Back to Journals » Clinical Ophthalmology » Volume 15
Pars Planectomy: Preliminary Report of a New Glaucoma Filtering Technique in Vitrectomized Eyes
Authors Wangsupadilok B, Tansuebchueasai N
Received 8 January 2021
Accepted for publication 5 February 2021
Published 23 February 2021 Volume 2021:15 Pages 791—798
DOI https://doi.org/10.2147/OPTH.S299347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Supplementary video of "Pars planectomy: a new glaucoma filtering technique" [ID 299347].
Views: 558
Boonchai Wangsupadilok, Natchada Tansuebchueasai
Department of Ophthalmology, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand
Correspondence: Natchada Tansuebchueasai
Department of Ophthalmology, Faculty of Medicine, Prince of Songkla University, Hatyai, Songkhla, Thailand
, 90110 Tel +66 74-451381
Fax +66 74-451389
Email [email protected]
Purpose: To propose a new filtering technique in vitrectomized eyes with glaucoma and report its clinical results and safety.
Methods: The medical records of 13 eyes that developed glaucoma following pars plana vitrectomy and underwent pars planectomy, from 2011 to 2018, at Songklanagarind hospital, Hatyai, Songkhla, Thailand were retrospectively reviewed. The main outcome measures were visual acuity (VA), intraocular pressure (IOP), number of glaucoma medications, and postoperative complications. Surgical success was defined as IOP value at the last visit of 6– 21 mmHg, regardless of anti-glaucoma medication usage, and without further glaucoma surgery.
Results: The mean follow-up duration was 47.7 ± 32.1 months (range, 0.3– 101.1 months). Preoperative BCVA increased from LogMAR 1.01 ± 0.85 to 1.2 ± 0.91 at the last visit (p = 0.233). The mean preoperative IOP was 28.15 ± 9.17 mmHg with an average of 3.46 ± 0.52 anti-glaucoma medications. At the final visit, the mean IOP was 14.08 ± 4.89 mmHg (p = 0.006) and the mean number of anti-glaucoma medications decreased to 1.31 ± 1.38 (p = 0.000). The probability of surgical success was 58.3%, 50%, and 37.5% at 1, 2, and 6 years after pars planectomy, respectively. Postoperative complications included vitreous hemorrhage in 1 eye (7.7%). No retina and pars plicata associated complications were found.
Conclusion: Pars planectomy is efficient and safe as well as requires a short learning curve. It should be considered as an alternative filtering surgery in glaucoma after vitrectomy, especially with an extensive limbal scar that might be a limitation in trabeculectomy and GDDs techniques and outcomes.
Keywords: pars planectomy, glaucoma filtering surgery, new filtering technique, vitrectomized glaucoma
Introduction
Secondary glaucoma is not an uncommon condition in vitrectomized eyes. Elevation of intraocular pressure (IOP) occurs in 19% to 28% after pars plana vitrectomy (PPV).1,2 Trabeculectomy has been considered as the standard procedure to lower IOP in glaucoma patients.3,4 However, history of previous ocular surgery, including vitrectomy, is associated with reduced effectiveness of filtration surgery.5,6 In addition, performing trabeculectomy in vitrectomized eyes is challenging as existing limbal conjunctival scars and scleral wounds can limit bleb location and extension. Previous conjunctival manipulation can also complicate the postoperative wound-healing process.5 All of these factors contribute to a higher bleb failure rate.
Glaucoma drainage devices (GDDs) are the primary surgical intervention for refractory glaucoma in vitrectomized eyes. The success rate for GDDs is high, although there can be serious complications.7–10 Complications tend to occur in eyes with multiple previous conjunctival surgeries, and include bleb encapsulation, which can cause subsequent device failure, and tube exposure following conjunctival erosion, which can lead to endophthalmitis.7,11 The financial costs of GDDs also present additional concerns, particularly in low- and middle-income countries.
We propose that moving the trabeculectomy site from the limbus to the pars plana area can allow aqueous to drain directly from the posterior chamber through the sclerotomy site at the pars plana into the posteriorly located subconjunctival bleb. In this article, we describe this new “Pars planectomy” technique and report preliminary clinical outcomes among patients who underwent the procedure at a tertiary hospital in southern Thailand.
