Back to Journals » Research Reports in Clinical Cardiology » Volume 6
P2Y12 inhibitors for acute coronary syndromes: current perspectives
Authors Nawarskas J, Newsome C, Anderson J, Ahmed B
Received 22 April 2015
Accepted for publication 26 May 2015
Published 9 October 2015 Volume 2015:6 Pages 123—143
DOI https://doi.org/10.2147/RRCC.S69478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Kones
James J Nawarskas,1 Cheyenne Newsome,2 Joe R Anderson,1 Bina Ahmed3
1Department of Pharmacy Practice and Pharmacy Administration, The University of New Mexico College of Pharmacy, 2Department of Pharmacy, The University of New Mexico Hospitals, 3Department of Internal Medicine, The University of New Mexico School of Medicine, The University of New Mexico, Albuquerque, NM, USA
Abstract: Antiplatelet therapies are a cornerstone for the management of acute coronary syndromes (ACSs), based largely on the prominent role that platelet activation and aggregation has on the pathophysiology of the disease. Dual-antiplatelet therapy involving an oral P2Y12 inhibitor plus aspirin is now considered standard of care for treating ACS. While clopidogrel has enjoyed nearly exclusive use as the P2Y12 inhibitor of choice for many years, the more powerful P2Y12 inhibitors prasugrel and ticagrelor have recently challenged clopidogrel as the preferred antiplatelet therapy for treating ACS. Both prasugrel and ticagrelor have proven to be superior to clopidogrel in reducing cardiovascular events in large clinical trials, albeit at the risk of increased bleeding. With the availability of these newer more potent agents, tailoring P2Y12 inhibition to be more patient specific becomes an intriguing possibility. Factors such as type of ACS presentation, patient comorbidities, use of concomitant medications, platelet reactivity, genetic predisposition, and cost should all be considered. In addition to oral agents, intravenous P2Y12 inhibition with cangrelor offers the advantage of quick onset and offset of action, but its clinical role is yet to be defined. Optimal medical and mechanical treatment of ACS hinges on suppressing platelet-related pathways, and P2Y12 inhibition plays a key role. As our understanding of ACS continues to evolve, there remains much to learn with respect to optimizing the use of these powerful drugs to most effectively help achieve the best clinical outcomes.
Keywords: P2Y12 inhibitors, acute coronary syndrome, ticagrelor, prasugrel, clopidogrel
Introduction
The predominant pathophysiological cause of acute coronary syndromes (ACSs) is atherosclerotic plaque rupture and subsequent arterial thrombosis. Platelets are the principal components of an arterial thrombus, and their rapid aggregation at the site of arterial damage leads to a constellation of events further contributing to thrombus progression and growth.1,2 Drugs that inhibit platelet aggregation would therefore be of theoretical benefit for treating ACS and clinical trials have indeed proven this to be the case. Clinical practice guidelines now state that oral antiplatelet therapies are foundational treatments for ACSs.3–6 The benefits of aspirin for treating ACS were established in 1988 when the Second International Study of Infarct Survival trial demonstrated that 160 mg/day of aspirin reduced vascular death, both alone and in combination with streptokinase, in patients with a suspected myocardial infarction (MI).7 Aspirin is now considered first-line therapy for all patients with ACS.3–6 The landscape changed with the publication of the CURE trial in 2001, which demonstrated that adding clopidogrel to aspirin reduced major adverse cardiovascular events by 20% compared to aspirin alone in patients suffering from an ACS without ST-segment elevation.8 Since then, other trials have supported the benefits of dual-antiplatelet therapy (DAPT) in various ACS settings.9–12
Despite the benefits of clopidogrel, it is not universally effective, which may in part be due to genetic variations in response. In addition, the magnitude of antiplatelet effect is moderate and it can take up to 8 hours to reach maximal effect after a 600 mg loading dose.13 These limitations of clopidogrel have led to the development and approval of alternative agents that also target the P2Y12 receptor. Prasugrel and ticagrelor, like clopidogrel, block the binding of adenosine diphosphate to the P2Y12 platelet receptor, thereby interfering with platelet activation and aggregation. However, both prasugrel and ticagrelor yield faster and more pronounced inhibition of platelet aggregation compared to clopidogrel (Table 1). In addition, ticagrelor does not need to be metabolically activated and prasugrel requires only one metabolic step for activation compared to two for clopidogrel. This reduces the potential for variations in response with prasugrel and ticagrelor compared to clopidogrel due to fewer potential drug interactions and lesser influence of genetic variability of drug-metabolizing enzyme activity. In addition, the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel – Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38 and the study of Platelet Inhibition and Patient Outcomes (PLATO) proved prasugrel and ticagrelor, respectively, to be superior to clopidogrel in terms of reducing ischemic events, albeit with a higher risk of bleeding.22,23
  | Table 1 Comparison of P2Y12 inhibitors |
Cangrelor is an intravenously administered investigational P2Y12 inhibitor with a very short half-life (3–6 minutes) and a rapid onset and offset of effect, with the platelet function normalizing within 60 minutes of drug discontinuation.24,25 As such, it is only being investigated for use in the acute setting. The CHAMPION studies comprise the major clinical trial data evaluating cangrelor for ACS treatment. CHAMPION PCI (n=8,877) and CHAMPION PLATFORM (n=5,362) were both placebo-controlled trials that compared cangrelor to a 600 mg loading dose of clopidogrel in ACS patients scheduled to undergo PCI.24,25 CHAMPION PCI administered the clopidogrel load at the start of PCI, whereas CHAMPION PLATFORM administered the clopidogrel load at the end of the procedure. The primary end point of death, MI, or ischemia-driven revascularization at 48 hours was comparable between the two groups in both studies. However, MI was the most frequently occurring end point in both of these studies, which is often difficult to adjudicate periprocedurally due to rising levels of baseline cardiac biomarkers associated with the index event. When the pooled results of the CHAMPION PCI and CHAMPION PLATFORM trials were analyzed in the non-STEMI population using the universal (vs protocol) definition of MI, cangrelor reduced the risk of the primary end point by 18% compared to clopidogrel (P=0.018).26,27 The CHAMPION PHOENIX trial more carefully defined periprocedural MI in comparing cangrelor to clopidogrel in 11,145 patients undergoing PCI (57% stable angina, 43% ACS) who had not received an oral P2Y12 inhibitor within 7 days before randomization.28 In this double-blind, placebo-controlled study, cangrelor reduced the primary end point of death, MI, ischemia-driven revascularization, or stent thrombosis at 48 hours compared to clopidogrel (4.7% vs 5.9%, P=0.005), with most of the benefit occurring through a reduction in MI and stent thrombosis. In addition, severe bleeding was not significantly increased with cangrelor. However, about 25% of patients in this trial received a 300 mg versus 600 mg loading dose of clopidogrel and 37% of patients in the clopidogrel group received the drug during or after PCI, raising concerns as to whether or not a sufficient antiplatelet effect was present during PCI in these patients.29 There are also concerns regarding, even with the attention given to defining MI, whether or not this study was able to accurately define periprocedural MI according to the universal definition, which requires at least two serum cardiac biomarker samples taken 6 hours apart in patients with elevated biomarkers before PCI; the median time from hospital admission to PCI was 4.4 hours in CHAMPION PHOENIX.26,29 Cangrelor is currently indicated as an adjunct to PCI for reducing the risk of periprocedural MI, repeat coronary revascularization, and stent thrombosis in patients who have not been treated with a P2Y12 inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.30 Cangrelor has also shown promise as a bridging agent in patients requiring surgery. In a double-blind, placebo-controlled study of 210 patients who required discontinuation of P2Y12 inhibitor for coronary artery bypass grafting (CABG) surgery, cangrelor provided sustained inhibition of platelet function throughout the preoperative period without an increase in major bleeds, although there were numerically more minor bleeding episodes with cangrelor.31 However, this was not a clinical outcome study and the results should be interpreted with that in mind.
So while DAPT for ACS has predominantly included aspirin and clopidogrel, the clinician currently has the option of choosing from among three different oral P2Y12 inhibitors for this indication. In addition, the role of intravenous ultra-fast acting P2Y12 inhibition with cangrelor holds promise, but still needs to be better defined and is not the focus of this review. This paper discusses different considerations the clinician, as well as health care system, must weigh when selecting the most appropriate oral P2Y12 inhibitor for each individual patient presenting with an ACS.
