Back to Journals » Drug Design, Development and Therapy » Volume 9
Pain relief and improved physical function in knee osteoarthritis patients receiving ongoing hylan G-F 20, a high-molecular-weight hyaluronan, versus other treatment options: data from a large real-world longitudinal cohort in Canada
Authors Petrella R, Wakeford C
Received 12 May 2015
Accepted for publication 27 July 2015
Published 15 October 2015 Volume 2015:9 Pages 5633—5640
DOI https://doi.org/10.2147/DDDT.S88473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Robert J Petrella,1,2 Craig Wakeford3
1Departments of Family Medicine, Medicine (Cardiology) and Kinesiology, University of Western Ontario; 2Aging, Rehabilitation & Geriatric Care Research Centre, Lawson Health Research Institute, London, ON; 3Health Economics and Health Outcomes, Sanofi-Aventis Inc., Laval, QC, Canada
Abstract: From the Southwestern Ontario database, one of the largest primary-care datasets in Canada, 1,263 patients with osteoarthritis (OA) of one or both knees were identified who received two consecutive series of intra-articular (IA) injections of hylan G-F 20 preparation and no other prescribed OA medications, and were evaluated fully between 2006 and 2012. A cohort of 3,318 demographically matched OA patients who had not been treated with IA injection therapy was identified from the same database for comparison. Responses to therapy were assessed by means of a 10-point visual analog scale (VAS) for pain at rest and after completion of a 6-minute walk test (6MWT), while physical capacity was measured by performance in the 6MWT itself. After two cycles of hylan G-F 20 therapy, the average VAS score for pain at rest declined from 7.82±1.27 at baseline to 4.16±1.51 (average change 3.66±1.78, significantly more than the reduction of 3.12±2.03 seen in the reference group [P<0.012]) and the average VAS score for pain after the 6MWT decreased by 5.56±1.74 points (from 9.58±0.4 at baseline to 4.02±1.67 at the final assessment), a significantly larger change than that seen in the reference group (Δ2.99±1.85; P<0.001 for intergroup comparison). Distance walked in the 6MWT increased on average by 115 m, significantly more than that seen in the reference group (Δ91 m; P<0.001 for intergroup comparison). These findings from a primary-care database suggest sustained benefits in terms of pain and physical function from repeat cycles of IA injections of hylan G-F 20 and no other prescribed OA medications in adults with OA of the knee.
Keywords: osteoarthritis, high-molecular-weight hyaluronic acid, intra-articular, 6-minute walk test, repeat treatment, pain relief
Introduction
There are currently around 4.6 million people diagnosed with osteoarthritis (OA) in Canada and an additional 400,000 cases are recorded each year.1 Many of these patients have OA of the knee joint(s). OA is not a lethal condition but is disabling and is associated with substantial impact on the lives of individuals and wider societal costs. Most of the Can$195 billion annual cost of OA is attributable to indirect costs such as loss of productive work time; the total economic cost of OA is predicted to double by 2020 and to double again by 2030.1
There is no curative intervention for OA; therapy is targeted toward pain relief and the preservation of function. Intra-articular (IA) injection of hyaluronic acid or high-molecular-weight hyaline analogs (HAs) has been evaluated as effective for these purposes for OA of the knee.2 However, there is currently a lack of consensus among experts about the weight of the supporting evidence for this intervention, and it is generally regarded as a supplementary therapy.3–6 Use of IA HA in knee OA is predicated on its ability to restore more normal viscoelastic and mechanical qualities of synovial fluid in osteoarthritic joints as well as various potentially favorable effects on inflammation.7 The therapeutic effects of HA, once established, may persist for 6 months or more.8 Some evidence of sustained benefit from repeat cycles of IA HA has been reported from clinical studies, but comparable experience from observational studies of real-world practice is lacking.9,10
The Southwestern Ontario (SWO) database, established in 1999, records both administrative and clinical patient-level data from a mixture of urban and rural catchment areas in London, Ontario, and nearby communities. This large dataset, which now includes approximately 10%–15% of the adult inhabitants of SWO, has been used to investigate patient demographic profiles and practice patterns in Canadian primary care. We now report our findings from a new analysis of the SWO database, examining the longer term effects of several cycles of IA HA injections on pain and mobility in patients with OA of the knee. Our investigation focused on the hylan G-F 20 preparation (Synvisc®; Sanofi Biosurgery, Cambridge, MA, USA). This is the most widely prescribed HA preparation in Canada and could be expected, on the basis of previous information,11 to generate a large patient sample.