Methods
The study protocol was approved by the Human Research Ethics Committee of the Faculty of Medicine, Prince of Songkla University (REC 63–286-2-1). The electronic medical records of 13 patients with medically uncontrolled vitrectomized glaucoma who underwent pars planectomy, from 2011 to 2018, at Songklanagarind hospital, Hatyai, Songkhla, Thailand were retrospectively reviewed. All were fluid-filled eyes. Exclusion criteria included: 1) history of laser procedure to reduce outflow resistance (trabeculoplasty); 2) history of laser procedure to reduce inflow (cyclophotocoagulation); 3) history of other filtering surgeries (including trabeculectomy and GDDs). For each patient, written informed consent was obtained prior to the operation. Due to the retrospective nature of the review, patient consent to review their medical records was waived. However, the data was maintained with confidentiality and complied with the Declaration of Helsinki.
Surgical Procedure
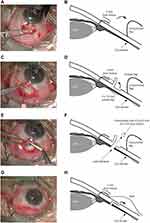
A fornix-based conjunctival incision was created at 2 mm from the limbus with a length of 5–6 mm, followed by blunt dissection which was done to create a subconjunctival space (see Figure 1A and B). A half-thickness scleral flap with a size of 2×1.5 mm2 was created at 4.5 mm from the limbus (the flap’s hinge was at 3 mm from the limbus) (Figure 1C and D). The sponge soaked in 0.4 mg/mL Mitomycin-C (MMC) was placed at the exposed area of the conjunctiva, Tenon’s capsule, and sclera for 4 minutes. The sponge was then removed, and exposed tissue was then washed off with 200 mL balanced salt solution. A pars plana window with a size of 0.5×0.5 mm2 was created with sclerotomy punch precisely at 3 mm from the limbus. Exposed uveal tissue was cauterized to create a uveal window connecting with the posterior chamber (Figure 1E and F). The scleral and conjunctival flap were closed with Nylon 10–0 monofilament sutures (Figure 1G and H). The full surgical procedure was presented in Supplementary Video S1. Postoperatively, all patients received a standard topical antibiotic and anti-inflammation for 6–8 weeks. Laser suture lysis was considered, based on IOP level and bleb contour, at follow-up visits.
Outcomes
At all preoperative and postoperative visits, all patients underwent standard ophthalmologic examinations which included: 1) best-corrected visual acuity (BCVA) with ETDRS chart; 2) Goldmann applanation tonometry; 3) biomicroscopy; and 4) fundus examination. A single glaucoma specialist (BW) performed all examinations and surgeries. We also recorded demographic data, BCVA, IOP, cup to disc ratio, number of glaucoma medications, history of previous vitreoretinal surgery, postoperative complications, and history of needling procedures (if performed).
VA was converted to logMAR value. Non-optotype visual acuities were converted to logMAR as follows: 1.9 for counting fingers (CF), 2.3 for hand motion (HM), 2.5 for light projection, 2.7 for light perception (LP), and 3 for no light perception (NLP).12 For office tonometry, IOP was measured with Goldmann applanation tonometer. In eyes with corneal irregularities that might affect the applanation accuracy, pneumatonometry (Tono-Pen; Reichert Technologies) was used instead. For fix-combination drugs, the number of medications was recorded as two. Preoperative variables were recorded one day before pars planectomy. The follow-up visits were scheduled at 1 day, 1–2 weeks, 1 month, 3 months, 6 months, and every 6 months afterward. More frequent visits were appointed if considered clinically necessary. After pars planectomy, if IOP was uncontrolled, medication and bleb needling were considered appropriately.
We defined surgical success based on an IOP value at the last visit of 6–21 mmHg, with or without anti-glaucoma medication, and no additional glaucoma surgery (filtering surgery and destruction of ciliary body). The IOP taken into consideration for success outcome was measured at least 3 months after pars planectomy to avoid the postoperative short-term fluctuation.
Statistical Analysis
Statistical analysis was performed using Stata MP version 16.1. Continuous baseline data were presented using mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate. Categorical data were presented using frequency and percentage. IOP over time was analyzed using mixed-effect, random-intercepted linear regression in which each eye was considered as a random element and time was considered to have a fixed effect. The success rate over various postoperative intervals was analyzed by Kaplan–Meier survival analysis. A p-value < 0.05 was considered significant.
Results
Thirteen eyes of 13 patients who underwent pars planectomy after medically uncontrolled secondary glaucoma after PPV were included in the study. Baseline characteristics are shown in Table 1. The mean follow-up duration was 47.7 ± 32.1 months (a median of 43.7 months; a range of 0.3–101.1 months). Among indications for performing 23-gauge PPV, trauma (46.2%) occupied the majority, followed by rhegmatogenous retinal detachment (RRD) (23%).