Type of ACS and treatment strategy
ST-elevation myocardial infarction
Primary percutaneous coronary intervention (PPCI) is the preferred reperfusion strategy for treating ST-elevation MI (STEMI), and clopidogrel, prasugrel, and ticagrelor all have evidence supporting their use in this scenario. The use and benefits of DAPT for patients undergoing PPCI in STEMI were established prior to the more widespread use of clopidogrel following the CURE trial in 2001. The ISAR and STARS trials published in 1997 and 1998, respectively, demonstrated the superiority of ticlopidine plus aspirin compared to both aspirin alone and aspirin plus warfarin in post-stent patients.32,33 The unfavorable hematologic side effect profile of ticlopidine has led to it being used only very rarely in contemporary practice, and clopidogrel has been shown to be an equally efficacious yet safer alternative.34 Consequently, DAPT with clopidogrel and aspirin has been considered the standard of care for many years for patients undergoing PPCI. That said, questions about the optimal dosing of aspirin and clopidogrel led to the conduct of the CURRENT-OASIS 7 trial involving 25,086 patients undergoing an invasive treatment strategy for ACS (29% STEMI, 71% unstable angina [UA] or non-STEMI).9 In this trial, doubling the dose of clopidogrel (600 mg loading dose followed by 150 mg daily for 6 days, then 75 mg daily) or using higher dose aspirin (300–325 mg daily) offered no efficacy advantage in reducing the primary end point of cardiovascular death, MI, or stroke at 30 days compared to standard-dose clopidogrel (300 mg load followed by 75 mg daily) or lower dose aspirin (75–100 mg daily). However, double-dose clopidogrel increased the incidence of major bleeding compared to standard-dose clopidogrel (2.5% vs 2.0%; hazard ratio [HR] 1.24, 95% confidence interval [CI] 1.05–1.46; P=0.01). In addition, a prespecified subgroup analysis of patients who underwent PCI for ACS (n=17,263) demonstrated that double-dose clopidogrel reduced the rate of the primary outcome by 14% (P=0.039) as well as the rate of definite stent thrombosis by 46% (P=0.0001), albeit with a 41% increase in the rate of major bleeding (P=0.009).12 The data supporting the use of prasugrel for STEMI emanate from the TRITON-TIMI 38 trial that enrolled 13,608 patients with ACS scheduled to undergo PCI.22,35 In this study overall, prasugrel plus aspirin was more effective than clopidogrel plus aspirin at reducing the incidence of the primary end point of cardiovascular death, nonfatal MI, or nonfatal stroke (9.9% vs 12.1%; HR 0.81, 95% CI 0.73–0.90; P<0.001), albeit with an increase in the risk of non-CABG-related major bleeds (2.4% vs 1.8%; HR 1.32, 95% CI 1.03–1.68; P=0.03).22 All-cause mortality did not differ between prasugrel and clopidogrel, and there was no increase in the risk of intracranial hemorrhage with prasugrel except in those patients with a history of a cerebrovascular event. Overall, the clinical benefits of prasugrel were independent of ACS type (ie, UA/non-STEMI or STEMI). Data from the prespecified cohort of patients who presented with STEMI and underwent PPCI (26% of patients) demonstrated a 21% reduction in the primary end point compared to clopidogrel at 15 months (P=0.02).22,35 Interestingly, non-CABG-related major bleeding was not increased with prasugrel in the STEMI cohort (1.0% prasugrel vs 1.3% clopidogrel; P=0.34).35
The landmark trial that assessed the safety and efficacy of ticagrelor was the PLATO trial.23 Overall, PLATO demonstrated that ticagrelor plus aspirin reduced the primary end point of vascular death, MI, or stroke by 16% compared to clopidogrel plus aspirin (P<0.001), although non-CABG-related major bleeding was greater with ticagrelor compared to clopidogrel (19% increase in risk using study-defined criteria; 25% increase in risk using TIMI-defined criteria; P=0.03 for both). There was a 22% risk reduction in all-cause mortality with ticagrelor treatment (4.5% vs 5.9%; P<0.001), but ticagrelor also increased the risk of intracranial hemorrhage by 87% (0.3% vs 0.2%; P=0.06). The STEMI cohort represented 38% of the entire study cohort, and this subgroup experienced efficacy similar to that of the overall population: a 13% risk reduction in the primary end point with ticagrelor (P=0.07) and an 18% reduction in all-cause mortality (P=0.05). Non-CABG-related major bleeding was not significantly different between ticagrelor and clopidogrel in this subgroup.36 An additional analysis showed that the reduction in MI seen with ticagrelor occurred primarily in patients who were admitted with STEMI, while those admitted with non-STEMI experienced a reduction in cardiovascular mortality but not MI.37
Although PPCI is the preferred treatment for STEMI, a good proportion of patients still receive fibrinolytic therapy for reperfusion. Among these patients, the strongest data support the use of clopidogrel. The CLARITY-TIMI 28 trial demonstrated that adding clopidogrel (300 mg load followed by 75 mg daily) to aspirin and fibrinolytic therapy was superior to placebo (with aspirin and fibrinolytic) in reducing the composite primary end point of an occluded infarct-related artery on angiography or death or recurrent MI before angiography (HR 0.64, 95% CI 0.53–0.76; P<0.001) in 3,491 patients being treated for ST-elevation MI.10 At 30 days, clopidogrel therapy reduced the composite end point of cardiovascular death, recurrent MI, or recurrent ischemia leading to urgent revascularization by 20% (P=0.03). The COMMIT trial randomized 45,852 patients with acute MI (87% with ST-elevation; 6% with bundle branch block) to receive either clopidogrel or placebo in addition to aspirin therapy.11 Patients undergoing primary PCI were excluded, and fibrinolytic therapy was administered to 54% of patients at some time before or after randomization. Overall, clopidogrel reduced the primary composite outcome of death, reinfarction or stroke by 9% (95% CI 0.86–0.97; P=0.002) and reduced death alone by 7% (95% CI 0.87–0.99; P=0.03) compared to placebo with no significant excess risk of bleeding. These benefits were present both in patients who did and did not receive fibrinolytic therapy, although numerically the benefit was more pronounced in patients who received fibrinolytic therapy (11% risk reduction in the primary end point with fibrinolytic therapy vs 7% without fibrinolytic therapy; P=0.4).
Non-ST-elevation ACS
Current clinical practice guidelines for the management of non-ST-elevation ACS divide disease management into two general treatment approaches: 1) an early invasive strategy (ie, coronary angiography with intent to perform immediate revascularization) and 2) an ischemia-guided strategy (ie, coronary angiography only if refractory or recurrent symptoms despite medical treatment or hemodynamic instability).3 Clopidogrel has been studied using both of these management approaches. The landmark CURE study compared clopidogrel plus aspirin to placebo plus aspirin in 12,562 patients suffering from non-ST-elevation ACS. Overall, clopidogrel plus aspirin was more beneficial than aspirin alone at reducing the composite end point of cardiovascular death, nonfatal MI, or stroke (HR 0.80, 95% CI 0.72–0.90; P<0.001) with a 38% increase in risk for major bleeding (P=0.001). The benefits of clopidogrel were independent of admitting diagnosis (75% of patients had UA, 25% non-STEMI) or whether or not patients were revascularized with PCI after randomization.8 The CURE trial employed primarily an ischemia-guided strategy, with PCI being performed at the discretion of the local investigator. Forty-four percent of patients underwent coronary angiography after randomization and 21% underwent PCI (14% during initial hospitalization, 7% after discharge). A median of 6 days had passed before PCI was performed in those receiving PCI during the initial hospitalization.38 For patients undergoing an early invasive management strategy for non-ST-elevation ACS, the results from the CURRENT-OASIS 7 trial can be applied to clopidogrel treatment.12 For non-ST-elevation ACS with an early invasive approach, both prasugrel and ticagrelor have evidence to support their use. In TRITON-TIMI 38 trial, all patients underwent PCI and an early invasive treatment strategy. Three-quarters of the cohort were patients with UA or non-STEMI and showed similar clinical benefits of prasugrel over clopidogrel independent of ACS type.22 Similarly in PLATO, the vast majority of patients were enrolled with UA or non-STEMI although only 72% underwent planned invasive treatment.23 The benefits of ticagrelor over clopidogrel were independent of whether an early invasive or ischemia-guided strategy was employed.39,40 A subgroup analysis from PLATO demonstrated that patients admitted with MI (either STEMI or non-STEMI) had significant reductions in major adverse cardiovascular events with ticagrelor plus aspirin versus clopidogrel plus aspirin, whereas those admitted with UA did not (HR 0.96, 95% CI 0.75–1.22).23 However, the study was underpowered for patients with UA, the test for interaction by clinical presentation of ACS was negative (P=0.41), and there is no biologically plausible explanation for this purported lack of benefit in UA patients.41 As such, patients with UA are believed to be appropriate candidates for ticagrelor therapy.3,5,41
For patients with non-ST-elevation ACS undergoing an ischemia-guided strategy (PCI optional), it is important to note that only ticagrelor (not prasugrel) is supported by evidence of benefit over clopidogrel. Twenty-eight percent of patients in the PLATO trial underwent an ischemia-guided treatment strategy, and ticagrelor was shown to be more beneficial than clopidogrel in that group.23,42 TRITON-TIMI 38 did not enroll patients undergoing an ischemia-guided strategy. Consequently, the TRILOGY-ACS trial was conducted in 7,243 patients with non-ST-elevation ACS who were not planned to undergo revascularization in order to compare the efficacy and safety of prasugrel plus aspirin to clopidogrel plus aspirin as part of medical therapy.43 After a median follow-up of 17 months, there was no difference in either the primary efficacy end point of cardiovascular death, MI, or stroke or bleeding rates between prasugrel and clopidogrel.
In summary, the clinical trial results as well as clinical practice guidelines support the use of clopidogrel, prasugrel, or ticagrelor for all types of ACS (Figure 1).3–6 Landmark trial data are reviewed in Table 2. Clopidogrel is efficacious for STEMI treatment regardless of whether PPCI or fibrinolysis is the chosen treatment approach. Similarly, clopidogrel is efficacious for non-ST-elevation ACS treatment regardless of whether an early invasive or ischemia-driven management strategy is selected. Prasugrel has not been adequately studied in patients receiving a fibrinolytic drug for STEMI and has not shown superior efficacy over clopidogrel for non-ST-elevation ACS undergoing an ischemia-guided strategy. Its use is more appropriately restricted to ACS patients undergoing PCI, especially patients with STEMI undergoing PPCI due to an efficacy advantage over clopidogrel without an increased risk of bleeding. Ticagrelor has been shown to be more efficacious than clopidogrel in most ACS settings but, like prasugrel, has not been adequately studied in STEMI patients receiving fibrinolytic therapy. This latter situation would therefore favor the administration of clopidogrel as the P2Y12 inhibitor of choice.
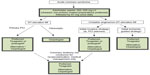  | Figure 1 Guideline-based recommendations for oral P2Y12 selection for acute coronary syndromes. |
Patient comorbidities
Diabetes
Patients with diabetes have been shown to have diminished responsiveness to clopidogrel, which is believed to be due to disease-mediated changes in drug pharmacokinetics that may involve reduced clopidogrel absorption and/or altered clopidogrel metabolism.44 While certainly not conclusive, there is some suggestion that patients with diabetes in large clinical trials receiving clopidogrel did not obtain as much benefit as patients without diabetes.45 For example, the 2,840 patients with diabetes in the CURE study benefited with clopidogrel treatment overall, but 14.2% of them experienced the primary end point while on clopidogrel compared to 7.9% of patients without diabetes.8 Similarly, the CREDO trial demonstrated clopidogrel to reduce the combined end point of death, MI, or stroke in both patients with (n=560) and without (n=1,556) diabetes who received clopidogrel for 1 year following elective bare-metal coronary artery stent placement, but the magnitude of benefit was greater in the patients who did not have diabetes (32.8% relative risk reduction) compared to those with diabetes (11.2% relative risk reduction).46 This phenomenon has not yet been shown with either prasugrel or ticagrelor. In contrast to the diminished clinical response that has been suggested with clopidogrel, the 3,146 patients with diabetes in TRITON-TIMI 38 trial showed benefit with prasugrel to a greater extent than those without diabetes: the primary end point was reduced by 30% with prasugrel in diabetic patients compared to a 14% reduction in nondiabetics, although there was no significant interaction between treatment effect and diabetes status (P=0.09).22,47 Prasugrel increased the risk of TIMI major bleeding by 43% in nondiabetics compared to clopidogrel (P=0.02), but there was no significant increase in major bleeding risk in diabetics (2.6% prasugrel vs 2.5% clopidogrel, P=0.81).47 The net clinical benefit (death, MI, stroke, non-CABG-related major bleed) with prasugrel was shown to be greater in diabetics compared to nondiabetics (26% vs 8% risk reduction, respectively, P=0.05).47 The 4,662 patients with diabetes in the PLATO trial received the same benefit with ticagrelor as the patients without diabetes.48 The risk reduction for the primary end point was 12% in the diabetic population and 17% in the patients without diabetes (P=0.49). The risk of non-CABG-related major bleeding was also comparable between diabetics and nondiabetics receiving ticagrelor. The PEGASUS-TIMI 54 trial also showed similar benefit with long-term ticagrelor therapy in both diabetic and nondiabetic patients with stable coronary artery disease (15% risk reduction in both groups).49 In aggregate, these data may be used to support the use of either prasugrel or ticagrelor over clopidogrel for treating ACS in a patient with diabetes.
