Back to Journals » Clinical Ophthalmology » Volume 14
Pars Plana Vitrectomy Reoperations for Complications of Proliferative Diabetic Retinopathy
Authors Al-khersan H, Venincasa MJ , Kloosterboer A, Sridhar J, Smiddy WE , Townsend JH , Flynn HW
Received 3 March 2020
Accepted for publication 1 May 2020
Published 10 June 2020 Volume 2020:14 Pages 1559—1563
DOI https://doi.org/10.2147/OPTH.S252285
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hasenin Al-khersan, Michael J Venincasa, Amy Kloosterboer, Jayanth Sridhar, William E Smiddy, Justin H Townsend, Harry W Flynn
Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, FL, USA
Correspondence: Harry W Flynn
Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, FL, USA
Tel +1 305-326-600
Email [email protected]
Objective: To report visual acuity in patients undergoing pars plana vitrectomy (PPV) reoperations for complications of proliferative diabetic retinopathy (PDR).
Design: Retrospective case series.
Subjects: Diabetic patients undergoing reoperation with PPV between 2015 and 2018 at a university referral center.
Methods: Patient charts were reviewed for indication for initial and repeat PPV, baseline clinical characteristics including gender, age, and lens status, and pre- and post-operative best-corrected visual acuity.
Main Outcome Measures: Best-corrected visual acuity at last follow-up.
Results: Of 538 eyes (409 patients) undergoing a PPV for diabetic retinopathy, 153 (28.4%) eyes had reoperation. Among the 130 eyes (119 patients) that met the inclusion criteria, 55 eyes (50 patients) underwent reoperation for complications of PDR, defined as non-clearing vitreous hemorrhage (NCVH) and/or tractional retinal detachment (TRD). Within this subgroup of 55 eyes, 19 (34.5%) eyes had an indication for the first surgery of NCVH. Fourteen (73.7%) of these NCVH eyes achieved a visual acuity of 20/80 or better. When the indication for the first surgery was TRD (33 eyes, 60%), 8 (24.2%) eyes achieved this same outcome (p=0.0011).
Conclusion: Approximately one of every four eyes treated with PPV for PDR will undergo repeat PPV during follow-up. VA outcomes after the repeat PPV were variable, with NCVH cases achieving better outcomes compared to TRD.
Keywords: vitreoretinal surgery, diabetic retinopathy, retinal detachment, retina
Background
Diabetic retinopathy is the leading cause of blindness among working-age adults.1 There are approximately 93 million people with diabetic retinopathy worldwide, 17 million of whom have proliferative diabetic retinopathy (PDR).2 The prevalence of diabetes is expected to continue to increase in the coming years.3
The advent of anti-vascular endothelial growth factor (VEGF) therapy has greatly improved outcomes in the treatment of diabetic retinopathy.4,5 From 2001 to 2012, while the general overall rate of vitrectomy among a large managed-care cohort rose by 31%, the rate of pars plana vitrectomy (PPV) among diabetics decreased by 43%.6 However, despite these improvements, many patients still undergo surgical intervention for complications of disease – primarily non-clearing vitreous hemorrhage (NCVH) and tractional retinal detachment (TRD).7,8
Since the advent of vitrectomy in the 1970s, surgical techniques and instrumentation have improved substantially, perhaps in part due to the use of smaller gauge vitrectomy systems and enhanced viewing systems.9–12 Nevertheless, prior investigations have shown that nearly a third of patients who undergo vitrectomy for TRD go on to require a second operation.13 The purpose of the present study was to characterize best-corrected visual acuity (BCVA) outcomes in such patients who undergo reoperation for complications of PDR.
Methods
A computer search of a single academic institution identified all charts of patients with a diagnosis code of PDR and a CPT® billing code of PPV from January 2015 to August 2018. Among this cohort, all eyes requiring repeat PPV were identified. These charts were reviewed to determine suitability for inclusion in the current study. Exclusion criteria included <3 months of follow-up and PPV performed at an outside institution.
