 2.472 (95% CI: 0.636–14.095). The FDA has concluded that the data is insufficient to validate an association between dapagliflozin and breast cancer because of various factors, including no screening mammography, breast cancer occurrence during the first year of therapy with dapagliflozin, the time from diabetes diagnosis, history of previous exposure to other antidiabetic agents, and the hormone receptor positivity of breast cancers.
Lactic acidosis is a rare metabolic complication that can occur with dapagliflozin–metformin XR, and is due to the accumulation of metformin; the risk for lactic acidosis is listed as a black box warning. It is a medical emergency that is fatal in about half of the cases.47 Declining kidney function is an important factor influencing metformin accumulation.66,67 Therefore, the use of metformin is considered to be contraindicated in many conditions that could increase the risk of developing lactic acidosis, such as chronic renal, hepatic, and cardiovascular disease.67
Dapagliflozin–metformin XR is contraindicated in patients with moderate-to-severe renal impairment (serum creatinine ≥1.5 mg/dL for men or ≥1.4 mg/dL for women, or estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), because both dapagliflozin and metformin are renally cleared, and metformin accumulation can lead to lactic acidosis.47,66–68 No dosing adjustments are indicated for patients with mild renal impairment (eGFR ≥60 mL/min/1.73 m2).47 Greater declines in kidney function lead to a proportional increase in the systemic exposure of dapagliflozin and its metabolite.69 Therefore, as renal function declines in chronic kidney disease, dapagliflozin is less efficacious at maintaining glycemic control.70
Case study
SR is a 47-year-old woman with a 1-year history of T2DM who presents today with recent weight gain (12 lb over the last year) and inadequate T2DM control. She was started on glipizide 5 mg and metformin 500 mg every morning and evening at the time of her diagnosis 1 year ago and was titrated up to glipizide 10 mg and metformin 1,000 mg twice daily. She was also advised to lose weight (at least 10 lb) but admits that she still consumes a high fat diet with refined sugars. She informed her provider that she had stopped taking her morning dose of glipizide because of dizziness in the early afternoon, which was often accompanied by sweating and a feeling of mild agitation. SR admitted that she usually misses breakfast and often eats a late lunch at fast-food restaurants. Lab results taken at this visit indicated that her A1C had dropped from 9.2 to 8.1 since starting glipizide 5 mg and metformin 1,000 mg. SR also takes lisinopril 10 mg daily and hydrochlorothiazide 25 mg daily for hypertension and atorvastatin 20 mg at bedtime for elevated cholesterol.
Assessment
Based on SR’s medical history, records, physical examination, and laboratory test results, she was assessed as follows:
- Uncontrolled T2DM (A1C >7%)
- Obesity (body mass index =37.8 kg/m2)
- Hyperlipidemia (controlled with atorvastatin)
- Hypertension (marginally controlled with lisinopril and hydrochlorothiazide)
- Normal urine microalbumin test (<30 mg albumin/24-hour urine collection)
- Self-care management/lifestyle deficits:
- Limited physical activity
- High fat and carbohydrate intake
- No self-monitoring of blood glucose (SMBG) program
- Poor understanding of diabetes
Discussion
SR presents with uncontrolled T2DM, recent weight gain, and comorbid conditions that usually accompany T2DM. While there has been improvement in her glycemic control as evidenced by the reduction in A1C, nonadherence to her current antidiabetic therapy may contribute to her inability to reach her glycemic goal. Including SR in the decision-making regarding her treatment would open up conversation about barriers she faces when attempting to manage her diabetes on a daily basis. SR should receive diabetes self-care management education, which focuses on healthy eating (especially the importance of eating breakfast every morning), physical activity, monitoring blood glucose levels, and adhering to medication therapy. SR should receive ongoing education and support to help her develop healthy coping skills and strong problem-solving skills. Developing and strengthening these behaviors will improve SR’s glycemic control and reduce her risk of complications.