Smoking
There is debate regarding a correlation between smoking status and response to antiplatelet therapy, platelet reactivity, and clinical outcomes. Smokers have been shown to have a more pronounced antiplatelet response to clopidogrel compared to nonsmokers, possibly through accelerated formation of the active metabolite of clopidogrel by cytochrome P450 (CYP) 1A2 enzyme induction.50–55 However, a large analysis of several patient cohorts reported that smoking was not associated with an enhanced platelet response to clopidogrel56 and clinical trial data have not convincingly proven that clopidogrel is more efficacious in smokers compared to nonsmokers.57–59 The antiplatelet effects of prasugrel are comparable in both smokers and nonsmokers, and prasugrel has been shown to elicit greater antiplatelet effects than clopidogrel regardless of smoking status.55 Prasugrel and clopidogrel were compared in a subanalysis of the TRILOGY-ACS trial to explore the relationship between smoking status, platelet reactivity and clinical outcomes.60 The 30-month analysis included 7,062 patients less than 75 years of age randomized to clopidogrel or prasugrel and evaluated clinical ischemic outcomes (cardiovascular death, MI, or stroke). Twenty-three percent of these patients (n=1,613) also had platelet function testing performed. In this study, current smokers had fewer comorbidities at baseline and nearly half quit smoking during follow-up. On-treatment platelet reactivity was lower with prasugrel compared to clopidogrel, but no significant interaction between smoking status and platelet reactivity was noted. The frequency of ischemic outcomes in smokers was significantly lower with prasugrel (11.7%) versus clopidogrel (18.6%), but no difference was observed in nonsmokers (13.8% vs 13.7%, respectively; P=0.002 for interaction). These findings are hypothesis generating but suggest a relationship between smoking and response to antiplatelet therapy with prasugrel. In the PLATO trial, the clinical benefits of ticagrelor were not affected by smoking status, with ticagrelor demonstrating a 17% reduction in risk of the primary end point compared to clopidogrel in smokers and an 11% reduction in risk in ex/nonsmokers (P=0.5).61
Other considerations
Prasugrel is contraindicated in patients with prior stroke or transient ischemic attack since it was detrimental to these patients in the TRITON-TIMI 38 trial: there was a 54% increase in the risk of the combined end point of death, MI, stroke, or non-CABG-related major bleeding compared to clopidogrel (P=0.04), including more intracranial bleeds (2.3% prasugrel, 0% clopidogrel; P=0.02).15,22 TRITON-TIMI 38 also showed that prasugrel did not provide any efficacy benefit in patients at least 75 years of age or among patients who weighed less than 60 kg with a tendency toward more major bleeding, although this was not statistically significant (non-CABG-related major bleeding was 4.3% with prasugrel, 3.3% with clopidogrel; P=0.10).22 Patients over 75 years of age also had an increased risk of fatal and symptomatic intracranial bleeds with prasugrel therapy (1.0% and 0.8%, respectively, with prasugrel vs 0.1% and 0.3%, respectively, with clopidogrel).15 These findings have led to the recommendation to avoid prasugrel in patients 75 years of age or older unless the benefits are believed to outweigh the risks and to consider lowering the maintenance dosage from 10 mg daily to 5 mg daily in patients weighing less than 60 kg.15 Ticagrelor was associated with an increased risk of intracranial hemorrhage in the PLATO trial and as such is contraindicated in patients with a history of intracranial hemorrhage.14 Ticagrelor is also contraindicated in patients with severe hepatic impairment and is to be used cautiously in patients with moderate hepatic impairment due to both an increased risk of bleeding due to a reduction in the synthesis of coagulation proteins as well as a probable increase in ticagrelor exposure due to reduced hepatic metabolism.14
Timing of therapy
Patients presenting with ACS often possess much uncertainty in terms of not only diagnosis but also the extent of coronary artery disease. This is especially true with non-ST-elevation ACS. Consequently, there may be reluctance to begin P2Y12 inhibitor therapy until coronary angiography has been performed and the coronary anatomy defined. The CREDO trial showed a reduction in the combined end point of death, MI, or urgent target-vessel revascularization when clopidogrel pretreatment (300 mg) was given more than 6 hours before PCI.46 A meta-analysis evaluating the efficacy and safety of clopidogrel pretreatment in patients undergoing PCI for either ACS or stable coronary artery disease demonstrated that pretreatment lowered the risk of major cardiac events by 23% compared to no pretreatment (P<0.001) without an increased risk of major bleeds.62 The Comparison of Prasugrel at the Time of PCI or As Pretreatment at the Time of Diagnosis in Patients with Non-STEMI (ACCOAST) trial investigated the efficacy of administering prasugrel either at the time of diagnosis (upstream) or after coronary angiography in 4,033 patients with non-ST-elevation ACS. The first loading dose of prasugrel (or placebo) was given a median of 4.4 (or 4.2) hours prior to angiography. The primary end point of cardiovascular death, MI, stroke, urgent revascularization, or glycoprotein IIb/IIIa inhibitor rescue therapy through day 7 did not differ between treatment strategies, although upstream treatment increased the risk of major bleeding by 90% (P=0.006).63
TRITON-TIMI 38 was designed as a PCI study, so randomization of study treatment (prasugrel or clopidogrel) occurred after the coronary artery anatomy was known. Study drug was started either during or within 1 hour after PCI in 74% of patients.22 In PLATO, randomization to ticagrelor or clopidogrel occurred at the time of diagnosis and treatment was started at a median of 15 minutes to just under 4 hours prior to PCI.23 The ATLANTIC study randomized 1,862 patients with STEMI going for coronary angiography to either prehospital (in the ambulance) or in-hospital (in the catheterization laboratory) ticagrelor administration.64 Randomization occurred at the time of diagnosis, and the median time from randomization to angiography was 48 minutes with a median difference of 31 minutes between the two treatment strategies with respect to ticagrelor administration. The coprimary end points were: 1) the proportion of patients who did not have a 70% or greater resolution of ST-segment elevation before PCI and 2) the proportion of patients who did not have TIMI flow grade 3 in the infarct-related artery at initial angiography. There was no difference between groups with either of the two co-primary end points or with major bleeds, but the rates of definite stent thrombosis were lower with prehospital treatment both within 24 hours of index PCI (0% vs 0.8%; P=0.008) as well as at 30 days (0.2% vs 1.2%; P=0.02).
These results suggest that consideration should be given to early loading of P2Y12 inhibitors in patients with STEMI who are receiving PPCI as the initial treatment strategy.65 For patients with non-ST-elevation ACS, delaying the administration of a loading dose is recommended if there is diagnostic uncertainty. Once a diagnosis is established, then either ticagrelor or clopidogrel can be administered, especially if the delay to angiography will be several hours. If prasugrel is to be used and angiography is planned within hours of presentation, then prasugrel can be given once coronary anatomy is known and the decision has been made to undergo PCI.65
Concomitant medications
Proton pump inhibitors
Proton pump inhibitors (PPIs) are substrates and inhibitors of CYP2C19, the same enzyme that plays a major role in clopidogrel activation. Concern therefore exists as to whether or not PPIs may interfere with the activation of clopidogrel and, hence, its pharmacologic effect. Several retrospective cohort studies and prospective randomized trials have evaluated the possible interaction between PPIs and clopidogrel. Some report a significantly increased risk (6%–18%) for negative cardiac-related outcomes and overall mortality (3%–9% increased risk) associated with concurrent use of PPIs and clopidogrel.66–71 In contrast, other clinical outcome studies have reported minimal or no impact of concurrent PPI and clopidogrel use on cardiovascular outcomes.72–75 The major study investigating this issue was the COGENT trial.75 This study randomized 3,873 patients with an indication for DAPT to receive aspirin with either clopidogrel plus omeprazole or clopidogrel plus placebo. The primary cardiovascular end point of the combination of cardiovascular death, MI, revascularization, or stroke occurred at similar rates in both groups (4.9% omeprazole vs 5.7% placebo; P=0.98). While these results seem to suggest no clinically meaningful interaction between omeprazole and clopidogrel, the study was prematurely terminated due to lack of funding, which limits its power. Given the conflicting data, the potential for negative outcomes from concomitant use with clopidogrel, and the availability of suitable alternatives for PPI therapy, it is recommended to avoid omeprazole or esomeprazole (stronger inhibitors of CYP2C19) in patients treated with clopidogrel.16,76,77 Pantoprazole is a potential alternative PPI to use in patients taking clopidogrel who require a PPI. Pantoprazole is a weaker inhibitor of CYP2C19 and has less effect on the activity of clopidogrel.16,76,77 Neither prasugrel nor ticagrelor relies heavily on CYP2C19 for metabolism, and accordingly, neither of these drugs exhibit any significant interactions with PPIs. Aside from potentially interfering with clopidogrel activation, PPIs have been accused of increasing the risk of MI independent of antiplatelet therapy.78–81 A database analysis examining MI risk over 120 days in 125,000 patients with prescriptions for PPIs and an equal number of matched control patients not using PPIs found PPI use to be significantly associated with a higher risk of MI (HR 1.58, 95% CI 1.11–2.25; P=0.011). However, with a number needed to harm of 4,357, the benefits of PPI therapy may very well outweigh this potential risk for many patients.82
Aspirin
The PLATO trial demonstrated that patients enrolled in North American sites did not benefit as much from ticagrelor therapy as those outside of North America. In fact, there was even suggestion that clopidogrel may be better than ticagrelor in these patients. The HR for North American participants was 1.25 (95% CI 0.93–1.67) compared to 0.80–0.86 for the rest of the world.23 This led to a delay in the approval of ticagrelor in the United States pending an explanation for this phenomenon. Subsequent analysis of data from PLATO demonstrated that the use of aspirin dosages of 300 mg/day or greater was substantially higher in the United States (53.6% of patients) compared to the rest of the world (1.7% of patients). Of 37 different baseline and post-randomization factors explored, aspirin dosage was the only factor that was able to explain the geographic disparity in results.83 When the results were analyzed in patients taking low-dose (≤100 mg/day) aspirin, consistent benefit was seen with ticagrelor, even in North American patients.83 Consequently, the product labeling for ticagrelor prohibits the usage of this drug to patients taking daily aspirin dosages in excess of 100 mg daily.14 To date, there remains no clear explanation as to why higher doses of aspirin may mitigate the benefits of ticagrelor and neither prasugrel nor clopidogrel has shown this phenomenon. In an effort to better describe this interaction, there exist some theories as to why ticagrelor may interact with aspirin.84 One theory is that P2Y12 inhibitors are somewhat reliant on prostacyclin for their antiplatelet effect and inhibiting prostacyclin with higher dosages of aspirin may therefore be counterproductive.83 Ticagrelor may be most susceptible to this interaction because of its relatively strong inhibition of P2Y12. Another theory is that ticagrelor is unique from other P2Y12 inhibitors in that it possesses off-target effects unrelated to platelet P2Y12 inhibition that may be affected by aspirin. For example, ticagrelor has been shown to inhibit vasoconstriction by acting on vascular smooth muscle cell P2Y12 receptors, an effect that was attenuated by higher but not lower doses of aspirin.84,85
Oral anticoagulants
Patients with ACS who have a need for oral anticoagulation (eg, atrial fibrillation, mechanical heart valve) represent a complicated treatment group. Logically, adding an antiplatelet medication to a patient taking an oral anticoagulant presents an increased bleeding risk.86,87 Clinical practice guidelines address this issue, but the deficiency of controlled clinical trials in this area makes it difficult to establish definitive recommendations. The WOEST trial was a randomized, open-label study comparing the safety and efficacy of clopidogrel alone and clopidogrel plus aspirin in 573 patients undergoing PCI who also had an indication for oral anticoagulation (69% atrial fibrillation, 10%–11% mechanical valve).88 The oral anticoagulant used was warfarin or a warfarin-like drug. After 1 year of treatment, the primary end point of any bleeding episode within 1 year of PCI occurred in 19.4% of patients receiving double therapy and 44.4% of patients receiving triple therapy (HR 0.36, 95% CI 0.26–0.50; P<0.0001). The combined secondary end point of death, MI, stroke, target-vessel revascularization, and stent thrombosis occurred in 11.1% of patients receiving double therapy and 17.6% of patients receiving triple therapy (P=0.025).