Methods
Detailed descriptions of the nature, size, and operation of the SWO database have appeared in other publications.12–14 This is an ongoing patient-level observational cohort of 350,000 patients registered at a mixture of urban and rural primary-care practices (n=87). Baseline information for all participants includes demographics, complete morbidity profile, medications, and other clinical data. Patient data are updated quarterly for all those attending their primary-care providers.
For the present investigation, patient data were extracted from the SWO database for the period 1 January 2006 to 31 December 2012. Within that time interval, all adult patients (≥18 years old) who had a diagnosis of OA of one or both knees and who were recorded as having initiated and received two consecutive courses of hylan G-F 20 at intervals of 6 months (±30 days) were identified. Hylan G-F 20 was administered according to the manufacturer-approved schedule, comprising either three injections, each of 2.0 mL (16 mg) at intervals of 1 week for three consecutive weeks, or a single-dose formulation that delivered 48 mg in a 6-mL injection. No other prescribed OA medications were permitted in the study group. Patients with bilateral OA received injection in one joint only.
Patients were also included if their treatment began in 2005 but was evaluated in 2006 or if treatment began in 2012 and was evaluated in 2013.
Additional inclusion conditions comprised a baseline mobility score and/or OA-related pain severity assessment up to 4 weeks before the start of therapy and a repeat assessment up to 4 weeks after completing therapy. Patients were excluded if no data were recorded for the post-injection efficacy/outcome measures for each course of treatment. The severity of OA at baseline was quantified based on the Kellgren–Lawrence rating system (1=mild; 4=severe) using radiographs that were obtained not more than 1 year prior to the first course of treatment.
A comparison cohort was retrospectively identified of patients with knee OA who had a baseline evaluation and then a treatment and follow-up evaluation during the same time frame (2006–2012) but who had no record of treatment with IA therapies (including, but not limited to, HA) before or during that interval. Persons meeting these criteria were matched on baseline characteristics to patients in the study cohort. In conformity with the requirements for inclusion in the HA group, patients in the comparison group had to have had at least two consecutive courses of OA-directed therapy at intervals of 6 months (±30 days). Qualifying prescriptions had to have been filled within 30 days of issuance, and prescribed medications had to be unchanged during the period of follow-up and assessment. Patients in the control group could have new or additional OA medications prescribed at the discretion of their physicians, with the exception of IA therapies, which were prohibited.
Patient responses to therapy in both groups were assessed by means of mobility, expressed as distance travelled in the 6-minute walk test (6MWT), 15 and a 10-point visual analog scale (VAS) score for pain at rest and after the 6MWT, with 0=no pain and 10=severe pain. Assessments of treatment effect were performed 8–30 days after the last injection in a treatment cycle. These methodologies are widely used in arthritis research to quantify symptom severity and limitation of function, and responses to therapy.16–18 The 6MWT, first proposed some 50 years ago,19 is applied across many chronic diseases and has normative data.20 Patients with bilateral OA were assessed by VAS only in the injected knee.
Data management, quality assurance, and data security
Data collated into the SWO database are extracted from charts at point of care and then entered into a proprietary SQL™ program, which includes data verification. Data collection is conducted by a designated data abstraction team that also conducts quarterly verification tests on a random sample of 10% of records. Error rates are less than 1.3% per annum.
Participating practices have consented to the centralized accrual of clinical data from patient records in conformity with institutional approval. As described in other reports, all records are anonymized to standards that meet or exceed current confidentiality requirements,13 including encrypted identifiers for all physicians and patients. Industry-standard data protection methods, also detailed elsewhere,13,14 are in place to ensure the security of data during Internet transmission.
