Back to Journals » Clinical and Experimental Gastroenterology » Volume 8
Partial hepatectomy induces delayed hepatocyte proliferation and normal liver regeneration in ovariectomized mice
Authors Umeda M, Hiramoto M, Imai T
Received 2 January 2015
Accepted for publication 3 April 2015
Published 2 July 2015 Volume 2015:8 Pages 175—182
DOI https://doi.org/10.2147/CEG.S80212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Andreas M. Kaiser
Makoto Umeda,1 Masaki Hiramoto,1,2 Takeshi Imai1
1Department of Aging Intervention, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan; 2Department of Biochemistry, Tokyo Medical University, Tokyo, Japan
Abstract: Estrogens play central roles in sexual development, reproduction, and hepatocyte proliferation. The ovaries are one of the main organs for estradiol (E2) production. Ovariectomies (OVXs) were performed on the female mice, and hepatocyte proliferation was analyzed. The ovariectomized mice exhibited delayed hepatocyte proliferation after partial hepatectomy (PH) and also exhibited delayed and reduced E2 induction. Both E2 administration and PH induced the gene expression of estrogen receptor α (ERα). The transcripts of ERα were detected specifically in periportal hepatocytes after E2 administration and PH. Moreover, the E2 concentrations and hepatocyte proliferation rates were highest in the proestrus period of the estrous cycle. Taken together, these findings indicate that E2 accelerated ERα expression in periportal hepatocytes and hepatocyte proliferation in the female mice.
Keywords: estrogen, ER, estrous cycle, hepatocyte proliferation, liver regeneration
Introduction
Estrogens have a number of functions in development, growth, and sex differentiation, and they play important roles in female reproduction and in nonreproductive tract tissues, including the gonads, brain, bone, adipose tissue, and cardiovascular system.1,2 Estrogens contain mainly three compounds; Estron (E1), Estradiol (E2), and Estriol (E3). E2 is most active hormone among these Estrogens. The target factors of estrogens are thought to consist of three proteins, the estrogen receptor α (ERα), estrogen receptor β (ERβ), and the G protein-coupled receptor 30 (GPR30).3–6
Several studies have implicated the liver as an estrogenic organ, and an ER activity7 (later called genomic reaction) has been detected in this organ before the first ER (later named ERα) was cloned from MCF7 (Michigan Cancer Foundation 7), human mammary cell line.7–13
The liver plays a pivotal role in mammalian homeostasis and has the ability to complement its original mass in response to several types of stress, including partial hepatectomy (PH).14,15 After PH, hepatocytes proliferate intensely for a few days, and the liver regenerates in 2 weeks.14,15
Estrogens induce hepatocyte proliferation in vitro in neonate and in vivo after PH.8,11,12 These multiple effects could be mediated by ERα rather than ERβ and GPR30 because ERα has been shown to be expressed in the mouse liver.16–18 Moreover, estradiol (E2) signaling in the liver is a genomic reaction (see above), which indicates that ERα and/or ERβ are targets for E2 signaling in the liver.17,18 The expression of ERβ has not been detected in the liver, but ERα transcripts were detected.19 Taken together, the target factor of E2 in the liver may be ERα.20
To elucidate estrogen signaling in the liver of the female mice, ovariectomies (OVXs) were first performed to decrease E2 production, because the ovary is the tissue that produces the most E2.21,22 Next, the female mice were treated with E2. We showed that circulating E2 is significantly increased after PH in both non-OVX and OVX female mice and demonstrated that ERα, the expression of which is enhanced by E2 administration, plays a crucial role in E2-induced hepatocyte proliferation and liver regeneration.
Materials and methods
Materials
β-Estradiol (052-04041; Wako Pure Chemical Industries, Ltd, Osaka, Japan), 4-hydroxytamoxifen (H7904; Sigma-Aldrich, St Louis, MO, USA), and ICI182780 (ICI, I4409; Sigma-Aldrich) were purchased. The E2 enzyme immunoassay kit (No 582251) was purchased from Funakoshi (Tokyo, Japan). EDTA (15111-45), NaCl (31320-34), and Tris (35406-91) were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). ERα-specific agonist 4,4′, 4′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT, CAS No: 263717-53-9) was purchased from Sigma-Aldrich (H6036). ERβ-specific agonist 2,3-bis(4-hydroxyphenyl)propionitrile (DPN) was purchased from Cayman Chemical, Ann Arbor, MI, USA/Funakoshi 10008842. The Hep G2 cell line from human hepatocellular carcinoma cell line was purchased from the American Type Culture Collection (HB-8065; ATCC, Manassas, VA, USA).
