Back to Journals » Cancer Management and Research » Volume 10
Overview of advances in vasculogenic mimicry – a potential target for tumor therapy
Received 5 February 2018
Accepted for publication 30 March 2018
Published 2 August 2018 Volume 2018:10 Pages 2429—2437
DOI https://doi.org/10.2147/CMAR.S164675
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Hong Ge,1 Hui Luo1,2
1Department of Radiation Oncology, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 2Division of Graduate, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Abstract: Vasculogenic mimicry (VM) describes the process utilized by highly aggressive cancer cells to generate vascular-like structures without the presence of endothelial cells. VM has been vividly described in various tumors and participates in cancer progression dissemination and metastasis. Diverse molecular mechanisms and signaling pathways are involved in VM formation. Furthermore, the patterning characteristics of VM, detected with molecular imaging, are being investigated for use as a tool to aid clinical practice. This review explores the most recent studies investigating the role of VM in tumor induction. Indeed, the recognition of these advances will increasingly affect the development of novel therapeutic target strategies for VM in human cancer.
Keywords: vasculogenic mimicry, mechanisms, molecular imaging, clinical significance, target therapy
Introduction
Blood supply contributes to cancer progression, recurrence, and metastasis. With vascular channel structures, nutrients such as glucose could be supplied to tumor tissues; meanwhile, metabolic wastes are evacuated. Previous research has shown that angiogenesis is not an exclusive method to nourish tumor tissues. It has been suggested that cancer stem cells transdifferentiate into endothelial-like cells that support the formation of the extracellular matrix (ECM), eventually inducing tumor-associated neovascularization – this process is called vascular mimicry (VM).1,2 VM represents a new channel that can mimic the embryonic vascular network pattern and provide sufficient blood supply to tumor tissues.3 VM has been depicted in numerous types of malignant tumors, such as glioblastoma, astrocytoma, non-functioning pituitary adenomas, head and neck cancer, lung cancer, breast cancer, esophageal carcinoma, gastric cancer, hepatocellular cancer, colorectal cancer, ovarian carcinoma, prostate cancer, gallbladder cancer, Ewing sarcoma, and osteosarcoma.2,4–11
Over the last few decades, several potential mechanisms of VM formation have been explored, such as epithelial–mesenchymal transition (EMT) and cancer stem cells (CSCs).12 The contribution of several protein factors, including epithelial cell kinase (ephrin type-A receptor 2 [EphA2]), focal adhesion kinase (FAK), hypoxia inducible factor 1-α (HIF-α), integrins laminin 5 (Ln-5) γ2 chain, matrix metalloproteinase (MMPs), phosphoinositide 3-kinase (PI3K), and vascular endothelial cadherin (VE-Cad), to VM formation have been investigated.3,13–16 Despite remarkable advances in identifying VM, the key explicit mechanisms of VM are not well characterized, and fully elucidating these mechanisms are of great importance. Lately, advancements in molecular imaging have facilitated the detection, diagnosis, and evaluation of cancer. Qualitative and quantitative investigations on the biological procedures of tumor tissue at the cellular and molecular level can be achieved.
Accordingly, significant features of VM in highly aggressive malignant tumors have been explored widely by various invasive or noninvasive molecular imaging techniques.17 Furthermore, VM formation is associated with unfavorable outcomes of malignant tumor.18 Currently, new drugs and therapeutic strategies targeting VM in cancer are still under development. This review focuses on the advances of concepts, mechanisms, molecular imaging techniques, and targeted therapy in VM that exist at present.