Statistical methods
The differences from pretreatment to posttreatment were computed for each group, and the mean changes were then compared between the two (independent) groups using Student’s t-test and the Wilcoxon rank test. Longitudinal regression analysis was performed to compare the changes in mobility and pain scores between the two groups, with adjustment for severity of OA according to the categorical variables of the Kellgren–Lawrence score and sex and the continuous variables of age at the first HA treatment, body mass index (BMI), comorbidity, and OA duration. A two-tailed P-value of <0.05 was used to indicate statistical significance.
All analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Inspection of the SWO database between 2006 and 2012 identified 20,187 patients who had a diagnosis of knee OA, 6,618 of whom were recorded as having been treated with IA HA. As illustrated in Figure 1, slightly fewer than half of these patients (46.6%) had received hylan G-F 20 and ~20% had undergone at least two consecutive series of treatment and assessment during 2006–2012.
  | Figure 1 CONSORT diagram for derivation of the study cohort. |
There was a slight preponderance of men among the patients who received consecutive courses of IA hylan G-F 20 therapy (Table 1). Two-thirds of patients had bilateral knee involvement; disease status was rated as established to advanced and severe (Kellgren–Lawrence scores 2–4, inclusive) in >90% of cases. Almost all patients (82%) were classified as either overweight (BMI 25–29 kg/m2) or expressly obese (BMI ≥30 kg/m2). The average age of patients at the first HA treatment was 68±15 years, and the average interval between the first diagnosis of OA and the first use of IA hylan G-F 20 was 5 years. Dyslipidemia (13%) and type 2 diabetes mellitus (11%) were conspicuous among baseline comorbidity findings. The demographic profile of the control group, also shown in Table 1, was substantially similar to that of the hylan G-F 20 group except for mean age, which was approximately 5 years lower in the reference cohort.
  | Table 1 Demographic details of the study cohort and reference group |
OA-related prescription medications recorded in the control group, as raw data, included prescription oral nonsteroidal anti-inflammatory drugs (NSAIDs) (n=2,355, 71%), acetaminophen (n=1,161, 35%), narcotics (primarily codeine phosphate, n=729, 22%), topical NSAIDs (n=197, 6%), glucosamine (n=430, 13%), chondroitin (n=132, 4%), and glucosamine with chondroitin (n=391, 12%).
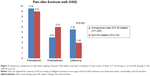
Findings for patients’ self-reported pain and objective data on mobility are depicted in Figures 2–4. Baseline scores did not differ significantly between groups. Pain at rest, pain after the 6MWT, and average distances travelled in the 6MWT all responded favorably to treatment in both groups, but in every instance, the mean effect in patients who underwent a repeat course of IA hylan G-F 20 therapy was significantly (P≤0.012) superior to that seen with control therapies.
Discussion
This study was designed to examine the clinical impact of HA in the real-world circumstances of Canadian primary care. Patients in the IA therapy group received no other prescribed OA medications, and patients in the control group received any prescribed OA therapy other than IA injections. Our research aimed to characterize the use of HA – specifically in the hylan G-F 20 preparation – as a practicable option in knee OA therapy in the context of a condition with a variable trajectory for which multiple different but non-definitive medical treatment modalities are available. We identified significant clinical benefit from two courses of hylan G-F 20 (administered within 6 months) in a contingent of patients with established knee OA in comparison to patients who did not receive IA therapy in this analysis of a large, longitudinal Canadian primary-care database.
Compared with reference medications, IA administrations of hylan G-F 20 were consistently associated with a significantly larger average reduction in patient-assessed pain both at rest (Figure 2) and after a 6MWT (Figure 3), and also with a significantly larger increase in distance travelled during a 6MWT (Figure 4). The treatment effect was most marked for the reduction in self-assessed pain after walking (Figure 3). These findings were obtained using simple, robust, and validated outcome measures, namely, a VAS for self-reported pain and a 6MWT for quantification of physical capacity. These instruments are widely employed in OA research, and although they do not offer the sophistication of some indices used in clinical trials, their simplicity, reliability, and reproducibility make them very suitable for use in primary care.