Basic demographic data, indications for surgery, details of each surgical procedure including trochar gauge, iatrogenic breaks, and use of laser/intraoperative anti-VEGF therapy, and VA outcomes were collected for each patient. Patients were categorized based on the indications for initial PPV and reoperation. Patients whose second PPV was for an indication of TRD or NCVH were categorized as having had a reoperation for complications of PDR. Epiretinal membranes without TRD were not included in the TRD category.
Visual acuities were converted to logMAR. Count fingers, hand motions, and light perception vision were assigned LogMAR values of 1.85, 2.3, and 2.7, respectively.14 Paired t-test was used to evaluate the mean change in BCVA after each operation and from baseline to final follow-up. ANOVA analysis was performed to assess differences in change in BCVA based on the choice of tamponade. Fischer’s exact test was used to compare patients achieving better than 20/80 Snellen BCVA. Lastly, multivariable linear regression was utilized to analyze patient factors correlating with BCVA outcomes. A P-value <0.05 was considered statistically significant. Statistical analysis was carried out using Stata 15.1 (StataCorp, College Station, Texas).
Institutional review board approval was obtained from the University of Miami. Surgical consent was obtained from all patients, and a waiver of patient consent was granted for chart review given the retrospective nature of the study. Patient confidentiality was maintained according to the Health Insurance Portability and Accountability Act. The study abided by provisions in The Declaration of Helsinki.
Results
The chart search identified 538 eyes (409 patients) with both a diagnosis of PDR and a PPV performed for a diabetic-related complication. Of these, 153 (28.4%) eyes required a second vitrectomy. Twenty-three eyes were excluded on the basis of limited follow-up or a vitrectomy performed at an outside institution. Of the nine eyes excluded for inadequate follow-up, six had an indication for PPV of TRD, one of combined TRD/rhegmatogenous retinal detachment (RRD), and two of NCVH.
Among the remaining 130 eyes (119 patients) with reoperation for PDR, the initial PPV indication was TRD in 96 (73.8%), NCVH in 31 (23.8%), and combined TRD/rhegmatogenous detachment in 3 (2.3%). Eyes with an indication for reoperation outside of NCVH or TRD such as oil removal were not included in the final analysis (Table 1). Eyes that underwent reoperation specifically for NCVH, TRD, or both were categorized as having had reoperation for persistent complications of PDR and analyzed further (55 eyes, 50 patients). Within this final study cohort, the majority of these eyes were from type II diabetics and had undergone various prior therapies for diabetic retinopathy (Table 2). The mean follow-up among the final study cohort was 130 weeks.
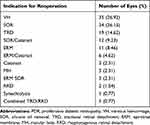 |
Table 1 Indications for Repeat Vitrectomy in Eyes with PDR |
 |
Table 2 Baseline Characteristics Among Patients Undergoing Reoperation for Complications of PDR |
Among the final study cohort, the initial indication for PPV was NCVH in 19 (35%) eyes, TRD in 33 (60%) eyes, and combined TRD/RRD in 3 (5.5%) eyes (Table 3). If the initial PPV was for NCVH, the second PPV was also for NCVH in 18 (95%) of 19 eyes while in the remaining eye (5.3%) it was for a new TRD. The mean time between operations in this group was 51 (±84) weeks. If the initial PPV was for TRD, reoperation was for NCVH in 18 (54.5%) of 33 eyes, for TRD in 14 (42.4%) eyes, and for combined TRD/RRD in one eye.
 |
Table 3 Indications for Repeat Vitrectomy Amongst Eyes Requiring Reoperation for Complications of PDR |
The baseline logMAR for eyes with an initial indication for PPV of TRD was 1.29 (±0.77) logMAR (Figure 1). There was a non-statistically significant worsening of VA at the final visit of 0.18 logMAR to 1.47 (±0.97) logMAR (p=0.32). The baseline logMAR for eyes with an initial indication for PPV of NCVH was 1.37 (±0.58) logMAR and improved by a mean of 0.96 logMAR to a final mean acuity of 0.40 (±0.54) logMAR (p<0.0001). Both groups worsened after the initial PPV and improved after the second PPV (Figure 2).