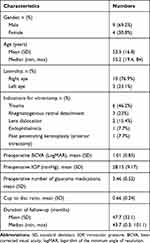 |
Table 1 Baseline Characteristics of the Study Patients |
Preoperative BCVA increased from LogMAR 1.01 ± 0.85 to 1.2 ± 0.91 at the last visit (p = 0.233). The mean preoperative IOP was 28.15 ± 9.17 mmHg with an average of 3.46 ± 0.52 anti-glaucoma medications. At the final visit, both mean IOP and number of anti-glaucoma medications significantly decreased to 14.08 ± 4.89 mmHg (p = 0.006) and 1.31 ± 1.38 (p = 0.000), respectively. Changes of mean IOP and number of glaucoma medications were shown in Figures 2 and 3.
 |
Figure 2 Changes of mean intraocular pressure after pars planectomy in vitrectomized eyes with glaucoma. |
 |
Figure 3 Changes of mean number of medications after pars planectomy in vitrectomized eyes with glaucoma. |
One patient had lost to follow up at 1 month postoperatively. With an exclusion of this patient, the mean postoperative IOP (14.42 ± 4.94 mmHg, p = 0.002) and the number of medications (1.42 ± 1.38, p = 0.000) still significantly decreased from the baseline. Figure 4 presents the results of the Kaplan–Meier survival curve analysis for 12 patients. The probability of surgical success at 6 months, 1, 2, and 6 years after pars planectomy was 66.7%, 58.3%, 50%, and 37.5%, respectively. Overall, seven (58.3%) of 12 eyes did not meet the success criteria. All were because of inadequate IOP control.
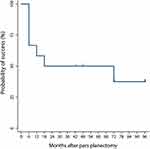 |
Figure 4 Kaplan–Meier survival curve of surgical outcomes for 12 patients who underwent pars planectomy. Vertical lines denote censored data (loss to follow-up). |
Regarding postoperative complications, we only found vitreous hemorrhage in 1 eye (7.7%), which resolved spontaneously. No retina and pars plicata associated complications were found. No eyes had lost their vision to no light perception postoperatively. Laser suture lysis was done in 7 eyes (53.8%). Bleb encapsulation was the most common cause of postoperative IOP rising, and 4 eyes (30.8%) had undergone needling with 0.1 mL of mitomycin C (0.4 mg/mL). The mean interval time for needling was 3.43 ± 2.06 months.
Discussion
Because of limitations in the efficacy of trabeculectomy and devastating complications that comes with GDDs placement in patients with glaucoma after vitrectomy, we proposed a new filtering technique called pars planectomy. Our preliminary report showed significantly decreased IOP and number of anti-glaucoma medications from the baseline with a high success rate and fewer complications.
IOP elevation after PPV occurs 19% and 28%, and some eyes can develop secondary glaucoma afterward.1,2 The study of Han et al13 has shown that eyes with greater acute postoperative IOP elevation (≥30 mmHg within 48 hours) are significantly prone to have sustained late IOP elevation (at least 6 weeks after the surgery). Presumed mechanisms include intraocular gas expansion, inflammation obstructing trabecular meshwork, silicone oil-related complications, steroid response, pupillary block, and exacerbation of pre-existing ocular conditions, like neovascular glaucoma (NVG), angle recession, and uveitic glaucoma.13 Koreen et al have studied eyes without neither aforementioned secondary causes nor history of glaucoma/glaucoma suspect and found that the incidence of late-onset open-angle glaucoma in uncomplicated vitrectomized eyes was 11.6%.14 With all aforementioned reports, glaucoma in vitrectomized eyes is not uncommon and glaucoma management, especially, when uncontrolled with medications, should be carefully taken into consideration.
After trabeculectomy, the aqueous outflow is bypassed into the subconjunctival bleb, hence the success rate depends on bleb survival. The most frequent cause of bleb failure is subconjunctival fibrosis which occurs as a result of postoperative inflammation and wound healing process to a greater extent than desired.5 With the use of anti-inflammation and antimetabolites to reduce postoperative scarring, the success rate has been increased but not to the guaranteed extent for all glaucoma eyes. Previous ocular surgery, including one with conjunctival incision procedures like vitrectomy, has been found to associate with reduced effectiveness of following filtration surgery.5,6 In some vitrectomized eyes that have undergone additional limbal incision surgeries, like scleral fixated intraocular lens implantation, extracapsular, and intracapsular cataract extraction, the limbal scar can cause technical difficulties, limitations in bleb location and extension, and subsequently poor bleb function when traditional trabeculectomy is performed. Previous conjunctival manipulation and breaking down of the blood-aqueous barrier also result in an exaggerated postoperative wound healing process.5 All of these factors contribute to a higher bleb failure rate.