While underpowered to detect a significant effect on cardiovascular outcomes, the WOEST trial did provide enough evidence to prompt some clinicians to omit aspirin from a post-stent regimen in patients in need of oral anticoagulation. This treatment approach is reflected as a Class IIb recommendation (benefit ≥ risk; treatment may be considered) in current guidelines for managing atrial fibrillation.89 These same guidelines as well as others also mention using a bare-metal stent, when appropriate, over a drug-eluting stent as a means of minimizing the duration of DAPT in patients needing triple antithrombotic therapy or who are at a high bleeding risk.4–6,89 Current clinical practice guidelines (most released before the publication of WOEST) neither discourage nor condone triple antithrombotic therapy,3–5,89 although guidelines from the European Society of Cardiology as well as the American College of Chest Physicians (both published prior to WOEST) are in favor of triple therapy in patients with atrial fibrillation and a CHADS2 or CHADS2-VASc score of ≥2.6,90 The American College of Chest Physicians recommend triple antithrombotic therapy for 1 month for a bare-metal stent and 3–6 months following drug-eluting stent placement, followed by discontinuation of the P2Y12 inhibitor.90
Clearly, more research is needed to help guide the clinician in managing the post-ACS patient with a need for oral anticoagulation. While most clinical practice guidelines are rather neutral in their recommendations, there are guidelines that are in favor of triple antithrombotic therapy in this situation. However, the evidence that is available would suggest using clopidogrel as the P2Y12 inhibitor of choice and warfarin (or warfarin-like compound) as the oral anticoagulant of choice should triple antithrombotic therapy be employed. This decision has to be made weighing the risks and benefits in each individual patient.
Personalized therapy
Platelet function testing
The reported incidence of patients with high platelet reactivity (HPR) while taking clopidogrel is reported to be between 4% and 30%.91–94 Five meta-analyses of prospective observational studies and subanalyses of randomized controlled studies involving >10,000 PCI patients have reported strong associations between HPR while on clopidogrel and adverse cardiovascular outcomes.95–99 Strategies have been tested to overcome poor responsiveness to clopidogrel, including increasing the clopidogrel dose or switching to a more potent P2Y12 inhibitor.
The Gauging Responsiveness with A VerifyNow assay – Impact on Thrombosis and Safety (GRAVITAS) study was the first large randomized prospective trial evaluating the clinical benefit of tailored clopidogrel treatment in patients undergoing PCI.100 GRAVITAS included 2,214 patients, most with stable coronary artery disease, who received a 600 mg clopidogrel loading dose before PCI with stent implantation. At 12–24 hours after PCI, patients receiving clopidogrel with HPR (defined as P2Y12 reaction units [PRU] ≥230 with VerifyNow P2Y12) were randomized to standard clopidogrel dosing (75 mg daily) or high-dose clopidogrel (150 mg daily). At 6 months, there was no difference in the primary composite efficacy end point of cardiovascular death, acute MI, or stent thrombosis (2.3% in both groups; P=0.97). There was also no difference in the primary safety end point of severe or moderate bleeding based on the GUSTO definition (1.4% high dose vs 2.3% standard dose; P=0.1). Criticisms of the GRAVITAS trial include an event rate much lower than expected, possibly causing the trial to be underpowered to detect a difference between treatments. Additionally, the threshold of PRU ≥230 to classify poor responders may have been too high. A post hoc analysis identified PRU ≥208 as being a more predictive cutoff value for greater risk of ischemic events.101 In this analysis, achieving an on-treatment PRU <208 was associated with a lower risk of cardiovascular events at both 2 months and 6 months whereas achieving a PRU <230 was not. In addition, increasing the clopidogrel dose to 150 mg was not sufficient to overcome a poor response to clopidogrel in many patients in the tailored treatment arm, as 36%–40% of patients remained poor (PRU ≥230) responders when platelet function testing was repeated at 1 month and 6 months.100 The ARCTIC trial evaluated the clinical utility of platelet function testing (using VerifyNow P2Y12) to adjust antiplatelet regimens for patients scheduled for PCI.102 This study randomized 2,440 patients to receive adjusted antiplatelet treatment based on platelet function testing compared with conventional antiplatelet dosing. One-third of patients in the monitoring group had HPR on clopidogrel (PRU ≥235) and received adjusted antiplatelet therapy with either high-dose clopidogrel or prasugrel. Platelet function testing was repeated at 14 days and 30 days after stent implantation, with further adjustments made in therapy. After 1 year of follow-up, there was no difference in the primary composite end point of death, MI, stroke/transient ischemic attack, urgent coronary revascularization, and stent thrombosis between the group who received platelet function monitoring compared with the group who had not received monitoring (34.6% vs 31.1%; P=0.10). There was also no difference in major bleeding (2.3% [monitored group] vs 3.3%; P=0.15). This study showed no benefit in adjusting platelet therapy based on platelet function testing.
While ARCTIC and GRAVITAS evaluated increasing the dosage of clopidogrel based on platelet function testing, the TRIGGER PCI trial sought to determine if prasugrel offered any benefit to patients with stable angina receiving a drug-eluting stent who were identified as poor clopidogrel responders. Poor responders to clopidogrel (PRU >208 with VerifyNow P2Y12) were randomized to either 75 mg clopidogrel or 10 mg prasugrel daily starting the morning after PCI. The trial was stopped for futility after enrollment of only 423 patients because of low 6-month major adverse cardiovascular event rates (0.5% in the clopidogrel arm and 0% in the prasugrel arm).103 In summary, the three largest studies evaluating changing antiplatelet therapy in poor responders based on platelet function testing have failed to show benefit with respect to clinical outcomes.