SR was previously prescribed a sulfonylurea and metformin, but discontinued the morning dose of the sulfonylurea because of hypoglycemia (unconfirmed by SMBG), which could have been precipitated by missing breakfast. The primary care provider decides to discontinue the glipizide because of hypoglycemia and considers other options for managing SR’s diabetes. Sulfonylureas and meglitinide can reduce postprandial elevations caused by increased carbohydrate intake, but they are also associated with weight gain.5,11,27 The thiazolidinediones effectively address insulin resistance but have also been associated with weight gain.5,11,32 The α-glucosidase inhibitors can help with the rise in postprandial hyperglycemia by reducing carbohydrate absorption in the gut.5,11,71 However, these inhibitors require a slow titration and are associated with multiple gastrointestinal side effects. The glucagon-like peptide-1 (GLP-1) agonists facilitate glycemic control by increasing glucose-dependent insulin secretion and promote satiety and slow gastric emptying resulting in weight loss.5,11,72 Dipeptidyl peptidase-4 (DPP-4) inhibitors also reduce A1C, without associated weight gain. SGLT2 inhibitors lower glucose concentrations and results in weight loss and modest reduction in blood pressure.5,11,72 SR has good renal and hepatic function and normal potassium levels. The findings on SR’s physical examination and results of her laboratory assessment are presented in Table 2.
GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT2 inhibitors can be used as monotherapy (although GLP-1 receptor agonists are not recommended as the first treatment for T2DM) or in combination with other antidiabetic agents, and they may be initiated at a lower dosage and uptitrated if additional glycemic control is necessary. The GLP-1 agonists, DPP-4 inhibitors, and SGLT2 inhibitors are all appropriate options for this patient. After reviewing the available options and discussing the need for improved glycemic control with the patient, the provider decides to prescribe dapagliflozin–metformin XR because it can be administered orally once a day, is associated with a low risk of hypoglycemia, and provides the added benefit of weight reduction and modest blood pressure lowering. The cholesterol panel should be closely monitored since SGLT2 inhibitors have been shown to increase LDL in clinical studies.47
Summary and conclusion
The prevalence of T2DM is increasing in the US.1,2 In spite of the large selection of antidiabetic agents available to treat T2DM, a large proportion of patients with T2DM still fail to achieve glycemic goals. Most patients with diabetes must have two or more oral medications and, in some cases, insulin in order to obtain glycemic control.11 In addition, many patients with T2DM have other comorbid conditions such as hypertension, hyperlipidemia, and other conditions that require treatment, thus increasing the pill burden. Streamlining the number of daily medications by using FDC therapy has been shown to improve medication adherence, patient satisfaction, and glycemic control.16–18
The case study described herein demonstrates that no single therapy fits every patient and underscores the need to individualize antidiabetic therapy based on the specific characteristics of the patient, as suggested by the recent ADA and AACE guidelines.5 Current treatment guidelines for patients with T2DM recommend the use of SGLT2 inhibitors as an alternative option or as an add-on agent when metformin is not tolerated or is inappropriate or fails to provide adequate glycemic control.5,11 Dapagliflozin in combination with metformin provides additional reduction in blood glucose concentrations with the added benefit of modest weight loss and reduction in systolic blood pressure. The available data on dapagliflozin–metformin XR support its use as a treatment option in patients with T2DM.
Disclosure
The authors report no conflicts of interest in this work.
References
CDC, Department of Health and Human Services [homepage on the Internet]. National Diabetes Statistics Report, 2014 [updated 2014; cited May 15, 2015]. Available from: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 3, 2015. | |
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. | |
Stumvoll M, Goldstein BJ, van Haeften TW. Pathogenesis of type 2 diabetes. Endocr Res. 2007;32(1–2):19–37. | |
American Diabetes Association. 4. Foundations of care: education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. Diabetes Care. 2015;38(Suppl 1):S20–S30. | |
Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan – 2015. Endocr Pract. 2015;21(5 Suppl 1):1–87. | |
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. | |
Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. | |
American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. | |
Egede LE, Gebregziabher M, Dismuke CE, et al. Medication nonadherence in diabetes longitudinal effects on costs and potential cost savings from improvement. Diabetes Care. 2012;35:2533–2539. | |
Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–1224. | |
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):1140–1149. | |
Piette JD, Heisler M, Wagner TH. Problems paying out-of-pocket medication costs among older adults with diabetes. Diabetes Care. 2004;27(2):384–391. | |
Jha AK, Aubert RE, Yao J, Teagarden JR, Epstein RS. Greater adherence to diabetes drugs is linked to less hospital use and could save nearly $5 billion annually. Health Aff (Millwood). 2012;31(8):1836–1846. | |
Benner JS, Chapman RH, Petrilla AA, Tang SS, Rosenberg N, Schwartz JS. Association between prescription burden and medication adherence in patients initiating antihypertensive and lipid-lowering therapy. Am J Health Syst Pharm. 2009;66(16):1471–1477. | |
Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713–719. | |
Balu S, Simko RJ, Quimbo RM, Cziraky MJ. Impact of fixed-dose and multi-pill combination dyslipidemia therapies on medication adherence and the economic burden of sub-optimal adherence. Curr Med Res Opin. 2009;25(11):2765–2775. | |
Hussein MA, Chapman RH, Benner JS, et al. Does a single-pill antihypertensive/lipid-lowering regimen improve adherence in US managed care enrollees? A non-randomized, observational, retrospective study. Am J Cardiovasc Drugs. 2010;10(3):193–202. | |
Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62(1):76–87. | |
Jarab AS, Almrayat R, Alqudah S, et al. Predictors of non-adherence to pharmacotherapy in patients with type 2 diabetes. Int J Clin Pharm. 2014;36(4):725–733. | |
Lopez JM, Annunziata K, Bailey RA, Rupnow MF, Morisky DE. Impact of hypoglycemia on patients with type 2 diabetes mellitus and their quality of life, work productivity, and medication adherence. Patient Prefer Adherence. 2014;8:683–692. | |
Grady S, Fox KM, Hardy E. Association of weight loss and medication adherence among adults with type 2 diabetes mellitus: SHIELD (Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes). Curr Ther Res Clin Exp. 2013;75:77–82. | |
Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present and future. Lancet. 2014;383(9922):1068–1083. | |
DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. | |
DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14(1):5–14. | |
Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27(2):136–142. | |
Cornell S. Continual evolution of type 2 diabetes: an update on pathophysiology and emerging treatment options. Ther Clin Risk Manag. 2015;11:621–632. | |
DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin dependent diabetes mellitus. N Engl J Med. 1995;333:541–549. | |
Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–579. | |
Bloomgarden ZT. Treatment of type 2 diabetes: the American Association of Clinical Endocrinologists Meeting, May 2002. Diabetes Care. 2002;25(9):1644–1649. | |
American Diabetes Association. Standards of medical care in diabetes – 2015. Diabetes Care. 2015;38(1):S33–S37, S42–S46. | |
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32(1):193–203. | |
Kahn SE, Haffner SN, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. | |
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–865. | |
Blonde L, San Juan ZT. Fixed-dose combinations for treatment of type 2 diabetes mellitus. Adv Ther. 2012;29(1):1–13. | |
Moulding T, Dutt AK, Reichman LB. Fixed-dose combinations of antituberculous medications to prevent drug resistance. Ann Intern Med. 1995;122:951–954. | |
Hutchins V, Zhang B, Fleurence RL, Krishnarajah G, Graham J. A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Curr Med Res Opin. 2011;27(6):1157–1168. | |
GLUCOVANCE® (glyburide and metformin hydrochloride) [full prescribing information]. Princeton, NJ: Bristol-Myers Squibb; 2010. Available from: http://packageinserts.bms.com/pi/pi_glucovance.pdf. Accessed July 21, 2015. | |
METAGLIP® (glipizide and metformin hydrochloride) [full prescribing information]. Princeton, NJ: Bristol-Myers Squibb; 2009. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021460s007lbl.pdf. Accessed July 21, 2015. | |
PRANDIMET® (repaglinide and metformin hydrochloride) [full prescribing information]. Princeton, NJ: Novo Nordisk; 2010. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022386s007lbl.pdf. Accessed July 21, 2015. | |
JANUMET® (sitagliptin and metformin hydrochloride) [full prescribing information]. Whitehouse Station, NJ: Merck and Co; 2010. Available from: https://www.merck.com/product/usa/pi_circulars/j/janumet/janumet_pi.pdf. Accessed July 21, 2015. | |
KOMBIGLYZE XR® (saxagliptin and extended-release metformin hydrochloride) [full prescribing information]. Princeton, NJ: Bristol-Myers Squibb; 2010. Available from: http://www.azpicentral.com/kombiglyze-xr/pi_kombiglyze_xr.pdf#page=1. Accessed July 21, 2015. | |