Hepatocyte proliferation in the female mice
The hepatocyte proliferation rates of the female mice were analyzed with bromodeoxyuridine (BrdU, M0744; Dako Denmark A/S, Glostrup, Denmark) immunohistochemistry (IHC, SK-4105; Vector Marketing Corporation, Gibbsboro, NJ, USA). The females were injected intraperitoneally with 50 mg/kg of BrdU 2 hours before dissection, and the livers were removed, rinsed, and embedded in the tissue-Tek OCT compound (Sakura Finetek Japan Co, Ltd, Tokyo, Japan). Ten-micrometer cryosections were fixed with 4% paraformaldehyde, incubated with an antibody against BrdU (No 11170376001, Hoffman-La Roche Ltd, Basel, Switzerland) that was diluted 50-fold in 0.1% bovine serum albumin/phosphate-buffered saline (BSA/PBS), revealed by CY3-conjugated donkey anti-rabbit IgG antibody, and mounted with Vectashield medium (Vector Laboratories Ltd, Burlingame, CA, USA). The numbers of BrdU-positive hepatocyte nuclei in at least five low-magnification microscopic fields of each sample (~2000 hepatocytes) were counted.23–25
In situ hybridization
The RNA probes were prepared. The ERα and ERβ cDNAs were cloned into pSG5 vector,26 and the 35S-labeled antisense probes were synthesized by T7 in vitro transcription and translation system (Promega Corporation, Fitchburg, WI, USA). The specimens were prehybridized for 2 hours at 50°C in prehybridization buffer (50% formamide, 0.3 M NaCl, 10 mM Tris–HCl at pH 6.8, 10 mM NaPO4 at pH 6.8, 5 mM Ethylenediaminetetraacetic aicd (EDTA), 1× Denhart’s, 10 mM DTT, 500 mg/mL yeast RNA, 100 mg/mL salmon sperm DNA, and 500 nmol/mL nonlabeled α-thio-UTP [DuPont, Wilmington, DE, USA]). After the RNase A treatment, the slides were washed for 1 hour in the washing buffer (50% formamide, 0.3 M NaCl, 10 mM Tris–HCl at pH 6.8, 10 mM NaPO4 at pH 6.8, 5 mM EDTA, 1× Denhart’s, and 10 mM DTT). The slides were subsequently washed in 2× Saline-Sodium Citrate buffer (SSC) for 15 minutes at room temperature, in 0.1× SSC for 15 minutes at 50°C, and then in 0.1× SSC for 30 minutes at room temperature. After dehydration of the sections, they were coated with Kodak NTB-2 emulsion, dried, and stored at 4°C. The exposure time ranged from 12 days to 15 days. Kodak D19 developer was used for 2 minutes at room temperature. The sections were then stained in toluidine blue, dehydrated in ethanol, and mounted under coverslips in Eukitt mounting medium.26–29
Reverse transcription polymerase chain reaction
Total liver RNA was extracted by the guanidium thiocyanate-phenol-chloroform method. cDNA was synthesized for 20 minutes at 50°C from 1 μg of RNA with Moloney murine leukemia virus reverse transcriptase. The transcribed cDNA was amplified by 30 cycles of PCR for ERα (5′-CGG CTG CCA CTT ACC TGG GAG CTC TCA GAT-3′ and 5′-GGG GAG CCT GGG AGC TCT CAG AT-3′), ERβ (5′-TCT CTG AGA GCA TCATGT CC-3′ and 5′-CAG CCT GGC CGT CAC TGT GA-3′), and hypoxanthine phosphoribosyltransferase (5′-GTA ATG ATC AGT CAA CGG GGG AC-3′ and 5′-CCA GCA AGC TTG CAA CCT TAA CCA-3′).24,30,31
Surgeries (PHs, orchiectomies, and OVXs)
Liver resection of the left and median lobes was performed following midventral laparotomy between 8 am and 11 am under isoflurane anesthesia as previously described.23,32 The bilateral OVX procedure was performed as follows: the mice were anesthetized with peritoneal injections of pentobarbital, one central lateral incision was made in the skin, and two lateral incisions were made in the muscle layer, and the ovaries were extracted through the incision and excised after ligation.