Conceptual progress of VM
Until relatively recently, angiogenesis was considered the sole method of tumor vascularization. Common anti-angiogenesis drugs primarily target endothelial cells, through inducing endothelial cell apoptosis and reducing the proliferation to “starve” tumors. However, the antitumor effects of angiogenesis inhibitors are unsatisfactory. With advances in tumor vascularization, other patterns of blood supply have been detected. In 1999, Maniotis et al first described highly patterned vascular tubes that were formed by highly aggressive uveal melanoma cells without the presence of endothelial cells lining these vessel-like channels.1 Since then, the concept of VM has been solidified as an alternative vascularization method that nourishes tumor tissues.12,19 Recently, choriocarcinoma was shown to utilize VM to increase nutrient retrieval from the blood. In this tumor type, the vessels were surrounded by neoplastic trophoblastic cells. In the marginal regions of tumor tissue, trophoblastic cells invaded host vessels and formed anastomoses.20 VM channels expressed high levels of tissue transglutaminase antigen 2 which enables tubular structure formation.21 CSCs can differentiate into other kinds of cell lineages. Cancer cells in VM express multipotent, stem cell-like phenotypes, including tumor and endothelial phenotypes.22 In melanoma stroma, both fibrovascular septa and VM are observed; VM is distinguished from fibrovascular septa by lamination, thickness, and immunohistochemistry.23
Remarkably, there is indirect evidence suggesting there is circulation of plasma through VM patterns in various types of cancer. Frenkel et al described blood circulation within VM channels with laser scanning confocal angiography in a choroidal melanoma.24 In plastic EW7 Ewing sarcoma tumors in the athymic Tie2-GFP transgenic mouse model, both fluorescent blood vessels and non-fluorescent VM tubes could be observed by intravital microscopy; moreover, the hemodynamics in the cancer-cell-lined channels could be visualized.25 Microstructures of VM by transmission electron microscopy illustrated empty spaces in the central region of the tubes; there were no internal vacuoles with organelle-poor cytoplasm, and desmosomes contributed to the establishment of the junction between VM and endothelial-like cells.26
Additionally, other characteristics of VM have been recently described. In a heterotopic malignant mesothelioma xenograft model in BALB/C node mice, a small number of vessels consisting of mouse endothelial cells were observed; however, further immunohistochemical (IHC) analysis using the human-specific mitochondria antibody MAB1273B revealed VM channels containing erythrocytes at the periphery of the tumor tissue.27 In multiple myeloma, Nico et al showed that mast cells contribute to the formation of VM channels.28 Co-culture of aggressive melanoma cell lines with mesenchymal stromal cells were shown to acquire endothelial cell-like properties, eventually forming VM channels; however, such a phenomenon was not detected in poorly aggressive melanoma cell lines.29 These data suggest that VM is a novel method for providing blood and nutrition to aggressive tumor tissues.
The composition of tumor cells and a basement membrane made VM different from the classical concept of angiogenesis. To date, VM can be characterized in tumor samples with IHC using a positive staining pattern of both CD31 and periodic acid–Schiff (PAS) as markers. Two distinctive types of VM in aggressive malignant tumors have been identified. Type 1, known as the tubular type, is composed of non-endothelial cell-lined blood tubes, and cancer cells were found lining the surface of the channels.30 Type 2, known as the patterned matrix type, comprises a basement membrane rich in fibronectin, collagens, and laminin, where the membrane is surrounded by tumor cells instead of endothelial cells.31
Furthermore, VM is used as a means to support tumor growth in the early stages of tumorigenesis. Both angiogenesis and VM are coordinately used in tumor tissues.10 In the VM–angiogenesis junction, VM serves as part of the functional microcirculation; cancer cells within the tumor-lined vascular channels can easily transfer into endothelial-lined blood vessels, thereby facilitating tumor invasion and metastasis.