This new evidence of a sustained treatment effect from multiple courses of IA HA in patients with OA of the knee complements findings from other controlled and naturalistic studies8–19,21–23 and offers clinical correlates to the structural and joint-preservation effects documented by Wang et al24 and Li et al.25 Our contribution is notable for originating from what, to the best of our knowledge, is one of the largest cohorts of patients to receive repeat cycles of IA HA and for being derived from a dataset that is considered broadly representative of practice in Canada where primary-care physicians are the first point of care of the health care system and serve as its gatekeepers. Assessments of the efficacy of IA HA from meta-analyses vary: our data align with the conclusions of Bannuru et al26 rather than those of Rutjes et al.27
Various authorities have examined the question of what represents a clinically meaningful difference in the treatment of OA. For pain, several commentators have identified the threshold for a relevant clinical effect as a reduction in pain from baseline of the order of 25%.28–31 This threshold was exceeded in this investigation, which recorded a 47% reduction in mean pain score at rest and a 59% reduction after the 6MWT. These reductions in pain severity also exceed the target of a 40% reduction in pain suggested by Tubach et al32 and are compatible with the Osteoarthritis Research Society International Standing Committee for Clinical Trials Response Criteria Initiative and the Outcome Measures in Rheumatology criteria for a moderate response in the context of a clinical trial.33
Comparison of our 6MWT results with normative data derived from healthy individuals20,34 reveals the extent of limitation of function that may be associated with OA of the knee. After adjustment for age and weight, normative estimates for a 6MWT distance are in the region of 550 m for men and 500 m for women. In our cohort, the average distance travelled before initiation of IA HA therapy was ~310 m, close to or below the lower limit of normal.34 The average increase of 115 m after completion of treatment is thus a substantial increase; however, these patients still had a clear limitation of function compared with healthy age- and weight-matched peers, reflecting the morbidity imposed by OA of the knee. Reference to Figure 3 leads us to conjecture that at least part of the increase in distance walked by patients treated with IA HA may be attributed to patient perceptions of less pain during movement.
Broadly similar results might be expected from repeat use of other HA formulations, but in the absence of data, no firm conclusion can be reached on this point and the possibility of meaningful variation from the present findings cannot be excluded.
As with any real-world study, there are various limitations on our insights into patient behavior and the possible operation of multiple confounding factors has to be acknowledged. Apart from the issuing of repeat prescriptions, we have no data on treatment compliance in the control group and we also have no information about adherence (ie, patients taking their medication as prescribed throughout the full course of treatment). Undertreatment of the control group is therefore possible and may have contributed to the observed intergroup differences. We have no means of assessing use of over-the-counter medications in either group, and while it might be conjectured that rates of usage would be similar in both groups (and so make no net contribution to the results), we have no way of confirming this supposition. We also have no data on either the possible participation of patients in any physical rehabilitation or mobilization programs or the use (or changes in use) of physical aids, including walking canes and joint braces (see Sun et al35 for an important recent contribution in this area as well as Fernandes et al36). One of us (RJP) has reported previously on the interaction between perception of pain, activity-modifying behavior, and limitation of physical function and knee-related quality of life.37 The effect of these subtle interactions can be considerable but was beyond the scope of our investigation. One possibility to consider is that willingness to complete a course of IA therapy may be accompanied by greater willingness to adhere to rehabilitation exercises and similar measures. More positively, the potential for assignment or concealment bias appears small: patients and prescribers were not blinded to treatment assignment but identification of patients for inclusion was undertaken retrospectively by personnel unconnected with the process of care and guided by specific criteria.
Willingness to undergo IA therapy may also be relevant to the potential for an injection placebo effect. Bannuru et al26 have recently explored this issue and reported that IA HA had the largest effect size of any intervention examined versus oral placebo. These authors posited a contribution of an injection placebo effect to their findings and suggested that such an influence might contribute up to 45% of the total effect size. Even so they identified what they described as “small but robust differences… between active treatments.”26 A contribution from an injection placebo effect cannot be excluded from our findings, but we are unable to make any estimate of its size.
Obesity is the only recognized modifiable risk factor for OA,1 and reference to Table 1 confirms that most of the patients in both groups of our survey were substantially overweight or obese. We have no insights into the participation of patients in formal or informal weight-loss programs during the period under study.
These considerations notwithstanding, this was substantially a comparison of IA HA versus oral NSAIDs or coxibs or analgesics. The differences in outcomes between the groups are best explained as an enduring effect of IA hylan G-F 20 therapy and are compatible with a role for viscosupplementation in response to waning of the effect of first-line drugs.