GDDs, another commonly performed filtering surgery, are usually considered as a primary surgical intervention in vitrectomized eyes with refractory glaucoma. The devices rechanneled aqueous route into a long tube that connects with a plate surrounded by the fibrous bleb away from the limbus. However, a device placement can also be difficult in certain cases with multiple previous conjunctival surgeries.
Our new technique, pars planectomy, was originally designed to overcome the aforementioned limitations by modifying the classic trabeculectomy technique. The surgical site was moved away from the limbus to the pars plana area, where the conjunctiva was usually left untouched, so that aqueous was drained directly from the posterior chamber through the sclerotomy site at the pars plana and finally into the more posteriorly located subconjunctival bleb.
The success probability of trabeculectomy with mitomycin C (MMC) in vitrectomized eyes was reported to be only 55.1% at 1 year and decreased to 45.3% after 2 years.15 With a similar definition, our result showed a success rate of 58.3% at 1 year and 50% at 2 years. Both studies were done in vitrectomized eyes, but the results might not be directly compared to ours because of the difference in study population. Neovascular glaucoma (NVG) (67%) and uveitic glaucoma (8%) occupied the majority in the former study of 116 eyes, while ours were trauma (46.2%) and RRD (23%) in 13 eyes. NVG and uveitis are known risk factors for trabeculectomy failure because continual neovascularization and ocular inflammation result in early conjunctival scarring and aggressive postoperative wound healing. Especially in vitrectomized eyes, vasoproliferative factors can easily diffuse into the anterior chamber and induce neovascularization and inflammation of the conjunctiva. This might explain the slightly less desirable success rate in the former study.
Regarding GDDs, many studies have reported successful results of Ahmed implant at 76.4–84.4% at the final visit.7,8 Park et al reported a similar success rate at 89.9% after 1 year and decreased to 74.8% after 2 years with Ahmed implant in vitrectomized NVG.9 The successful result of Baerveldt was also reported at the mean cumulative success probability of 80% at 6 months postoperatively, which remained stable throughout the follow-up.10 In our study, the cumulative success rate was 37.5% at the final visit, which is lower compared to GDDs. However, we found that the mean preoperative IOP and number of anti-glaucoma medications significantly decreased at final visit, which is similar to previous studies in GDDs.7–10
It is widely reported in many studies that placing such devices come with a higher risk of destructive complications. More severe complications required surgical interventions occur 12% more in the vitrectomized group.9 Bleb encapsulation, which can later cause device failure, tends to occur more in eyes with previous conjunctival surgery. Tube exposure following conjunctival erosion can lead to endophthalmitis that, in some cases, necessitates enucleation.7 Although the mechanism is not clear, it has been reported more in eyes that have undergone multiple conjunctival surgeries earlier.11 Hyphema, hypotony, choroidal detachment, suprachoroidal hemorrhage, decompression retinopathy, corneal decompensation, and phthisis bulbi were also reported.7–10 The only postoperative complication found in our study was vitreous hemorrhage (7.7%), which was much less in severity and incidence compared to those reported in GDDs. No retina and pars plicata associated complications were found. However, as vitrectomized eyes have low scleral rigidity, sudden hypotony and even hemorrhagic choroidal detachments can occur in procedures that drain through the pars plana or via a single chamber, especially in high myopia and pediatric eyes. The use of an anterior chamber maintainer or viscoelastic injection before performing sclerotomy can prevent sudden pressure change, and hence avoid such complications.
Pars planectomy also has some advantages over trabeculectomy and GDDs. Firstly, the limbal conjunctiva and stem cells were spared. This would be beneficial in cases that are prone to develop ocular surface disease or cases that require further limbal surgery, like scleral fixated intraocular lens implantation, extracapsular, and intracapsular cataract extraction. Secondly, with posteriorly diffused bleb, patients are less likely to develop symptomatic and overhanging bleb. Lastly, modified from traditional trabeculectomy, pars planectomy only requires a short learning curve and no additional instruments or devices. In an economic aspect, this is another advantage over GDDs, especially in developing countries.