Mechanisms of VM activating invasion and metastasis
VM plays an essential role in the process of tumor invasion and metastasis. After the initiation of VM, a mosaic vessel made of tumor cells and endothelial cells gradually forms. Vessels connect and merge with host vessels, and cancer cells could obtain oxygen and nutriment via VM – consequently inducing cancer growth (Figure 1).32 Moreover, VM contributes to portal vein invasion in hepatocellular carcinoma through the expression of several proteins, including Notch1, MMP-2, and MMP-9.33 In head and neck squamous cell cancer, tumor cells express both VM and endothelial-specific markers and respond to vascular endothelial growth factor (VEGF) and endostatin. These features can be enhanced by transforming growth factor-β1, facilitating the acquisition of an endothelial cell type in a subpopulation of cells. Additionally, tumor cell mobility and invasiveness were heightened.34 In non–small cell lung cancer, Dickkopf-1 significantly induces VM formation and promotes cancer cell growth, migration, and metastasis via overexpression of EMT- and CSC-associated proteins.35 Maspin has a positive correlation with VM; deregulated maspin facilitates tumor cell invasion and metastasis in non–small cell lung cancer.36 In melanoma cells, hypoxia induces the release of mitochondrial reactive oxygen species and promotes activation of the Met proto-oncogene, thereby enhancing VM formation and resulting in tumor cell spread, increased motility, invasion, and metastasis.37 In the hypoxic microenvironment, HIF-1α induces VM formation via the upregulation of lysyl oxidases, such as in hepatocellular cancer, and promotes tumor cell metastasis and progression.38 Overexpression of miRNA-124 represses VM formation via inhibition of MMP-2, MMP-9, and VEGF; further analysis indicated that miRNA-124 is important in preventing tumor cell migration.39 Witkiewicz et al demonstrated that VM resembled lymphatic vessels or veins in morphology; moreover, endothelial cells were replaced by fibroblasts, thereby facilitating tumor metastasis.40 In esophageal squamous cell carcinoma, researchers used RNA silencing, and observed a positive correlation between VM channels and N-cadherin expression.41 Genes involved in metastatic and invasive behavior could be influenced by the tumor microenvironment. On being exposed to a laminin-rich niche, highly invasive uveal melanoma cells can form VM channels; however, a downregulation of genes including CD44, thrombospondin 1, and cyclin E2 were observed, thereby reducing melanoma cell invasion.42
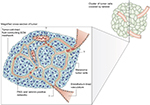  | Figure 1 The melanoma fluid-conducting extracellular matrix. Notes: In mice with aggressive melanoma (blue cells). The endothelium-lined vessels (pink) are closely opposed to the fluid-conducting meshwork formed by tumor cells. Tumor cells can remodel the vasculature, which becomes leaky and leads to the extravascular accumulation of erythrocytes and plasma (red). Reprinted by permission from Springer Nature, Nature Reviews Cancer, Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma, Hendrix MJ, Seftor EA, Hess AR, Seftor RE, COPYRIGHT 2003.77 ?, he specific mechanism of VM are still under investigation. |
Research of VM using molecular imaging
The development of noninvasive imaging is essential for cancer detection and diagnosis during the early stages of the disease. Molecular imaging provides a better way to understand the biologic processes governing VM at a cellular and molecular level in living organisms. This use of this technique has become more applicable for cancer in the past few decades. VM can develop vascular anastomosis with microcirculation in the tumor tissues. Theoretically, contrasting agents can easily enter the VM tube, thereby enabling visualization of imaging patterns. This technique brings hope to a variety of imaging techniques, especially the molecular imaging technology.