The nature of the SWO database precludes the investigation of treatment-related adverse events, and we can make no direct comment on this aspect of therapy. In general, however, although viscosupplementation is not risk free,38 the recorded side-effect profile of this intervention is in notable contrast to the risk of systemic side effects that may arise with prolonged use of both NSAIDs and coxibs.39,40 Incidences of such adverse events are detrimental to both the risk-to-benefit profile of these drugs and their cost-effectiveness.
Conclusion
Repeated treatment at 6-month intervals with IA injections of hylan G-F 20 with no other prescribed OA medications improved pain and mobility in a contingent of patients with OA of the knee identified in a large real-world database; the degrees of improvement in both pain and mobility were significantly greater than those achieved in matched peers treated with prescribed OA medications but not IA injection.
Acknowledgments
Sanofi provided financial support for database analysis and for the preparation of this report. The authors thank Dr Nazanin Mehin, Sanofi Biosurgery, for constructive comments and advice in the development of this report. Writing and editorial services in the preparation of this report were provided by Hughes associates, Oxford, UK.
Disclosure
RJP reports consultancy fees from Amgen, AstraZeneca, Carbylan BioSurgery, Inc., Novartis, Sanofi Biosurgery, and Pfizer, plus speaking engagements for Carbylan BioSurgery, Inc.; Sanofi Biosurgery; and Pfizer.
CW was a salaried employee of Health Economics and Health Outcomes at Sanofi-Aventis Inc., Canada, during the time that this research was initiated, devised, and conducted and during the preparation of this report.
The authors report no other conflicts of interest in this work.
References
Bombardier C, Hawker G, Mosher D. The Impact of Arthritis in Canada: Today and Over the Next 30 Years. Toronto, ON: Arthritis Alliance of Canada; 2011. | ||
Bruyère O, Burlet N, Delmas PD, Rizzoli R, Cooper C, Reginster JY. Evaluation of symptomatic slow-acting drugs in osteoarthritis using the GRADE system. BMC Musculoskelet Disord. 2008;9:165. | ||
McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. | ||
Richmond J, Hunter D, Irrgang J, et al; American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee (nonarthroplasty). J Am Acad Orthop Surg. 2009;17:591–600. | ||
Hochberg MC, Altman RD, April KT, et al; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465–474. | ||
Hunter DJ. Viscosupplementation for osteoarthritis of the knee. N Engl J Med. 2015;372:1040–1047. | ||
Lùrati A, Laria A, Mazzocchi D, Re KA, Marrazza M, Scarpellini M. Effects of hyaluronic acid (HA) viscosupplementation on peripheral Th cells in knee and hip osteoarthritis. Osteoarthritis Cartilage. 2015;23:88–89. | ||
Navarro-Sarabia F, Coronel P, Collantes E, et al; AMELIA Study Group. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Ann Rheum Dis. 2011;70:1957–1962. | ||
Waddell DD, Cefalu CA, Bricker DC. A second course of hylan G-F 20 for the treatment of osteoarthritic knee pain: 12-month patient follow-up. J Knee Surg. 2005;18:7–15. | ||
Petrella RJ. Hyaluronic acid for the treatment of knee osteoarthritis: long-term outcomes from a naturalistic primary care experience. Am J Phys Med Rehabil. 2005;84:278–283. | ||
Petrella RJ, Gill D, Wakeford C. Health care resource utilization in the management of knee osteoarthritis with hyaluronic acid in a Canadian real-world population [abstract]. ISPOR 19th Annual International Meeting, Montreal, Que., Canada, May 31–June 4, 2014. Value Health. 2014;17(3):A49. | ||
Petrella R, Michailidis P. Retrospective analysis of real-world efficacy of angiotensin receptor blockers versus other classes of antihypertensive agents in blood pressure management. Clin Ther. 2011; 33:1190–1203. | ||
Petrella RJ, Blouin J, Davies B, Barbeau M. Prevalence, demographics, and treatment characteristics of visual impairment due to diabetic macular edema in a representative Canadian cohort. J Ophthalmol. 2012;2012:159167. | ||