Animal study compliance
All experiments were performed in accordance with the ethical guidelines for animal care of the National Center for Geriatrics and Gerontology (NCGG). The experimental protocols were approved by the Animal Care Committee of the NCGG. All the surgeries were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize the suffering of the animals. E2 injections were decreased from five repetitions to a single injection to decrease the suffering of the sacrificed and operated mice.
Hep G2 cell culture
Hep G2 cells from a human liver carcinoma were maintained in a-minimal essential medium (11900-073; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal calf serum (FCS, CC3008-504, Cell Culture Technology, Tokyo, Japan). The cells were plated at a density of 1.5–3×106 cells/60-mm dish, and after 48 hours, the culture medium was exchanged for a-minimal essential medium without phenol red supplemented with 0.5% charcoal-treated FCS. After 24 hours, the cells were treated with β-estradiol or an equal volume (0.01 [v/v]%) of vehicle (EtOH, 14712-34; Nacalai Tesque, Inc.).20,25 Phenol red is a known phytoestrogen. The FCS contains higher endogenous estrogen concentrations, and the charcoal treatment reduces this concentration in the FCS.
Statistical analysis
The values are reported as the mean ± SEM. Statistical significance (ANOVA and Student’s t-test) is indicated as follows: *P<0.05; **P<0.005; ***P<0.0001. Nonsignificant differences (P>0.05) are indicated as NS.23–25,28
Results
PH induces E2 concentration in orchiectomized and OVX mice
Previous studies indicate that PH induces E2 concentration in human beings, rats, and male mice (Figure 1).9,11,12,20 E2 is converted from testosterone by aromatase, and the highest expression of aromatase is observed in the ovary and the testis; however, other tissues (eg, the gonads, placenta, adipose tissue, etc) also weakly produce aromatase.21,22 These observations suggested that no PH-induced E2 production was observed in orchiectomized (ORC) mice. So, the male mice were operated with ORC, and then, these ORC mice were operated with PH. After PH, plasmas were collected, and circulating E2 concentrations were measured (Figure 1A). Plasma E2 was strongly elevated after 6–48 hours in the control mice after PH (open squares) and was also induced after 24–48 hours in the ORC mice (filled triangles with dotted lines), which indicates that the E2 induction following PH was delayed and reduced in the ORC mice. PH-induced E2 concentrations were mainly from testes (of control mice) and majorly from nontestes organs (of ORC mice, eg, gonads, adipose tissue; Figure 1C).
Female mice have endogenous higher E2 concentration than male mice; so, OVXs were performed (Figure 1B) to analyze the effects of E2 concentration after PH. E2 concentrations were significantly decreased in the OVX mice (Figure 1B). After PH, E2 concentrations were induced in both OVX and control females. The E2 peaks in control females were ~80 pg/mL after 6–48 hours, and those of OVX mice were ~30 pg/mL after 24–48 hours, indicating delayed and small E2 induction by OVX. Taken together, PH induced E2 concentration mainly from testes and ovaries, and partly and delayed from other organs (unknown) in Figure 1C. Taken together, this is the first observation where PH triggered E2 concentration in both ORC and OVX animals.