Interestingly, evidence of VM has been reported in human tumors by using novel molecular imaging technologies. In a study that included 18 patients diagnosed with choroidal melanomas, patients were injected with indocyanine green as a contrasting agent prior to being imaged with a confocal scanning laser ophthalmoscope. The results demonstrated microvasculature patterns within the tumor; the tubular structures correlated well with the histopathological appearance of VM.43 Frenkel et al conducted indocyanine green laser scanning confocal angiography on patients with posterior choroidal melanoma. They found that fluid enters VM by leakage instead of through endothelial cell-lined vessels; furthermore, fluid accumulated in VM was never characterized as a stagnant pool.24 Polylactic acid – a nanoparticle agent – is showing promising advantages for targeted ultrasound imaging. This nanoparticle could be conjugated to the anti-human epidermal growth factor receptor 2 (HER2) antibody, thereby allowing for HER-positive breast tumor cells to be specifically bound.44 In accordance with this finding, imaging of VM channels by high-resolution ultrasound using targeted nanoparticles may be explored. With the development of intravascular macromolecular contrasting agents for magnetic resonance imaging (MRI), the diagnosis of VM tubes by techniques such as time-course dynamic micro-magnetic resonance angiography analysis could be achieved. Shirakawa et al used this technology to investigate the hemodynamics of VM in a newly established human inflammatory breast cancer xenograft in BALA/c nude mice. The results revealed that tumor center exhibited a signal that gradually increased in intensity, which is consistent with histological features of VM in the same area. The group also observed the presence of a VM-angiogenesis junction by transmission electron microscopy and IHC.45 Similar results were observed in Kobayashi et al’s study, and the data also suggested VM is involved in tumor tissue perfusion.46 Yamamoto et al described the radiological features of VM in malignant gliomas using the newly developed MRI. Anaplastic oligodendroglioma illustrating hyperintensity on fluid-attenuated inversion recovery images as well as histological diagnosis proved these were VM.17 Thus, due to remarkable progress made in this endeavor, imaging features of VM should be further explored as a tool for clinical application.
Significance of VM expression in clinical practice
Identification of VM formation is of paramount importance to daily clinical practice. Previous studies have attempted to measure microvascular density to quantify the degree of angiogenesis and its relationship to prognosis; however, the results are rather controversial.47,48 Part of the rationale is that the tumor microcirculation is lined not only by endothelial cells but also with tumor cells; VM contributes to the intratumor heterogeneity in aggressive cancer. Moreover, standard VM assessment is based on the quantification of positive PAS and negative CD31 staining of vessel-like structures; this is different from the commonly used tumoral microvascular density assessment. In human gliomas, VM formation is highly aggressive and acts as a complementary strategy for providing tumor tissues with blood supply in poorly vascularized areas.49 In prostatic cancer, the correlation between VM and histologic grading was insignificant, and the correlation between VM and perineurial invasion was rather weak. This observation may be due to the composition of prostatic tissue, which contains an abundance of smooth muscle fibers, as well as the staining methods used.50
VM has been demonstrated as an unfavorable survival factor and a marker of poor prognosis in various cancers. In colorectal carcinoma, VM was shown to be a strong, independent, prognostic factor of survival.7 A meta-analysis by Cao et al comprising 15 types of malignant tumors revealed that patients with VM-positive cancer showed a less favorable 5-year overall survival than patients with VM-negative cancer, especially in advanced-stage cancer.18 In patients with non–small cell lung cancer, CD133 expression and VM formation were significantly high and associated with tumor differentiation, lymph node metastasis, clinical stage as well as prognosis; therefore, VM could be used as a prognostic marker in clinical practice.51 Indeed, VM could also be used as a prognostic marker in ovarian cancer, inflammatory breast cancer, and gastric adenocarcinoma.52–54 In hepatocellular carcinoma, VM promotes cancer progression after orthotopic liver explant.55 Data on laryngeal squamous cell carcinoma suggested VM was a poor prognostic factor of disease-specific and metastasis-free survival; further analysis revealed that VM contributed to tumor progression through promoting lymph node metastasis.56 In uveal melanoma samples, Chromosome 3 aberrations played an important role in the formation of VM networks; identification of Chromosome 3 with fine needle aspiration could be used in prognostic assessment.57 There is a strong correlation between increased expression of HER2 and the positive rate of VM in invasive breast cancer. Furthermore, VM was associated with a positive nodal status and advanced clinical stages.58 However, other studies suggested different outcomes. Massi et al indicated that, in patients with pT3 and pT4 cutaneous melanoma, there is no evidence of VM as a prognostic factor.59
In some biopsy samples of malignant cancer or benign diseases, clinical evidence of VM is detected. VM tubes that contained red blood cells were found in malignant mesothelioma, cerebral cavernous malformations, and anaplastic oligodendroglioma.17,27 In benign nevi, IHC analysis of VM was shown in a minority of cases as compared to that of melanomas and was associated with unfavorable survival.60 In a patient diagnosed with multiple probable cerebral cavernous malformations, a slightly enlarged hyperintense region on the right frontal lobe persisted after therapy. Postoperative histological diagnosis demonstrated an anaplastic oligodendroglioma and cerebral cavernous malformations with VM.17 Therefore, early identification of radiological features of VM is essential for clinical decision making.