The TRILOGY-ACS platelet function substudy investigated the relationship between platelet function testing and clinical outcomes in 2,564 patients with ACS who were medically managed without revascularization and randomized in the TRILOGY-ACS trial to receive either prasugrel or clopidogrel in addition to aspirin therapy.104 Platelet function testing was performed at baseline, at 2 hours, and at 1 month, 3 months, 6 months, 12 months, 18 months, 24 months, and 30 months after randomization using the VerifyNow P2Y12 test. Prasugrel provided a greater antiplatelet effect than clopidogrel at all time points, as evidenced by lower PRU. At 30 months, the primary composite efficacy end point of cardiovascular death, MI, or stroke was 17.2% in the prasugrel group and 18.9% in clopidogrel group (P=0.29). There was also no significant correlation between PRU value and presence or absence of primary efficacy event rate. Overall, this trial found that medically managed ACS patients treated with prasugrel have lower platelet reactivity than patients treated with clopidogrel, but this was not associated with a difference in ischemic outcomes. In addition to potentially being underpowered, this study assessed PRU at 2 hours, well before steady-state drug concentrations are achieved, and correlated this PRU with clinical events occurring out to 5 days. Thus, early clinical events were being attributed to a time period during which the drug did not exert its maximal effect.105 In addition to adjusting P2Y12 inhibitor therapy to decrease ischemic events, there is also potential to adjust therapy to decrease bleeding events. In the TRITON-TIMI 38 and PLATO trials, the use of prasugrel or ticagrelor was associated with a higher rate of bleeding than with clopidogrel.22,23 Dosage adjustments based on platelet measurements may be able to prevent bleeding by avoiding excessive platelet inhibition, but has yet to be fully investigated.106 There are several platelet function tests available with different methodologies. In selecting a test that will be useful in clinical practice for P2Y12 inhibitors, it should be simple to perform; have rapid, highly reproducible results; be cost-effective; and provide meaningful prognostic or treatment course information.107 For P2Y12 inhibitor therapy, it would be best to use a platelet function test that directly measures ADP-stimulated activity. There are currently four ADP-stimulated assays (light transmission aggregometry, Multiplate, vasodilator-stimulated phosphoprotein [VASP], and VerifyNow P2Y12) that were shown to predict clinical outcomes in patients after PCI.106–114 Light transmission aggregometry has poor standardization, and VASP has a cumbersome testing process; thus, VerifyNow P2Y12 or Multiplate would seem to be the most preferable tests.106 There is also a discrepancy for the ideal cutoff level in terms of whether a PRU of >208 or >235 is more acceptable to define poor response to antiplatelet therapy.115–117 Additionally, the ideal time to perform platelet function testing after initiation of drug therapy is unknown. A complete discussion of platelet function tests is beyond the scope of this article, but has recently been reviewed elsewhere.118
There are several drawbacks to tailored antiplatelet treatment using platelet function testing, including cost, availability of tests, increased workload, and lack of universal agreement on cutoff values to define poor response to antiplatelet therapy. More importantly, however, trials that have studied alternative regimens for patients with HPR, ie, increasing clopidogrel dosage or changing to another P2Y12 inhibitor, have not convincingly shown benefits of such strategies. As such, there is uncertainty as to how to proceed with a patient who demonstrates HPR while on clopidogrel. Given this uncertainty, current guidelines do not recommend the use of routine platelet function testing as an HPR screening tool for patients on clopidogrel. However, they do allow the clinician leeway in performing such testing in patients considered high risk for poor clinical outcomes, such as patients undergoing high-risk PCI procedures (eg, treatment of extensive and/or very complex disease).3,5,119,120 Should platelet function testing show HPR while these patients are on clopidogrel therapy, some providers would desire a switch to either prasugrel or ticagrelor even in the absence of data demonstrating a convincing clinical benefit with such a strategy. Some would discourage such practice claiming that “[…] no treatment with proven efficacy and safety should be replaced by new treatments, even if theoretically more rational, prior to demonstration of their efficacy, safety and favourable cost–benefit ratio.”121 Others would argue that the likelihood of harm of switching from clopidogrel to another P2Y12 inhibitor in a patient not at high risk for bleeding is low and that given the limitations of current studies, the benefit of such a switch simply has not been realized in a clinical trial setting. Given these considerations, testing for HPR while on clopidogrel and adjusting therapy based on these results is not currently recommended for all patients but is not an unreasonable course of action for high-risk patients.119–121
Genetic testing
Clopidogrel is a prodrug that requires two-step oxidative metabolism by the CYP system to be converted into its active form (Figure 2).122,123 Carriers of CYP2C19 loss-of-function alleles have reduced activity of the enzyme necessary for clopidogrel activation.124,125 The prevalence of poor-metabolizer genotypes varies by race. Reported ranges for poor-metabolizer genotypes are from 20% to 30% in White individuals, from 30% to 45% in African American individuals, and up to 50%–65% in East Asians.126–129 In 2010, the US Food and Drug Administration (FDA) added a boxed warning to the label of clopidogrel, including a reference to patients who do not effectively metabolize the drug, and therefore may not receive its full clinical benefits based on their genetic composition.130 There have been discrepancies in the evidence linking the CYP2C19 loss-of-function allele to an increased risk of cardiovascular events. Early trials reporting a strong association between CYP2C19 loss-of-function alleles and poor cardiovascular outcomes are thought to have overemphasized the effect of loss-of-function CYP mutations and clinical outcomes with clopidogrel due to bias and population diversity.131–134 Two more recent meta-analyses did not indicate substantial influence of the presence of CYP2C19 loss-of-function alleles on major adverse cardiac events in patients taking clopidogrel.135,136 The ABCB1 gene is an additional variant that has been shown to impact clopidogrel efficacy. ABCB1 encodes for P-glycoprotein efflux pumps, which decrease drug absorption. Patients with high expression of ABCB1 have reduced concentrations of active clopidogrel metabolite137 and increased rates of cardiovascular events.138 Prasugrel undergoes rapid intestinal and serum metabolism to an intermediate that is subsequently converted to an active metabolite primarily by not only CYP3A4 and CYP2B6 but also by CYP2C19, CYP2C9, and CYP2D6.139–141 Cuisset et al evaluated the effect of CYP2C19 genetic variants on response to prasugrel and found that carriers of the loss-of-function CYP2C19*2 allele had a higher rate of HPR than noncarriers (16% vs 4%; P=0.01), a factor that increases the risk for major adverse cardiovascular events.142 This is somewhat in contrast to the RESET GENE trial, a crossover study in which 32 patients with HPR on 75 mg clopidogrel received either high-dose clopidogrel (150 mg daily) or prasugrel (10 mg daily) for 2 weeks.143 In this study, there were few CYP2C19*2 noncarriers who exhibited HPR on either therapy (12.5% had HPR on clopidogrel, 0% on prasugrel; P=0.274), but a significant difference was seen in the percentage of CYP2C19*2 carriers exhibiting HPR while on high-dose clopidogrel (43.7%) versus prasugrel (0%; P=0.003).143 A genetic substudy of TRITON-TIMI 38 evaluated if reduced function CYP alleles were associated with adverse cardiovascular outcomes in a cohort of 1,466 subjects allocated to prasugrel.124 This analysis found no significant associations between any of the 54 tested CYP genotype alleles and the composite end point of cardiovascular death, MI, or stroke or any of these individual end points. Another analysis of 1,461 patient taking prasugrel in TRITON-TIMI 38 evaluated the impact of ABCB1 and found no significant association between ABCB1 genotype and clinical outcomes.144 While clopidogrel and prasugrel both require metabolism to be activated, only about 15% of a given clopidogrel dose is available for activation since the majority of clopidogrel is converted to an inactive metabolite (Figure 2). This is in contrast to prasugrel, which does not have a known inactive metabolite, leaving the majority of a given dose available for metabolic activation.140,145 Since clopidogrel has less substrate for enzymatic activation, it may be more reliant on such activation for its pharmacologic effect, making it potentially more sensitive to CYP2C19 and ABCB1 mutations than prasugrel.
Alexopoulos et al compared the relative antiplatelet effects of high-dose clopidogrel and prasugrel in a randomized, crossover trial enrolling 71 post-PCI patients with HPR (PRU ≥235 with VerifyNow P2Y12).146 Patients received a clopidogrel loading dose prior to PCI, and platelet reactivity was measured 24 hours after the procedure to determine HPR. Patients were then randomized to 150 mg/day of clopidogrel or prasugrel with platelet function testing performed 30 days later, at which time patients were crossed over to receive the alternative regimen. After 30 days, platelet reactivity was significantly lower in patients treated with prasugrel than those with clopidogrel (129.4 PRU vs 201.7 PRU; P<0.001). Of note, the difference in magnitude of platelet function suppression between prasugrel and clopidogrel was greater in patients with >1 loss-of-function CYP2C19 allele (122.9 mean PRU difference) than those without any loss-of-function alleles (47.5 mean PRU difference). The rates of HPR were also lower with prasugrel versus clopidogrel in both carriers and noncarriers of a CYP2C19 loss-of-function allele. This study demonstrated prasugrel to be a more viable option than high-dose clopidogrel for PCI patients with HPR while on standard clopidogrel therapy.
Ticagrelor does not require hepatic metabolism for activation. The main metabolite of ticagrelor is also active and makes up 30%–40% of the plasma concentration of ticagrelor.147 An analysis of 174 patients enrolled in the ONSET/OFFSET and RESPOND studies who underwent genetic testing showed lower platelet reactivity with ticagrelor compared to clopidogrel regardless of CYP2C19 genotype.148 A genetic analysis of 10,285 patients from the PLATO trial found that patients with high expression of ABCB1 or a CYP2C19 loss-of-function allele had a nonsignificant trend toward better outcomes with ticagrelor.149 For patients with high expression of ABCB1, event rates for the composite outcome of cardiovascular death, MI, or stroke were 8.8% with ticagrelor vs 11.9% with clopidogrel (P=0.01). In patients with a CYP2C19 loss-of-function allele, the event rate with ticagrelor was 8.6% versus 11.2% with clopidogrel (P=0.038). In the clopidogrel group, the event rate at 30 days was higher in patients with a loss-of-function CYP2C19 allele compared to those without a loss-of-function allele (5.7% vs 3.8%, P=0.028). Not unexpectedly, the event rate in patients receiving ticagrelor was the same in those with and without CYP2C19 loss-of-function alleles.149
Given the potential increased risk of events in patients who are poor clopidogrel metabolizers, there have been investigations into solutions to overcome or circumvent this pathway. Several studies have demonstrated that increasing the clopidogrel dosage does not completely overcome the variability in platelet inhibition.146,150–152 The utility of prasugrel in CYP2C19*2 carriers was assessed by the RAPID GENE trial, which randomized patients undergoing PCI for ACS or stable angina to rapid point-of-care genotyping (n=91) or standard treatment (n=96).153 Patients in the rapid genotyping group were screened for the CYP2C19*2 allele. If present, 10 mg prasugrel daily was given and if absent, then 75 mg/day of clopidogrel was given, which was also the treatment given to patients in the standard treatment group. The primary end point was the proportion of CYP2C19*2 carriers with HPR (VerifyNow P2Y12 PRU value >234) after 1 week. The CYP2C19*2 allele was present in 23 individuals in each group (genotyping and standard care). None of the CYP2C19*2 carriers receiving prasugrel in the genotyping group had HPR after 1 week of treatment compared to seven (30%) of the CYP2C19*2 carriers allocated to standard clopidogrel treatment (P=0.009).153
The RAPID GENE trial was small, and while it indicated that genotyping can identify many patients with poor response to clopidogrel, there are genetic and environmental factors that also affect platelet inhibition.154–156 It is estimated that the CYP2C19 allele explains only about 10% of the variation in platelet response.157–159 Therefore, it is unlikely that genetic testing alone would give an adequate picture of a patient’s likeliness to have sufficient platelet inhibition with clopidogrel or guide a therapeutic strategy. Previously, genetic testing was limited by long turnaround time for results. However, now two point-of-care CYP2C19 tests, the Spartan RX (Spartan Bioscience Inc., Ottawa, ON, Canada) and Verigene (Nanosphere, Inc., Northbrook, IL, USA), identify loss-of-function CYP2C19*2 and *3 alleles. The differences between the two systems are that Verigene uses whole blood and can genotype several CYP2C19 variants, whereas Spartan uses a buccal swab and can detect only the *2, *3, and *17 variants. The time to get results is also shorter for the Spartan system (1 hour vs 3 hours).107 Despite the technical improvements over the years, genetic testing is still expensive and often not covered by insurance companies, which limits its use. However, the main factor limiting the use of genetic testing for antiplatelet therapy is conclusive evidence of efficacy and safety of tailoring regimens based on genetic information. Owing to this lack of outcome data, current clinical practice guidelines for genetic testing mirror those for platelet function testing: it is not recommended for routing screening, but a clinician may opt to perform testing for CYP2C19 loss-of-function alleles in patients at high risk for poor clinical outcomes with the caveat that it is relatively unknown how to proceed with that information.3,119
Cost
Cost is a major factor that compounds the decision of which P2Y12 inhibitor to use. While clopidogrel is available generically and is less expensive than prasugrel or ticagrelor, these newer agents have been shown to be more effective than clopidogrel at reducing the risk of cardiovascular events in most subsets of patients. Thus, the cost of the medication is not the only consideration, as the costs of recurrent event rates must also be brought into the equation.