Among eyes that underwent the initial PPV for a TRD, 10 (30.3%) had an initial BCVA >20/80 while at last follow-up only 8 (24.2%) eyes had a BCVA of >20/80 (p=0.58). In this group, if the reoperation was for persistent TRD (14 eyes), the BCVA worsened by a mean of 0.09 (±0.52) LogMAR (p=0.51). Meanwhile, those that underwent the second PPV for NCVH demonstrated mean improvement in vision of −1.16 (± 0.86) LogMAR (p<0.0001). Among eyes with an initial indication for PPV of NCVH, 3 of 19 (16%) eyes had initial BCVA of 20/80 or better. In this group, 14 of 19 (73.7%) eyes reached a final Snellen VA of 20/80 or better (p=0.0003).
The initial PPV consisted of 23-gauge instrumentation in 16 (29%) eyes, 25-gauge in 38 (69%) eyes, and 27-gauge in 1 (1.8%) eye. The initial PPV included the use of silicone oil tamponade in 18 (33%) eyes, SF6 in 8 (16%) eyes, C3F8 in 6 (11%) eyes, and no gas or oil in 23 (41%) eyes. Among the 18 eyes that received an oil tamponade after the initial PPV, 10 had oil extraction at the subsequent PPV while 8 had oil exchange. The BCVA worsened by a mean of 0.90 logMAR in eyes that had silicone oil exchange at reoperation; the BCVA improved by a mean of −0.36 logMAR (p=0.0018) in eyes that had oil removed at reoperation.
The mean IOP was 16.1mmHg at baseline and 16.7mmHg at the final visit. At the final visit, 16 (29.1%) eyes were using at least one topical IOP-lowering medication. Four (7.3%) eyes had been documented to have neovascular glaucoma at baseline, and an additional four eyes developed neovascular glaucoma during the follow-up period. Of these four, two had received pre-operative intravitreal bevacizumab.
Prior to the first PPV, 12 eyes (21.8%) were pseudophakic. By the conclusion of the study, 52 eyes (95.4%) were pseudophakic. A single patient was left aphakic. In multivariable linear regression, the type of diabetes, baseline lens status, total follow-up duration, and prior treatment with PRP were not found to correlate significantly with mean change in BCVA (Table 4). Initial indication for surgery of TRD as well as the use of silicone oil correlated with poor BCVA outcomes. Worse baseline BCVA was associated with a greater mean change in BCVA.
 |
Table 4 Multivariable Regression of Patient Factors Correlating with Changes in Visual Acuity |
Discussion
The outcomes of PPV for diabetic complications have generally improved, probably for many reasons including improved surgical techniques, instrumentation, and visualization. Still, many patients with PDR require PPV and even then some have poor visual and anatomic results and will need a reoperation.15–17 Of the 538 charts reviewed for inclusion in the present study, approximately one in four eyes undergoing vitrectomy for PDR had a second vitrectomy for various indications including cataract extraction and silicone oil removal. The rate of reoperation was similar to that observed in The Drive UK Study, where approximately one in four eyes also had a second vitrectomy.13
As clinical intuition would suggest, results of reoperation depended on the indication for initial PPV. A majority of NCVH reoperations yielded successful visual results, but only a minority of TRD reoperations did so. Other than TRD as an initial indication for surgery, the use of silicone oil also portended worse BCVA outcomes. This is likely due to the fact that silicone oil is more likely to be used in more advanced TRD.18,19 Furthermore, a worse initial BCVA correlated with greater improvement in vision. A ceiling effect in improvement in vision among eyes with better initial acuities most likely explains this finding. Treatment with PRP prior to initial visit did not correlate with BCVA outcomes as was also observed in a study by Yorston et al.17
The current study shares the limitations of most retrospective studies. Data collection was limited by the information documented in the medical record. Since the present study included multiple surgeons at a university center, biases of case selection and surgeon preference were involved.