Blood supply plays an important role in tumor growth and metastasis; however, the therapeutic efficiency of drugs directed toward endothelial cells is unsatisfactory.61 The discovery of VM in tumor blood supply provided new insights in cancer biology. In Merkel cell carcinoma, VM-rich regions are illustrated as resistant to conventional chemotherapeutic agents.62 VM channels decrease cancer latency and increase intratumoral cisplatin delivery but may also reduce drug efficacy.8 Therefore, VM may be considered a useful target for treating cancer.
Therapeutic targeting of VM
Present treatment strategies such as chemotherapy are inefficient in aggressive cancer. Residual tumor cells may form VM channels, thereby providing oxygen and nutrients, which support cell proliferation and cancer progression. Thus, VM is a promising target for developing novel anticancer therapeutics, and many drugs have been investigated. Angiogenesis inhibitors including anginex, TNP-470, and endostatin were delivered to both human melanoma cell lines and human endothelial cell lines in vitro; marked inhibition on the vascular cord and tube formation were observed in endothelial cells as compared to melanoma cells. Detailed analysis showed higher mRNA and protein levels for two putative endostatin receptors – α5 integrin and heparin sulfate proteoglycan 2 – in endothelial cells compared to melanoma cells.63 The Rho kinase inhibitor fasudil induces VM destruction in B 16 melanoma cell xenograft.64 Fasudil blocks the RhoA/ROCK signaling pathway and VM formation is inhibited.11 Nicotinamide can effectively inhibit the formation of VM channels as well as destroy preexisting channels by downregulating VE-Cad expression. VM formation was suppressed for up to a month after exposure to nicotinamide. Although melanoma cell proliferation was significantly inhibited, it is worth noting the opposite effect of increased invasion capacity of the tumor cells by nicotinamide.65 When cultured on an aggressive tumor cell-preconditioned three-dimensional matrix, VM formation was induced in poorly aggressive tumor cells. Chemically modified tetracycline has been reported to be capable of targeting the tumor microenvironment by inhibition of MMP activity. Generation of Laminin-5-γ2 (Ln-5γ2) chain promigratory fragments is suppressed, thereby blocking its gene expression of poorly aggressive melanoma cells; as a consequence, VM formation is inhibited.66 The inhibition of VM by verteporfin is due to the suppression of MMP-2 and VE-Cad in human pancreatic ductal adenocarcinoma cell lines.67 Nanostructured functional drug-loaded liposomes, modified with an HIV peptide lipid-derivative conjugate, containing epirubicin and celecoxib, DSPE-PEG2000-PTDHIV-1, downregulated VM expression via the inhibition of VE-Cad, FAK, EphA2, HIF-1α, and MMP-9 in invasive breast cancer xenografts in nude mice.68 Paclitaxel-loaded liposomes modified with tandem peptides were observed to induce the destruction of VM channels in glioma cells.69 Doxycycline downregulated the expression of VE-Cad and MMPs and inhibited VM in hepatocellular carcinoma.70 In clear-cell renal cell carcinoma, the tumor was efficiently targeted by sunitinib – both in vitro and in vivo; however, resistant tumor cells emerged with a more aggressive phenotype and high level of vascularization during maintenance treatment. VM formation, autonomous pro-tumoral gene expression, a stroma-rich variant, and a favorable tumor microenvironment are the main reasons for this change. Second-line therapy with everolimus enhanced the inhibition of sunitinib-resistant tumors by suppressing VM channels.71 In a malignant glioma tumor model, the bispecific immunotoxin VEGF165-ephrin A1-PE38KDEL was delivered by human mesenchymal stem cells. As a result, VM formation and tumor growth was effectively inhibited; this provided a novel strategy for treatment of malignant gliomas.72 In melanoma cells, cilengitide – an inhibitor of αν integrins – reduced VM formation by decreasing ECM invasion as well as VEGF-A and MMP-9 secretion.73 Similarly, genistein was shown to inhibit VM by downregulation of VE-Cad in uveal melanoma cells.74 Thalidomide is efficient in inhibiting VM channels and induces tumor cell necrosis by regulation of the NF-κB signaling pathway.75 Antioxidants such as resveratrol can decrease the level of VEGF and caspase-3 – thus, minimizing capillary formation.76
The effects of antiangiogenic compounds on VM formation were also assessed. The inhibitory effects of Endostar – a recombinant human endostatin – on VM formation of melanoma cells (Figure 2) and glioblastoma cells were shown to be insignificant.9,77 In an ovarian cancer model, short-term bevacizumab treatment demonstrated antitumor effects; however, it also contributed to cancer progression due to increased HIF-1α expression and VM formation.78 Apropos the above, angiogenesis inhibitors have failed to prevent progression and growth of malignant tumors, which also demonstrates that VM formation – as a form of microcirculation – plays an important role in tumor progression.