Crespin et al performed a cost-effectiveness analysis to estimate the 5-year medical costs and outcomes for a cohort of 100,000 ACS patients enrolled in Medicare receiving either: 1) genotype-driven or 2) ticagrelor-only treatment.160 With genotype-driven therapy, patients received clopidogrel unless they had a CYP2C19*2 mutation, in which event they received ticagrelor. Data comparing the clinical performance of ticagrelor and clopidogrel were derived from PLATO for the first 12 months of therapy. After 12 months, event rates were assumed to be equal for ticagrelor and clopidogrel treatment. Both bleeding risk and cardiovascular event rates were included. Outcomes were life years and quality-adjusted life years (QALYs) gained. Costs assumed in this study were $200 for genotyping and $30 and $164 for a 1-month supply of clopidogrel and ticagrelor, respectively. Results yielded a favorable result for universal ticagrelor. After 5 years of therapy, the incremental cost-effectiveness ratio (ICER) for universal ticagrelor was $10,059 per QALY versus genotype-driven treatment. The conclusion from this analysis was that prescribing ticagrelor universally increases QALYs for ACS patients at a cost below the typically accepted threshold for a cost-effective treatment (<$50,000 per QALY). This model had several limitations. The first was that the event rate in patients stratified to clopidogrel in the genotype-directed therapy was based on a modeled estimate and not actual rates observed in a clinical trial. An additional limitation of the trial is the cost calculation for ticagrelor of $164/month, which is only half of the current average wholesale price in the United States.
A similar analysis by Reese et al used a simulated cohort of patients with ACS undergoing PCI and evaluated results of patients receiving either: 1) genotype-guided therapy, 2) clopidogrel-only therapy, or 3) prasugrel-only therapy.161 In the genotype-guided strategy, patients with at least one CYP2C19 loss-of-function allele received prasugrel and patients with two functional CYP2C19 alleles received clopidogrel. The 15-month analysis examined the end points of a cardiovascular event, a bleeding event, or no event. This analysis based event probabilities on the TRITON-TIMI 38 trial, which included a genetic substudy. Drug cost estimates in this analysis for a 1-month supply of generic clopidogrel and prasugrel were $30 and $186, respectively. This study found that genotype-driven therapy was less costly compared to prasugrel for all patients (ICER: −$27,160) but was not less costly compared with clopidogrel for all patients (ICER: $2,300). An advantage of this study over Crespin et al is that it used genetically determined data. A limitation of this model was that the prasugrel price used in the study was half of the current average wholesale price of prasugrel in the United States.
The above trials suggest that ticagrelor is more cost-effective than genotype-driven therapy, but clopidogrel was more cost-effective than genotype-driven therapy or prasugrel therapy. The conclusions of these analyses, however, are dependent on a number of assumptions that are used in building these models. Also, the cost-effectiveness of these agents depends not only on their rates of effectiveness but also on their direct cost. Both ticagrelor and prasugrel will not be available generically for several years, while there are multiple generic manufacturers of clopidogrel. This will likely cause increased divergence in cost over time, which will need to be considered in selecting an agent.
The desire to use the most effective P2Y12 inhibitor while keeping drug costs at a minimum has led many clinicians to consider beginning a patient on either ticagrelor or prasugrel and then switching over to clopidogrel at a later time. The use of a more potent platelet inhibitor, such as prasugrel or ticagrelor, in the early stages of an ACS may provide more benefit at a time when countering enhanced platelet activation and aggregation is most important. Once this acute phase has passed, switching to a less powerful but more affordable agent (clopidogrel) would perhaps come without loss of clinical efficacy outside of the acute phase and lower the risk of bleeding over the long-term. However, Kerneis et al demonstrated that switching from prasugrel to clopidogrel after 15 days increased on-treatment platelet reactivity in 300 ACS patients.162 This trial was not designed to assess clinical outcomes, and as such, no conclusions can be drawn in this regard. Similarly, the POBA SWITCH study involving 20 patients with ACS and very low platelet reactivity while on prasugrel (measured by VASP) demonstrated an increase in platelet reactivity when switched to clopidogrel after 1 month.163 However, after the switch, most patients still maintained a level of platelet inhibition that may be considered acceptable (ie, VASP platelet reactivity index <50%). The ongoing SWAP-4 trial (ClinicalTrials.gov Identifier NCT02287909) is investigating the switch from ticagrelor to clopidogrel. Unfortunately, this is also a pharmacodynamic rather than a clinical trial. Larger, outcome-driven trials are needed before the practice of switching P2Y12 inhibitors can be recommended.
Conclusion
Over the last 2 decades, there has been considerable evolution in antiplatelet therapies for ACS. In addition to aspirin, oral P2Y12 inhibitors have proven efficacious in reducing recurrent cardiovascular events. Initially, P2Y12 inhibition was primarily achieved with clopidogrel followed by the development, study, and use of the more potent P2Y12 inhibitors, prasugrel and ticagrelor. While prasugrel and ticagrelor are more efficacious compared to clopidogrel, this comes at the risk of increased bleeding, which can be of significant clinical consequence. Many factors need to be weighed when choosing an optimal DAPT regimen taking into account patient-specific characteristics, comorbidities, concomitant medication use, and cost. More individualized assessment of platelet reactivity and pharmacogenetics offers promise in guiding drug selection, but incorporating this information into clinical practice has been elusive to date. Intravenous cangrelor offers the advantage of a quick onset and offset of drug effect, but its role in ACS treatment is currently rather limited. As our understanding of ACS continues to evolve, there remains much to learn with respect to optimizing the use of these powerful drugs to most effectively help achieve the best clinical outcomes.
Disclosure
The authors have no conflicts of interest to disclose.
References
Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8(10):1175–1180. | |
Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276(6):618–632. | |
Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):2354–2394. | |
O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–e425. | |
Hamm CW, Bassand JP, Agewall S, et al; for the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2011;32(23):2999–3054. | |
Steg PG, James SK, Atar D, et al; for the Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569–2619. | |
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2(8607):349–360. | |
Yusuf S, Zhao F, Mehta SR, et al; for the Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502. | |
The CURRENT-OASIS 7 Investigators. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010; 363(10):930–942. | |
Sabatine MS, Cannon CP, Gibson CM, et al; for the CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352(12):1179–1189. | |
Chen ZM, Jiang LX, Chen YP, et al; for the COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: randomized placebo-controlled trial. Lancet. 2005; 366(9497):1607–1621. | |
Mehta SR, Tanguay JF, Eikelboom JW, et al; on behalf of the CURRENT-OASIS 7 Investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233–1243. | |
Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease. The ONSET/OFFSET Study. Circulation. 2009;120(25):2577–2585. | |
Brilinta® (ticagrelor) tablets, for oral use [prescribing information]. Wilmington, DE: AstraZeneca LP; 2015. | |
Effient® (prasugrel) tablets, for oral use [prescribing information]. Indianapolis, IN: Eli Lilly and Company; 2013. | |
Plavix® (clopidogrel bisulfate) tablets [prescribing information]. Bridgewater, NJ: Bristol-Myers Squibb Sanofi Pharmaceuticals Partnership; 2013. | |
Akers WS, Oh JJ, Oestreich JH, Ferraris S, Wethington M, Steinhubl SR. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol. 2010;50(1):27–35. | |
Greenbaum AB, Grines CL, Bittl JA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J. 2006;151(3):689.e1–689.e10. | |
Parodi G, Valenti R, Bellandi B, et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients. RAPID (Rapid Activity of Platelet Inhibitor Drugs) Primary PCI Study. J Am Coll Cardiol. 2013;61(15):1601–1606. | |
Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50(19):1852–1856. | |
Wiviott SD, Trenk D, Frelinger AL, et al; for the PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading- and maintenance dose clopidogrel in patients with planned percutaneous coronary intervention: the prasugrel in comparison to clopidogrel for inhibition of platelet activation and aggregation-thrombolysis in myocardial infarction 44 trial. Circulation. 2007;116(25):2923–2932. | |
Wiviott SD, Braunwald E, McCabe CH, et al; for the TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. | |
Wallentin L, Becker RC, Budaj A, et al; for the PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. | |
Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361(124):2318–2329. | |
Bhatt DL, Lincoff AM, Gibson CM, et al; CHAMPION PLATFORM Investigators. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361(124):2330–2341. | |
Thygesen K, Alpert JS, White HD; on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007; 50(22):2173–2195. | |
White HD, Chew DP, Dauerman HL, et al. Reduced immediate ischemic events with cangrelor in PCI: a pooled analysis of the CHAMPION trials using the universal definition of myocardial infarction. Am Heart J. 2012;163(2):182.e–190.e. | |
Bhatt DL, Stone GW, Mahaffey KW, et al; for the CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303–1313. | |
Lange RA, Hillis LD. The duel between dual antiplatelet therapies. N Engl J Med. 2013;368(14):1356–1357. | |
Kengreal™ (cangrelor) for injection, for intravenous use [prescribing information]. Parsippany, NJ: The Medicines Company; 2015. | |
Angiolillo DJ, Firstenberg MS, Price MJ, et al; for the BRIDGE Investigators. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery. A randomized controlled trial. JAMA. 2012;307(3):265–274. | |
Schömig A, Neumann FJ, Walter H, et al. Coronary stent placement in patients with acute myocardial infarction: comparison of clinical and angiographic outcome after randomization to antiplatelet or anticoagulant therapy. J Am Coll Cardiol. 1997;29(1):28–34. | |
Leon MB, Baim DS, Popma JJ, et al; for the Stent Anticoagulation Restenosis Study Investigators. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. N Engl J Med. 1998;339(23):1665–1671. | |
Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; for the CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting. The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation. 2000;102(6):624–629. | |
Montalescot G, Wiviott SD, Braunwald E, et al; for the TRITON-TIMI 38 Investigators. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373(9665):723–731. | |
Steg PG, James S, Harrington RA, et al; for the PLATO Study Group. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention. A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122(21):2131–2141. | |
DiNicolantonio JJ, Serebruany VL. Exploring the reduction in myocardial infarctions in the PLATO trial: which patients benefited on ticagrelor vs clopidogrel? Int J Cardiol. 2013;165(3):396–397. | |
Mehta SR, Yusuf S, Peters RJ, et al; for the Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527–533. | |
Cannon CP, Harrington RA, James S, et al; for the PLATelet inhibition and patient Outcomes (PLATO) investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375(9711):283–293. | |
Lindholm D, Varenhorst C, Cannon CP, et al. Ticagrelor vs clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: results from the PLATO trial. Eur Heart J. 2014;35(31):2083–2093. | |
National Institute for Health and Care Excellence (NICE). Ticagrelor for the Treatment of Acute Coronary Syndromes. NICE Technology Appraisal Guidance 236; 2011. Available from: https://www.nice.org.uk/guidance/ta236/resources/guidance-ticagrelor-for-the-treatment-of-acute-coronary-syndromes-pdf. Accessed May 19, 2015. | |
James SK, Roe MT, Cannon CP, et al; PLATO Study Group. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ. 2011;342:d3527. | |
Roe MT, Armstrong PW, Fox KA, et al; for the TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367(14):1297–1309. | |
Angiolillo DJ, Jakubowski JA, Ferreiro JL, et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol. 2014;64(10):1005–1014. | |
Patti G, Proscia C, Di Sciascio G. Antiplatelet therapy in patients with diabetes mellitus and acute coronary syndrome. New insights from randomized trials. Circ J. 2014;78(1):33–41. | |
Steinhubl SR, Berger PB, Mann JT 3rd, et al; CREDO Investigators. Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention. A randomized controlled trial. N Engl J Med. 2002;288(19):2411–2420. | |
Wiviott SD, Braunwald E, Angiolillo DJ, et al; for the TRITON-TIMI 38 Investigators. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38. Circulation. 2008;118(16):1626–1636. | |
James S, Angiolillo DJ, Cornel JH, et al; for the PLATO study group. Ticagrelor vs clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient outcomes (PLATO) trial. Eur Heart J. 2010;31(24):3006–3016. | |
Bonaca MP, Bhatt DL, Cohen M, et al; for the PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–1800. | |
Tantcheva-Poór I, Zaigler M, Rietbrock S, Fuhr U. Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharmacogenetics. 1999;9(2):131–144. | |
Bliden KP, Dichiara J, Lawal L, et al. The association of cigarette smoking with enhanced platelet inhibition by clopidogrel. J Am Coll Cardiol. 2008;52(7):531–533. | |
Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109(25):3171–3175. | |
Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64:1917–1921. | |
Rollini F, Franchi F, Cho JR, et al. Cigarette smoking and antiplatelet effects of aspirin monotherapy versus clopidogrel monotherapy in patients with atherosclerotic disease: results of a prospective pharmacodynamic study. J Cardiovasc Transl Res. 2014;7(1):53–63. | |
Gurbel PA, Bliden KP, Logan DK, et al. The Influence of smoking status on the pharmacokinetics and pharmacodynamics of clopidogrel and prasugrel. The PARADOX Study. J Am Coll Cardiol. 2013;62(6):505–512. | |
Hochholzer W, Trenk D, Mega JL, et al. Impact of smoking on antiplatelet effect of clopidogrel and prasugrel after loading dose and on maintenance therapy. Am Heart J. 2011;162(3):518.e–526.e. | |
Desai NR, Mega JL, Jiang S, Cannon CP, Sabatine MS. Interaction between cigarette smoking and clinical benefit of clopidogrel. J Am Coll Cardiol. 2009;53(15):1273–1278. | |
Berger JS, Bhatt DL, Steinhubl SR, et al. Smoking, clopidogrel, and mortality in patients with established cardiovascular disease. Circulation. 2009;120(23):2337–2344. | |
Sibbald M, Yan AT, Huang W, et al. Association between smoking, outcomes, and early clopidogrel use in patients with acute coronary syndrome: insights from the global registry of acute coronary events. Am Heart J. 2010;160(5):855–861. | |
Cornel JH, Ohman EM, Neely B; TRILOGY ACS Investigators. Impact of smoking status on platelet function and clinical outcomes with prasugrel vs clopidogrel in patients with acute coronary syndromes managed without revascularization: insights from the TRILOGY ACS trial. Am Heart J. 2014;168(1):76.e–87.e. | |
Cornel JH, Becker RC, Goodman SG, et al. Prior smoking status, clinical outcomes, and the comparison of ticagrelor with clopidogrel in acute coronary syndromes – insights from the PLATelet inhibition and patient outcomes (PLATO) trial. Am Heart J. 2012;164(3):334.e–342.e. | |
Bellemain-Appaix A, O’Connor SA, Silvain J, et al; for the ACTION group. Association of clopidogrel pretreatment with mortality, cardiovascular events, and major bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2012;308(23):2507–2517. | |
Montalescot G, Bolognese L, Dudek D, et al; for the ACCOAST Investigators. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med. 2013;369(11):999–1010. | |
Montalescot G, van’t Hof AW, Lapostolle F, et al; for the ATLANTIC Investigators. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371(11):1016–1027. | |
Wiviott SD, Steg PG. Clinical evidence for oral antiplatelet therapy in acute coronary syndromes. Lancet. 2015;386(9990):292–302. | |
Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301(9):937–944. | |
Pezalla E, Day D, Pulliadath I. Initial assessment of clinical impact of a drug interaction between clopidogrel and proton pump inhibitors. J Am Coll Cardiol. 2008;52(12):1038–1039. | |
Evanchan J, Donnally MR, Binkley P, Mazzaferri E. Recurrence of acute myocardial infarction in patients discharged on clopidogrel and a proton pump inhibitor after stent placement for acute myocardial infarction. Clin Cardiol. 2010;33(3):168–171. | |
Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180(7):713–718. | |
Gaglia MA Jr, Torguson R, Hanna N, et al. Relation of proton pump inhibitor use after percutaneous coronary intervention with drug-eluting stents to outcomes. Am J Cardiol. 2010;105(6):833–838. | |
Gupta E, Bansal D, Sotos J, Olden K. Risk of adverse clinical outcomes with concomitant use of clopidogrel and proton pump inhibitors following percutaneous coronary intervention. Dig Dis Sci. 2010;55(7):1964–1968. | |
Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton pump inhibitors – a cohort study. Ann Intern Med. 2010;152(6):337–345. | |
O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374(9694):989–997. | |
Zairis MN, Tsiaousis GZ, Patsourakos NG, et al. The impact of treatment with omeprazole on the effectiveness of clopidogrel drug therapy during the first year after successful coronary stenting. Can J Cardiol. 2010;26(2):e54–e57. | |
Bhatt DL, Cryer BL, Contant CF, et al; for the COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363(20):1909–1917. | |
FDA. FDA Reminder to Avoid Concomitant Use of Plavix (Clopidogrel) and Omeprazole. Maryland: US Food and Drug Administration; 2010. | |
FDA. Interaction between Esomeprazole/Omeprazole and Clopidogrel Label Change. Maryland: US Food and Drug Administration; 2015. | |
Lundell L, Miettinen P, Myrvold HE, et al; Nordic GERD Study Group. Comparison of outcomes twelve years after antireflux surgery or omeprazole maintenance therapy for reflux esophagitis. Clin Gastroenterol Hepatol. 2009;7(12):1292–1298. | |
Goodman SG, Clare R, Pieper KS, et al; Platelet Inhibition and Patient Outcomes Trial Investigators. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125(8):978–986. | |
Charlot M, Ahlehoff O, Norgaard ML, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med. 2010;153(6):378–386. | |
Juurlink DN, Dormuth CR, Huang A, et al. Proton pump inhibitors and the risk of adverse cardiac events. PLoS One. 2013;8(12):e84890. | |
Shih CJ, Chen YT, Ou SM, Li SY, Chen TJ, Wang SJ. Proton pump inhibitor use represents an independent risk factor for myocardial infarction. Int J Cardiol. 2014;177(1):292–297. | |
Mahaffey KW, Wojdyla DM, Carroll K, et al; on behalf of the PLATO Investigators. Ticagrelor compared with clopidogrel by geographic region in the platelet inhibition and patient outcomes (PLATO) trial. Circulation. 2011;124(5):544–554. | |
Thomas MR, Storey RF. Impact of aspirin dosing on the effects of P2Y12 inhibition in patients with acute coronary syndromes. J Cardiovasc Transl Res. 2014;7(1):19–28. | |
Grzesk G, Kozinski M, Tantry US, et al. High-dose, but not low-dose, aspirin impairs anticontractile effect of ticagrelor following ADP stimulation in rat tail artery smooth muscle cells. Biomed Res Int. 2013;2013:928271. | |
Lamberts M, Gislason GH, Olesen JB, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol. 2013;62(11):981–989. | |
Sørensen R, Hansen ML, Abildstrom SZ, et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet. 2009; 374(9706):1967–1974. | |
Dewilde WJM, Oirbans T, Verheugt FWA, for the WOEST study investigators, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomized, controlled trial. Lancet. 2013;381(9872):1107–1115. | |
January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–e267. | |
You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy in atrial fibrillation. Chest. 2012;141(2 Suppl):e531S–e575S. | |
Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107(23):2908–2913. | |
Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: a review of the evidence. J Am Coll Cardiol. 2005;45(8):1157–1164. | |
Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45(2):246–251. | |
Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007;154(2):221–231. | |
Schwarz UR, Geiger J, Walter U, Eigenthaler M. Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets – definition and detection of ticlopidine/clopidogrel effects. Thromb Haemost. 1999;82(3):1145–1152. | |
Sofi F, Marcucci R, Gori AM, Giusti B, Abbate R, Gensini GF. Clopidogrel non-responsiveness and risk of cardiovascular morbidity. An updated metaanalysis. Thromb Haemost. 2010;103(4):841–848. | |
Aradi D, Komócsi A, Vorobcsuk A, et al. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: systematic review and meta-analysis. Am Heart J. 2010; 160(3):543–551. | |
Brar SS, ten Berg J, Marcucci R, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol. 2011;58(19):1945–1954. | |
Yamaguchi Y, Abe T, Sato Y, Matsubara Y, Moriki T, Murata M. Effects of VerifyNow P2Y12 test and CYP2C19*2 testing on clinical outcomes of patients with cardiovascular disease: a systematic review and meta-analysis. Platelets. 2013;24(5):352–361. | |
Price MJ, Berger PB, Teirstein PS, et al; GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305(11):1097–1105. | |