Limitations of this study are retrospective design, small sample size, variation of the etiologic factors for PPV, and, most importantly, the lack of a control group. We would like to emphasize that the results of this study cannot be generalized and present no evidence that our proposed technique offers any advantages over conventional trabeculectomy in terms of efficacy or safety.
In conclusion, pars planectomy should be considered as an alternative filtering surgery to control IOP in vitrectomized glaucoma, especially with an extensive limbal scar that might compromise trabeculectomy and GDDs outcomes and techniques. It is efficient and safe as well as requires a short learning curve. Further studies with more patients and longer follow-up are needed to determine long-term results. However, we did not intend this technique to replace trabeculectomy or GDDs or to recommend it as a primary surgical option. Selecting the most appropriate surgical technique for each patient depends on many factors, including surgeon’s experience and comfort, certain eye condition, patient’s expectation, postoperative interventions, possible risk of complications, and economic concerns.
Disclosure
All authors report no conflicts of interest for this work.
References
1. Weinberg RS, Peyman GA, Huamonte FU. Elevation of intraocular pressure after pars plana vitrectomy. Albr Von Graefes Arch Klin Exp Ophthalmol. 1976;200(2):157–161. doi:10.1007/BF00414365
2. Aaberg TM, Van Horn DL. Late complications of pars plana vitreous surgery. Ophthalmology. 1978;85:126–140. doi:10.1016/S0161-6420(78)35683-9
3. Cairns J. Trabeculectomy: preliminary report of a new method. Am J Ophthalmol. 1968;66(4):673–679.
4. Razeghinejad MR, Fudemberg SJ, Spaeth GL. The changing conceptual basis of trabeculectomy: a review of past and current surgical techniques. Surv Ophthalmol. 2012;57(1):1–25. doi:10.1016/j.survophthal.2011.07.005
5. Broadway DC, Chang LP. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma. 2001;10(3):237–249. doi:10.1097/00061198-200106000-00017
6. Takihara Y, Inatani M, Fukushima M, Iwao K, Iwao M, Tanihara H. Trabeculectomy with Mitomycin C for Neovascular Glaucoma: prognostic Factors for Surgical Failure. Am J Ophthalmol. 2009;147:5. doi:10.1016/j.ajo.2008.11.015
7. Erçalik NY, Imamoǧlu S. Ahmed glaucoma valve implantation in vitrectomized eyes. J Ophthalmol. 2018. doi:10.1155/2018/9572805
8. Hong J-W, Choi G-J. Ahmed valve implantation for refractory glaucoma following pars plana vitrectomy. Korean J Ophthalmol. 2005;19(4):293–296.
9. Park UC, Park KH, Kim DM, Yu HG. Ahmed glaucoma valve implantation for neovascular glaucoma after vitrectomy for proliferative diabetic retinopathy. J Glaucoma. 2011;20(7):433–438. doi:10.1097/IJG.0b013e3181f3eb06
10. Van Aken E, Lemij H, Vander Haeghen Y, de Waard P. Baerveldt glaucoma implants in the management of refractory glaucoma after vitreous surgery. Acta Ophthalmol. 2010;88(1):75–79. doi:10.1111/j.1755-3768.2008.01428.x
11. Al-Torbak AA, Al-Shahwan S, Al-Jadaan I, Al-Hommadi A, Edward DP. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. 2005;89(4):454–458. doi:10.1136/bjo.2004.049015
12. Reed GF. Numerical Imputation for Low Vision States. Invest Ophthalmol Vis Sci. 2006;47:1236–1240.
13. Han DP, Lewis H, Lambrou FH, Mieler WF, Hartz A. Mechanisms of intraocular pressure elevation after pars plana vitrectomy. Ophthalmology. 1989;96(9):1357–1362. doi:10.1016/S0161-6420(89)32715-1
14. Koreen L, Yoshida N, Escariao P, et al. Incidence of, risk factors for, and combined mechanism of late-onset open-angle glaucoma after vitrectomy. Retina. 2012;32(1):160–167. doi:10.1097/IAE.0b013e318217fffb
15. Inoue T, Inatani M, Takihara Y, Awai-Kasaoka N, Ogata-Iwao M, Tanihara H. Prognostic risk factors for failure of trabeculectomy with mitomycin C after vitrectomy. Jpn J Ophthalmol. 2012;56(5):464–469. doi:10.1007/s10384-012-0171-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.