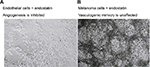  | Figure 2 The therapeutic effects of endostatin (an angiogenesis inhibitor) on microvascular endothelial cells (A), lack of vessel networks) compared with melanoma cells (B), VM formation was unaffected) in three-dimensional gels of collagen I in vitro. Note: Reprinted by permission from Springer Nature, Nature Reviews Cancer, Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma, Hendrix MJ, Seftor EA, Hess AR, Seftor RE, COPYRIGHT 2003.77 |
miRNAs are small non-coding RNA molecules, which contain approximately 22 nucleotides, that promote post-transciptional gene silencing.79 Accordingly, miRNAs play a crucial role in modulating cancer cell migration, invasion, and tumor angiogenesis. miRNA-9 is a tissue-specific miRNA localized in tissues of the central nervous system. In glioma cell lines, miRNA-9 inhibits VM formation and tumor growth by controlling Stathmin expression.80 A study by Wan et al revealed miRNA-124 inhibits VM formation of cervical cancer cells by targeting AmotL1 and suppressing the EMT process.39 As noted earlier, the VE-Cad complex is involved in tumor VM formation. miRNA-27b acts as an inhibitor of VM in ovarian cancer cells by suppressing VE-Cad expression.81 The full spectrum of mechanisms of miRNAs to tumor VM inhibition remains to be elucidated.
Over recent years, traditional Chinese medicines have been investigated in treating various cancers. Lycorine hydrochloride is effective against capillary-like tube formations in a melanoma cell line model by hindering VE-Cad expression.82 Dehydroeffusol was found to significantly inhibit VM formation by downregulating MMP-2.83 Other drugs, including grape-seed proanthocyanidins, isoxanthohumol, ginsenoside Rg3, norcantharidin, paris polyphylla, and curcumin have been well characterized and possess antitumor effects (Table 1).84–89
Conclusion and future vision
Although initially described in aggressive uveal melanoma, VM has led to profound changes in our understanding of tumor nourishment in a variety of cancers. The phenomenon illustrates that tumor cells can transdifferentiate due to a multipotent, CSC-like phenotype and generate ECM-rich, negative CD31, and positive PAS vascular networks. These channels are distinct microvessels that differ from host vessels and provide adequate blood supply which promotes tumor growth. As such, VM plays an essential role in facilitating tumor invasion and metastasis. Realizing its importance in tumorigenesis, researchers have been investigating VM with the aid of molecular imaging and, thus far, the results have been promising. Furthermore, VM was considered an independent, unfavorable, prognostic factor of clinical practice; VM is associated with aggressive behavior and easily metastasizes to distant sites. To date, anti-angiogenic therapies focus primarily on tumor blood vessels that are formed by endothelial cells, VM formation is one of the reasons for the limited therapeutic effects of existing anticancer drugs; although enormous efforts have been made in an attempt to eliminate VM formation and some drugs demonstrate a promising result, research into treatment strategies to counter VM is still ongoing. Much remains to be learned to delineate the role of VM in tumor progression on the cellular level and to translate these findings into novel therapies that target VM in the treatment of cancer.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant nos. 81372436 and 81773230); the Science and Technology Innovative Research Group of Zhengzhou city (grant no. 121PCXTD524), and the Joint Research Funds of Henan province and the Minister of Health of China (grant no. 201201009). The authors would like to thank Kyle Vaugh Laster (Hormel Cancer Institute, USA) for editing the language of the present manuscript.