Van Uum S, Hurry M, Petrella R, Koch C, Dranitsaris G, Lacroix A. Management of patients with Cushing’s disease: a Canadian cost of illness analysis. J Popul Ther Clin Pharmacol. 2014;21:e508–e517. | ||
Six Minute Walk Test (6MWT). Atlanta, GA, USA: American College of Rheumatology. Available from: https://www.rheumatology.org/Practice/Clinical/Clinicianresearchers/Outcomes_Instrumentation/Six_Minute_Walk_Test_(6MWT). Accessed February 8, 2015. | ||
Moe RH, Fernandes L, Osterås N. Daily use of a cane for two months reduced pain and improved function in patients with knee osteoarthritis. J Physiother. 2012;58:128. | ||
Foti C, Cisari C, Carda S, et al. A prospective observational study of the clinical efficacy and safety of intra-articular sodium hyaluronate in synovial joints with osteoarthritis. Eur J Phys Rehabil Med. 2011;47:407–415. | ||
Chen WL, Hsu WC, Lin YJ, Hsieh LF. Comparison of intra-articular hyaluronic acid injections with transcutaneous electric nerve stimulation for the management of knee osteoarthritis: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94:1482–1489. | ||
Balke B. A simple field test for the assessment of physical fitness. REP 63-6. Rep Civ Aeromed Res Inst US. 1963;53:1–8. | ||
Casanova C, Celli BR, Barria P, et al; Six Minute Walk Distance Project (ALAT). The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37:150–156. | ||
Raynauld JP, Torrance GW, Band PA, et al; Canadian Knee OA Study Group. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (Part 1 of 2): clinical results. Osteoarthritis Cartilage. 2002;10:506–517. | ||
Pal S, Thuppal S, Reddy KJ, et al. Long-term (1-year) safety and efficacy of a single 6-mL injection of hylan G-F 20 in Indian patients with symptomatic knee osteoarthritis. Open Rheumatol J. 2014;8:54–68. | ||
Pavelka K, Uebelhard D. Efficacy evaluation of highly purified intra-articular hyaluronic acid (Sinovial(®)) vs Hylan G-F20 (Synvisc(®)) in the treatment of symptomatic knee osteoarthritis. A double-blind, controlled, randomized, parallel-group non-inferiority study. Osteoarthritis Cartilage. 2011;19:1294–1300. | ||
Wang Y, Hall S, Hanna F, et al. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011;12:195. | ||
Li P, Raitcheva D, Hawes M, et al. Hylan G-F 20 maintains cartilage integrity and decreases osteophyte formation in osteoarthritis through both anabolic and anti-catabolic mechanisms. Osteoarthritis Cartilage. 2012;20:1336–1346. | ||
Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46–54. | ||
Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180–191. | ||
Brander VA, Gomberawalla A, Chambers M, Bowen M, Nuber G. Efficacy and safety of hylan G-F 20 for symptomatic glenohumeral osteoarthritis: a prospective, pilot study. PM R. 2010;2:259–267. | ||
Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–138. | ||
Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69:113–119. | ||
Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29–33. | ||
Tubach F, Ravaud P, Martin-Mola E, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res (Hoboken). 2012;64:1699–1707. | ||
Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2014;12:389–399. | ||
Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. | ||
Sun K, Song J, Manheim LM, et al. Relationship of meeting physical activity guidelines with quality-adjusted life-years. Semin Arthritis Rheum. 2014;44:264–270. | ||
Fernandes L, Hagen KB, Bijlsma JW, et al; European League Against Rheumatism (EULAR). EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72:1125–1135. | ||
Hamilton CB, Maly MR, Clark JM, Speechley M, Petrella RJ, Chesworth BM. Activity-modifying behaviour mediates the relationship between pain severity and activity limitations among adults with emergent knee pain. Physiotherapy Can. 2013;65:12–19. | ||
Goldberg VM, Coutts RD. Pseudoseptic reactions to hylan viscosupplementation. Clin Orthop. 2004;419:130–137. | ||
Atchison JW, Herndon CM, Rusie E. NSAIDs for musculoskeletal pain management: current perspectives and novel strategies to improve safety. J Manag Care Pharm. 2013;19(9 suppl A):S3–S19. | ||
Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16:821–847. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.