E2 administration and the estrous cycle induced E2 concentration and hepatocyte proliferation
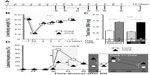
PH induces E2 concentration9,11,12,20 (Figure 1) as well as hepatocyte proliferation (Figure 2).14,15 E2 was administered to the OVX mice, and hepatocyte proliferation was analyzed (Figure 2A). E2 administration induced hepatocyte proliferation in the OVX mice in a dose-dependent manner (Figure 2A and B).32 The target factors of E2 are ERα, ERβ, and GPR30,3–6 and the ICI182780 (ICI) is one of the selective ER modulators (SERMs). ICI is an antagonist for ERα and ERβ, but an agonist for GPR30.5,6,33,34 ICI reduced hepatocyte proliferation (Figure 3B), which indicates that the target(s) of E2 action is related to hepatocyte proliferation that was not GPR30 but could have been ERα and/or ERβ. Moreover, no ERβ expression was observed in the male livers.19,20
  | Figure 3 SERM administration regulated hepatocyte proliferation. |
E2 is primarily produced by the ovaries in female mice.21,22 Generally, E2 production is dependent on the estrous cycle of nonpregnant mice, and E2 production is elevated during the proestrus period and reduced in the estrous cycle.35,36 The estrous cycles, E2 concentrations, and hepatocyte proliferations of nonpregnant female mice were analyzed (Figure 2B). The peak E2 concentrations and hepatocyte proliferations occurred during the proestrus phase/period (Figure 2B). E2 was administered to the OVX mice, and E2 concentrations and hepatocyte proliferations were analyzed (Figure 2C). Both the E2 concentrations and hepatocyte proliferations were similarly elevated in an E2-dependent manner (Figure 2A). Taken together, both E2 administration and the estrous cycle induced E2 concentration and induced E2 concentration lead hepatocyte proliferation (Figure 2C).
E2 and SERM administration regulated hepatocyte proliferation
To access the target factor for hepatocyte proliferation, several SERMs were administrated to hepatocytes (Figure 3). At first, E2 (filled bar in Figure 3A) and ICI (ERα and β antagonist, GPR30 agonist, gray bar in Figure 3A) were administrated to B6 WT mice (Figure 3A). E2-stimulated hepatocyte proliferation and ICI-inhibited hepatocyte proliferation indicated that hepatocyte proliferation was due to ERα and/or ERβ, not due to GPR30 (Figure 3C). Other SERM, such as PPT (ERα-specific agonist, triangles, CAS No: 263717-53-9)37 induced HepG2 cell proliferation significantly, but no induction was observed with ERβ-specific agonist DPN (circles, CAS No: 1428-67-7), indicating that ERα is the E2 target factor for E2-induced hepatocyte proliferation (Figure 3C). Using ERα and ERβ knock out (KO) (and their control) mice,31 we confirmed that E2-induced hepatocyte proliferation was via ERα, not ERβ (data not shown and manuscript in preparation). Moreover, hepatocyte-specific ERα KO mice23,27 were established and analyzed, resulting in similar results (data not shown and manuscript in preparation). Taken together, E2-induced hepatocyte proliferation might be via hepatocyte-ERα with ligand and receptor analyses.
E2 administration and PH induced ERα expression in periportal hepatocytes
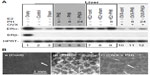
The mRNA expressions of ERα and ERβ in the liver were analyzed using reverse transcription polymerase chain reaction (RT-PCR) (Figure 4A) and in situ hybridization (Figure 4B). First, ERβ mRNA was detected in the positive control of ovary, and not amplified from liver samples (Figure 4A).19 PH and E2 administration induced the transcripts of ERα (Figure 4A). Note that both E2 injection and PH induced ERα expression synergetically. Delayed induced expression of ERα was observed in OVX mice at 24 hours after PH, similar to delayed E2 induction in OVX mice (Figure 1B). These observations suggested that PH induced delayed E2 concentration and ERα expression.
Moreover, E2 and PH induced ERα expression mainly in the hepatocytes located in the periportal area (Figure 4B), and these hepatocytes are known to actively proliferate after PH.38
Delayed hepatocyte proliferation after PH in OVX mice
No significant differences in liver weight were observed between the OVX and control mice before or after PH (Figure 5A and B). Hepatocytes are the main parenchymal cells of the liver, and DNA from the recovered livers was extracted and analyzed. The liver DNA quantities were restored by 4 days after PH in the OVX and control mice, and no significant differences were observed between the OVX and control mice (Figure 5C); these findings indicate that OVX had no significant effect on the liver regeneration. Moreover, hepatocyte proliferation following PH was analyzed based on BrdU incorporation. The rate of hepatocyte proliferation in the control mice was significantly elevated 36–44 hours after PH, but that of the OVX mice was significantly higher at 52 hours, and 60–72 hours after PH (Figure 5D). These data indicate that the OVX mice displayed slightly delayed hepatocyte proliferation (Figure 5D) but no significant change in liver weight/DNA amount recovery (Figure 5B and C).