Reoperations after initial PPV for complications for PDR are not rare. In the present study, approximately one in four eyes that underwent an initial PPV for PDR required a second vitrectomy. As expected, cases more likely to yield favorable results are those with less complex baseline conditions such as NCVH as compared to TRD. Characterizing rates of reoperation and outcomes is critical both in guiding treatment and appropriately counseling patients.
Disclosure
Dr Jayanth Sridhar reports personal fees from Alcon, Alimera, Regeneron, and Oxurion, outside the submitted work. The authors report no other financial disclosures or possible conflicts of interest related to the present work.
References
1. Kobrin Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmol Epidemiol. 2007;14(4):179–183. doi:10.1080/09286580701396720
2. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi:10.2337/dc11-1909
3. Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6–12. doi:10.1089/pop.2015.0181
4. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 Phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi:10.1016/j.ophtha.2011.12.039
5. Jansen ME, Hariprasad SM, Singer MA. Treatments for diabetic macular edema: past, present, and future. Ophthalmic Surg Lasers Imaging Retina. 2016;47(9):794–800. doi:10.3928/23258160-20160901-01
6. Wubben TJ, Talwar N, Blachley TS, et al. Rates of vitrectomy among enrollees in a United States managed care network, 2001–2012. Ophthalmology. 2016;123(3):590–598. doi:10.1016/j.ophtha.2015.11.001
7. Berrocal MH, Acaba LA, Acaba A. Surgery for Diabetic Eye Complications. Curr Diab Rep. 2016;16(10):99. doi:10.1007/s11892-016-0787-6
8. Sharma T, Fong A, Lai TY, Lee V, Das S, Lam D. Surgical treatment for diabetic vitreoretinal diseases: a review. Clin Exp Ophthalmol. 2016;44(4):340–354. doi:10.1111/ceo.12752
9. Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25(2):208–211. doi:10.1097/00006982-200502000-00015
10. Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y. A 27–gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology. 2010;117(1):93–102. doi:10.1016/j.ophtha.2009.06.043
11. Oshima Y, Awh CC, Tano Y. Self-retaining 27-gauge transconjunctival chandelier end illumination for panoramic viewing during vitreous surgery. Am J Ophthalmol. 2007;143(1):166–167. doi:10.1016/j.ajo.2006.07.051
12. Ribeiro RM, Teixeira AG, Diniz B, et al. Performance analysis of ultrahigh-speed vitreous cutter system. Retina. 2013;33(5):928–932. doi:10.1097/IAE.0b013e31826f069e
13. Gupta B, Sivaprasad S, Wong R, et al. Visual and anatomical outcomes following vitrectomy for complications of diabetic retinopathy: the DRIVE UK study. Eye (Lond). 2012;26(4):510–516. doi:10.1038/eye.2011.321
14. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–1240. doi:10.1167/iovs.05-0981
15. Brown GC, Tasman WS, Benson WE, McNamara JA, Eagle RC. Reoperation following diabetic vitrectomy. Arch Ophthalmol. 1992;110(4):506–510. doi:10.1001/archopht.1992.01080160084037
16. Williams DF, Williams GA, Hartz A, Mieler WF, Abrams GW, Aaberg TM. Results of vitrectomy for diabetic traction retinal detachments using the en bloc excision technique. Ophthalmology. 1989;96(6):752–758. doi:10.1016/S0161-6420(89)32813-2
17. Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92(3):365–368. doi:10.1136/bjo.2007.124495
18. Ramezani A, Ahmadieh H, Rozegar A, et al. Predictors and outcomes of vitrectomy and silicone oil injection in advanced diabetic retinopathy. Korean J Ophthalmol. 2017;31(3):217–229. doi:10.3341/kjo.2016.0018
19. Glaser BM. Silicone oil for proliferative vitreoretinopathy: does it help or hinder? JAMA Ophthalmol. 1988;106(3):323–324.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