Price MJ, Angiolillo DJ, Teirstein PS, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the gauging responsiveness with a VerifyNow P2Y12 assay: impact on thrombosis and safety (GRAVITAS) trial. Circulation. 2011;124(10):1132–1137. | |
Collet JP, Cuisset T, Rangé G, et al; ARCTIC Investigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367(22):2100–2109. | |
Trenk D, Stone GW, Gawaz M. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (testing platelet reactivity in patients undergoing elective stent placement on clopidogrel to guide alternative therapy with prasugrel) study. J Am Coll Cardiol. 2012;59(24):2159–2164. | |
Gurbel PA, Erlinge D, Ohman EM; TRILOGY ACS Platelet Function Substudy Investigators. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA. 2012;308(17):1785–1794. | |
Price MJ. Measured drug effect and cardiovascular outcomes in patients receiving platelet P2Y12 receptor antagonists. Clarifying the time and place for intensive inhibition. N Engl J Med. 2012;308(17):1806–1808. | |
Aradi D, Storey RF, Komócsi A, et al; Working Group on Thrombosis of the European Society of Cardiology. Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur Heart J. 2014;35(4):209–215. | |
Siller-Matula JM, Trenk D, Schrör K, et al; EPA (European Platelet Academy). EPA (European Platelet Academy). Response variability to P2Y12 receptor inhibitors: expectations and reality. JACC Cardiovasc Interv. 2013;6(11):1111–1128. | |
Bonello L, Tantry US, Marcucci R, et al; Working Group on High On-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56(12):919–933. | |
Bonello L, Paganelli F, Arpin-Bornet M, et al. Vasodilator-stimulated phosphoprotein phosphorylation analysis prior to percutaneous coronary intervention for exclusion of postprocedural major adverse cardiovascular events. J Thromb Haemost. 2007;5(8):1630–1636. | |
Blindt R, Stellbrink K, de Taeye A, et al. The significance of vasodilator-stimulated phosphoprotein for risk stratification of stent thrombosis. Thromb Haemost. 2007;98(6):1329–1334. | |
Freynhofer MK, Brozovic I, Bruno V, et al. Multiple electrode aggregometry and vasodilator stimulated phosphoprotein- phosphorylation assay in clinical routine for prediction of postprocedural major adverse cardiovascular events. Thromb Haemost. 2011;106(2):230–239. | |
Sibbing D, Braun S, Morath T, et al. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol. 2009;53(10):849–856. | |
Stone GW, Witzenbichler B, Weisz G, et al; ADAPT-DES Investigators. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382(9892):614–623. | |
Breet NJ, van Werkum JW, Bouman HJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303(8):754–762. | |
Campo G, Fileti L, de Cesare N, et al. Long-term clinical outcome based on aspirin and clopidogrel responsiveness status after elective percutaneous coronary intervention: a 3T/2R (tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel) trial substudy. J Am Coll Cardiol. 2010;56(18):1447–1455. | |
Campo G, Parrinello G, Ferraresi P, et al. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol. 2011;57(25):2474–2483. | |
Brar SS, ten Berg J, Marcucci R, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention a collaborative meta-analysis of individual participant data. J Am Coll Cardiol. 2011;58(19):1945–1954. | |
Chan NC, Eikelboom JW, Ginsberg JS, et al. Role of phenotypic and genetic testing in managing clopidogrel therapy. Blood. 2014;124(5):689–699. | |
Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011; 124(23):e574–e651. | |
Holmes DR Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “Boxed Warning.” A report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association. Circulation. 2010;122(5):537–557. | |
Cattaneo M. Response variability to clopidogrel: is tailored treatment, based on laboratory testing, the right solution? J Thromb Haemost. 2012;10(3):327–336. | |
Savi P, Nurden P, Nurden AT, Levy-Toledano S, Herbert JM. Clopidogrel: a review of its mechanism of action. Platelets. 1998; 9(3–4):251–255. | |
Sharma RK, Reddy HK, Singh VN, Sharma R, Voelker DJ, Bhatt G. Aspirin and clopidogrel hyporesponsiveness and non-responsiveness in patients with coronary artery stents. Vasc Health Risk Manag. 2009;5:965–972. | |
Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119(19):2553–2560. | |
Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354–362. | |
Wallentin L. P2Y12 inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur Heart J. 2009;30(16):1964–1977. | |
Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41(12):913–958. | |
Dandara C, Masimirembwa CM, Magimba A, et al. Genetic polymorphism of CYP2D6 and CYP2C19 in east- and southern African populations including psychiatric patients. Eur J Clin Pharmacol. 2001;57(1):11–17. | |
Myrand SP, Sekiguchi K, Man MZ, et al. Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther. 2008;84(3):347–361. | |
FDA. FDA Drug Safety Communication: Reduced Effectiveness of Plavix (Clopidogrel) in Patients Who are Poor Metabolizers of the Drug. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm. Accessed May 19, 2015. | |
Hulot JS, Collet JP, Silvain J, et al. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J Am Coll Cardiol. 2010;56(2):134–143. | |
Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001; 29(3):306–309. | |
Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821–1830. | |
Sofi F, Giusti B, Marcucci R, Gori AM, Abbate R, Gensini GF. Cytochrome P450 2C19*2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: a meta-analysis. Pharmacogenomics J. 2011;11(3):199–206. | |
Bauer T, Bouman HJ, van Werkum JW, Ford NF, ten Berg JM, Taubert D. Impact of CYP2C19 variant genotypes on clinical efficacy of antiplatelet treatment with clopidogrel: systematic review and meta-analysis. BMJ. 2011;343:d4588. | |
Zabalza M, Subirana I, Sala J, et al. Meta-analyses of the association between cytochrome CYP2C19 loss- and gain-of-function polymorphisms and cardiovascular outcomes in patients with coronary artery disease treated with clopidogrel. Heart. 2012;98(2):100–108. | |
Taubert D, von Beckerath N, Grimberg G, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80(5):486–501. | |
Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360(4):363–375. | |
Farid NA, Smith RL, Gillespie TA, et al. The disposition of prasugrel, a novel thienopyridine, in humans. Drug Metab Dispos. 2007;35(5):1096–1104. | |
Rehmel JL, Eckstein JA, Farid NA, et al. Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug Metab Dispos. 2006;34(4):600–607. | |
Riley AB, Tafreshi MJ, Haber SL. Prasugrel: a novel antiplatelet agent. Am J Health Syst Pharm. 2008;65(11):1019–1028. | |
Cuisset T, Loosveld M, Morange PE, et al. CYP2C19*2 and *17 alleles have a significant impact on platelet response and bleeding risk in patients treated with prasugrel after acute coronary syndrome. JACC Cardiovasc Interv. 2012;5(12):1280–1287. | |
Sardella G, Calcagno S, Mancone M, et al. Pharmacodynamic effect of switching therapy in patients with high on-treatment platelet reactivity and genotype variation with high clopidogrel dose versus prasugrel. Circ Cardiovasc Interv. 2012;5(5):698–704. | |
Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376(9749):1312–1319. | |
Kurihara A, Hagihara K, Kazui M, Ishizuka T, Farid NA, Ikeda T. In vitro metabolism of antiplatelet agent clopidogrel: cytochromeP450 isoforms responsible for two oxidation steps involved in the active metabolite formation. Drug Metab Rev. 2005;37(Suppl 2):99. [abstract]. | |
Alexopoulos D, Dimitropoulos G, Davlouros P, et al. Prasugrel overcomes high on-clopidogrel platelet reactivity post-stenting more effectively than high-dose (150-mg) clopidogrel. The importance of CYP2C19*2 genotyping. JACC Cardiovasc Interv. 2011;4(4):403–410. | |
de Labriolle A, Doazan JP, Lemesle G, Bonello L. Genotypic and phenotypic assessment of platelet function and response to P2Y12 antagonists. Curr Cardiol Rep. 2011;13(5):439–450. | |
Tantry US, Bliden KP, Wei C, et al. First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: the ONSET/OFFSET and RESPOND genotype studies. Circ Cardiovasc Genet. 2010;3(6):556–566. | |
Wallentin L, James S, Storey RF, et al; PLATO Investigators. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010; 376(9749):1320–1328. | |
Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading- and maintenance dose clopidogrel in patients with planned percutaneous coronary intervention: the prasugrel in comparison to clopidogrel for inhibition of platelet activation and aggregation- thrombolysis in myocardial infarction 44 trial. Circulation. 2007;116(25):2923–2932. | |
Lhermusier T, Lipinski MJ, Tantry US, et al. Meta-analysis of direct and indirect comparison of ticagrelor and prasugrel effects on platelet reactivity. Am J Cardiol. 2015;115(6):716–723. | |
Aradi D, Tornyos A, Pintér T, et al. Optimizing P2Y12 receptor inhibition in patients with acute coronary syndrome on the basis of platelet function testing. Impact of prasugrel and high-dose clopidogrel. J Am Coll Cardiol. 2014;63(11):1061–1070. | |
Roberts JD, Wells GA, Le May MR, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet. 2012;379(9827):1705–1711. | |
Hulot JS, Fuster V. Antiplatelet therapy: personalized medicine for clopidogrel resistance? Nat Rev Cardiol. 2009;6(5):334–336. | |
Gurbel PA, Tantry US. Do platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents?: platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents. Circulation. 2012;125(10):1276–1287. | |
Liang ZY, Han YL, Zhang XL, Li Y, Yan CH, Kang J. The impact of gene polymorphisms and high on treatment platelet reactivity on clinical follow up: outcomes in patients with acute coronary syndrome after drug eluting stent implantation. EuroIntervention. 2013;9(3):316–327. | |
Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–858. | |
Hochholzer W, Trenk D, Fromm MF, et al. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. 2010;55(22):2427–2434. | |
Bouman HJ, Harmsze AM, van Werkum JW, et al. Variability in on-treatment platelet reactivity explained by CYP2C19*2 genotype is modest in clopidogrel pretreated patients undergoing coronary stenting. Heart. 2011;97(15):1239–1244. | |
Crespin DJ, Federspiel JJ, Biddle AK, Jonas DE, Rossi JS. Ticagrelor versus genotype-driven antiplatelet therapy for secondary prevention after acute coronary syndrome: a cost-effectiveness analysis. Value Health. 2011;14(4):483–491. | |
Reese ES, Daniel Mullins C, Beitelshees AL, Onukwugha E. Cost-effectiveness of cytochrome P450 2C19 genotype screening for selection of antiplatelet therapy with clopidogrel or prasugrel. Pharmacotherapy. 2012;32(4):323–332. | |
Kerneis M, Silvain J, Abtan J, et al. Switching acute coronary syndrome patients from prasugrel to clopidogrel. J Am Coll Cardiol Intv. 2013;6(2):158–165. | |
Deharo P, Pons C, Pankert M, et al. Effectiveness of switching ‘hyper responders’ from Prasugrel to Clopidogrel after acute coronary syndrome: The POBA (Predictor of Bleeding with Antiplatelet drugs) SWITCH study. Int J Cardiol. 2013;168(5):5004–5005. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.