Discussion
PH induced increases in E2 concentrations in ORC and OVX living organisms
This is the first study that PH induced E2 production in ORC and OVX animals. In the papers in the 1970s and 1980s, and in our latest studies, no observation of E2 induction in ORC/OVX rodents or human beings was reported.
E2 administration and PH induced ERα expression in the livers of the female mice
The first ERα was cloned by Professor Pierre Chambon in 1986. The first Francavilla’s study was published in 1984; they did not know the ERα itself. Recently ERα expression is induced in male mice, indicating that this is the first study to report ERα expression in females.
Hepatocyte proliferation was also observed in the proestrus period of the estrous cycle
There are no studies about hepatocyte proliferation in the estrous cycle, and this is the first case of hepatocyte proliferation that was observed in sex cycles of females.
Conclusion
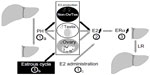
The possible mechanisms of PH- and E2-induced hepatocyte proliferation were identified (Figure 6). PH (step 1a), the estrous cycle (step 1b), and E2 injection (step 1c) stimulated increases in E2 concentrations (step 2), ERα expression in the periportal hepatocytes (step 3), and hepatocyte proliferation.
Future perspective
Genetical study
Our previous and latest studies showed that PH induced ERα expression in rodents of both sexes. Using genetically modified mice disrupting ER genes, the hepatic ER function in liver regeneration was contributed (manuscript in preparation).
During pregnancy
We demonstrated that induced E2 production in the estrus period of the estrous cycle triggered hepatocyte proliferation. In general, when are the females exposed highest E2 concentration? It is during pregnancy. The livers during pregnancy were analyzed, and hepatocyte proliferation was observed as expected (manuscript in preparation).
Acknowledgments
We are grateful to our department members in the NCGG for helpful discussions. This work was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and a grant from the Japan Science and Technology Agency (JST) to TI. The funders had no role in study design, data collection and analysis, the decision to publish, or manuscript preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
Ciocca DR, Roig LM. Estrogen receptors in human nontarget tissues: biological and clinical implications. Endocr Rev. 1995;16(1):35–62. | |
Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. | |
Antal MC, Petit-Demoulière B, Meziane H, Chambon P, Krust A. Estrogen dependent activation function of ERβ is essential for the sexual behavior of mouse females. Proc Natl Acad Sci U S A. 2012;109(48):19822–19827. | |
Adlanmerini M, Solinhac R, Abot A, et al. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci U S A. 2014; 111(2):E283–E290. | |
Chimento A, Sirianni R, Casaburi I, Pezzi V. GPER signaling in spermatogenesis and testicular tumors. Front Endocrinol (Lausanne). 2014;30:5. | |
Vaucher L, Funaro MG, Mehta A, et al. Activation of GPER-1 estradiol receptor downregulates production of testosterone in isolated rat Leydig cells and adult human testis. PLoS One. 2014;9:e92425. | |
Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9(6):1980–1989. | |
Fisher B, Gunduz N, Saffer EA, Zheng S. Relation of estrogen and its receptor to rat liver growth and regeneration. Cancer Res. 1984;44(6):2410–2415. | |
Francavilla A, Eagon PK, DiLeo A, et al. Sex hormone-related functions in regenerating male rat liver. Gastroenterology. 1986;91(5):1263–1270. | |
Kahn D, Gavaler JS, Makowka L, et al. Does hyperprolactinemia affect hepatic regeneration independent of sex steroids? J Lab Clin Med. 1988;112(5):644–651. | |
Francavilla A, Polimeno L, DiLeo A, et al. The effect of estrogen and tamoxifen on hepatocyte proliferation in vivo and in vitro. Hepatology. 1984;9(4):614–620. | |
Francavilla A, Gavaler JS, Makowka L, et al. Estradiol and testosterone levels in patients undergoing partial hepatectomy. A possible signal for hepatic regeneration? Dig Dis Sci. 1989;34(6):818–822. | |