ACTOPLUS MET® (pioglitazone hydrochloride and metformin hydrochloride) and ACTOPLUS MET XR® (pioglitazone hydrochloride and metformin hydrochloride extended-release) [full prescribing information]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.; 2010. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021842s014s015lbl.pdf. Accessed July 21, 2015. | |
AVANDAMET® (rosiglitazone and metformin hydrochloride) [full prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; 2008. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Avandamet/pdf/AVANDAMET-PI-MG.PDF. Accessed July 21, 2015. | |
AVANDARYL® (rosiglitazone and glimepiride) [full prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; 2009. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Avandaryl/pdf/AVANDARYL-PI-MG.PDF. Accessed July 21, 2015. | |
DUETACT® (pioglitazone and glimepiride) [full prescribing information]. Deerfield, IL: Takeda Pharmaceuticals America; 2010. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021925s010s011lbl.pdf. Accessed July 21, 2015. | |
INVOKAMET® (canagliflozin and metformin hydrochloride) [full prescribing information]. Titusville, NJ: Janssen Pharmaceuticals; 2014. Available from: http://www.invokanahcp.com/invokamet/prescribing-information.pdf. Accessed July 22, 2015. | |
XIGDUO XR® (dapagliflozin and metformin hydrochloride extended-release) [full prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2014. Available from: http://www.azpicentral.com/xigduo/pi_xigduoxr.pdf. Accessed July 22, 2015. | |
Bailey CJ, Gross JL, Peiters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomized, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–2233. | |
Bailey CJ, Gross JL, Hennicken D, et al. Dapagliflozin add on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102 week trial. BMC Med. 2013;11:43. | |
Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012;66(5):446–456. | |
Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015–2022. | |
Matthaei S, Bowering K, Rohwedder K, Grohl A, Parikh S; Study 05 Group. Dapagliflozin improves glycemic control and reduces body weight as add-on therapy to metformin plus sulfonylurea: a 24-week randomized, double-blind clinical trial. Diabetes Care. 2015;38:365–372. | |
Jabbour S, Hardy E, Sugg J, Parikh S; Study 10 Group. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37(3):740–750. | |
Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217–2224. | |
Wilding JP, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156(6):405–415. | |
Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27:479–484. | |
Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27:473–478. | |
Parikh S, Wilding J, Jabbour S, Hardy E. Dapagliflozin in type 2 diabetes: effectiveness across the spectrum of disease and over time. Int J Clin Pract. 2015;69(2):186–198. | |
Leiter LA, Cefalu WT, de Bruin TW, et al. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc. 2014;61:1252–1262. | |
Dziuba J, Alperin P, Racketa J, et al. Modeling effects of SGLT-2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obes Metab. 2014;16:628–635. | |
Ptaszynska A, Hardy E, Johnnson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013; 125(3):181–189. | |
AstraZeneca. Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58). Available from: https://clinicaltrials.gov/ct2/show/NCT01730534. Accessed January 21, 2016. | |
FDA. FDA Drug Safety Communication: FDA Warns that SGLT2 Inhibitors for Diabetes May Result in a Serious Condition of Too Much Acid in the Blood [May 15, 2015]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed August 15, 2015. | |
SGLT2 inhibitors: PRAC makes recommendations to minimise risk of diabetic ketoacidosis. European Medicines Agency. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/SGLT2_inhibitors/human_referral_prac_000052.jsp&mid=WC0b01ac05805c516f. Accessed June 18, 2015. | |
FDA. FDA Advisory Committee Meeting Regarding Dapagliflozin 5 mg and 10 mg Tablets. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM378076.pdf. Accessed August 16, 2015. | |
Richy FF, Sabido-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: a retrospective cohort study. Diabetes Care. 2014;37(8):2291–2295. | |
Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2003;163(21):2594–2602. | |
Kasichayanula S, Liu X, LaCreta F, Griffen SC, Boulton DW. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet. 2014;53(1):17–27. | |
Kasichayanula S, Liu X, Pe Benito M, et al. The influence of kidney function on dapagliflozin exposure, metabolism and pharmacodynamics in healthy subjects and in patients with type 2 diabetes mellitus. Br J Clin Pharmacol. 2013;76(3):432–444. | |
Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–971. | |
DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. | |
Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies. Diabetes Obes Metab. 2009;11(S3):26–34. | |
Bailey CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci. 2011;32(2):63–71. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