Kahn D, Eagon PK, Porter LE, et al. Effect of tamoxifen on hepatic regeneration in male rats. Dig Dis Sci. 1989;34(1):27–32. | |
Steer CJ. Liver regeneration. FASEB J. 1995;9(14):1396–1400. | |
Michalpoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–66. | |
Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–970. | |
Ciana P, Di Luccio G, Belcredito S, et al. Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol Endocrinol. 2001;15(7):1104–1113. | |
Ciana P, Raviscioni M, Mussi P, et al. In vivo imaging of transcriptionally active estrogen receptors. Nat Med. 2003;9(1):82–86. | |
Vacca M, D’Amore S, Graziano G, et al. Clustering nuclear receptors in liver regeneration identifies candidate modulators of hepatocyte proliferation and hepatocarcinoma. PLoS One. 2014;9:e104449. | |
Uebi T, Umeda M, Imai T. Estrogen induces estrogen receptor alpha expression and hepatocyte proliferation in the livers of the male mice. Genes Cells. 2015;20(3):217–223. | |
Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S. Aromatase expression and role of estrogens in male gonad: a review. Reprod Biol Endocrinol. 2003;1:35. | |
Bulun SE, Lin Z, Imir G, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359–383. | |
Imai T, Jiang M, Kastner P, Chambon P, Metzger D. Selective ablation of retinoid X receptor alpha in hepatocytes impairs their lifespan and regenerative capacity. Proc Natl Acad Sci U S A. 2001;98(8):4581–4586. | |
Imai T, Takakuwa R, Marchand S, et al. Peroxisome proliferators-activated receptor γ is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci U S A. 2004;101(13):4543–4547. | |
Umeda M, Uebi T, Maekawa N, Handa H, Imai T. PGJIFs, new mitochondrial PGJ2 target proteins, regulate cell proliferation. J Biosci Med. 2013;1(3):11–15. | |
Dollé P, Duboule D. Two gene members of the murine HOX-5 complex show regional and cell-type specific expression in developing limbs and gonads. EMBO J. 1989;8(5):1507–1515. | |
Imai T, Chambon P, Metzger D. Inducible site-specific somatic mutagenesis in mouse hepatocytes. Genesis. 2000;26(2):147–148. | |
Imai T, Jiang M, Chambon P, Metzger D. Impaired adipogenesis and lipolysis in the mouse upon Cre-ERT2-mediated selective ablation of RXR alpha in adipocytes. Proc Natl Acad Sci U S A. 2001;98(1):224–228. | |
Niederreither K, Fraulob V, Garnier JM, Chambon P, Dollé P. Differential expression of retinoic acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech Dev. 2002;110(1–2):165–171. | |
Imai T, Matsuda K, Shimojima T, et al. ERC-55, a binding protein for the papiloma virus E6 oncoprotein, specifically interacts with vitamin D receptor among nuclear receptors. Biochem Biophys Res Commun. 1997;233(3):765–769. | |
Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. | |
Higgins GM, Anderson RM. Experimental pathology of the liver I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. | |
Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106(4):1377–1388. | |
Movérare-Skrtic S, Börjesson AE, Farman HH, et al. The estrogen receptor antagonist ICI 182,780 can act both as an agonist and an inverse agonist when estrogen receptor α AF-2 is modified. Proc Natl Acad Sci U S A. 2014;111(3):1180–1185. | |
Blendinger K. Physiology and pathology of the estrous cycle of the bitch. Italian Companion Animal Veterinary Association. In: Proceedings of the SCIVAC Congress, Rimini, Italy; 2007:73–77. | |
Santmyire BR, Venkat V, Beinder E, Baylis C. Impact of the estrus cycle and reduction in estrogen levels with aromatase inhibition, on renal function and nitric oxide activity in female rats. Steroids. 2010;75(12):1011–1015. | |
Kitamura N, Araya R, Kudoh M, et al. Beneficial effects of estrogen in a mouse model of cerebrovascular insufficiency. PLoS One. 2009; 4(4):e5159. | |
Ferri D, Moro L, Mastrodonato M, et al. Ultrastructural zonal heterogeneity of hepatocytes and mitochondria within the hepatic acinus during liver regeneration after partial hepatectomy. Biol Cell. 2005;97(4):277–288. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.