Author contributions
Both HL and HG have contributed to the conception and design, data acquisition, and interpretation; drafting the article and final approval of the final version to be submitted; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
Maniotis AJ, Folberg R, Hess A, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–752. | ||
Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–828. | ||
Seftor RE, Seftor EA, Koshikawa N, et al. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61(17):6322–6327. | ||
Di Michele J, Rotondo F, Kovacs K, et al. Vasculogenic mimicry in clinically non-functioning pituitary adenomas: a histologic study. Pathol Oncol Res. 2017;23(4):803–809. | ||
Sun W, Fan YZ, Zhang WZ, Ge CY. A pilot histomorphology and hemodynamic of vasculogenic mimicry in gallbladder carcinomas in vivo and in vitro. J Exp Clin Cancer Res. 2011;30:46. | ||
Sharma N, Seftor RE, Seftor EA, et al. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate. 2002;50(3):189–201. | ||
Baeten CI, Hillen F, Pauwels P, de Bruine AP, Baeten CG. Prognostic role of vasculogenic mimicry in colorectal cancer. Dis Colon Rectum. 2009;52(12):2028–2035. | ||
Williamson SC, Metcalf RL, Trapani F, et al. Vasculogenic mimicry in small cell lung cancer. Nat Commun. 2016;7:13322. | ||
Liu Z, Li Y, Zhao W, Ma Y, Yang X. Demonstration of vasculogenic mimicry in astrocytomas and effects of Endostar on U251 cells. Pathol Res Pract. 2011;207(10):645–651. | ||
Upile T, Jerjes W, Radhi H, et al. Vascular mimicry in cultured head and neck tumour cell lines. Head Neck Oncol. 2011;3:55. | ||
Xia Y, Cai X, Fan J, et al. RhoA/ROCK pathway inhibition by fasudil suppresses the vasculogenic mimicry of U2OS osteosarcoma cells in vitro. Anticancer Drugs. 2017;28(5):514–521. | ||
Liu Q, Qiao L, Liang N, et al. The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. J Cell Mol Med. 2016;20(9):1761–1769. | ||
Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor RE, Hendrix MJ. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther. 2006;5(2):228–233. | ||
Liu K, Sun B, Zhao X, et al. Hypoxia induced epithelial-mesenchymal transition and vasculogenic mimicry formation by promoting Bcl-2/Twist1 cooperation. Exp Mol Pathol. 2015;99(2):383–391. | ||
Zang M, Zhang Y, Zhang B, et al. CEACAM6 promotes tumor angiogenesis and vasculogenic mimicry in gastric cancer via FAK signaling. Biochim Biophys Acta. 2015;1852(5):1020–1028. | ||
Hess AR, Seftor EA, Seftor RE, Hendrix MJ. Phosphoinositide 3-kinase regulates membrane Type 1-matrix metalloproteinase (MMP) and MMP-2 activity during melanoma cell vasculogenic mimicry. Cancer Res. 2003;63(16):4757–4762. | ||
Yamamoto J, Shimajiri S, Miyaoka R, Nishizawa S. Pitfalls of conservative treatments of multiple probable cerebral cavernous malformations (CCMs): clinicopathological features of CCMs coexisting with vasculogenic mimicry in an anaplastic oligodendroglioma. Brain Tumor Pathol. 2014;31(3):215–221. | ||
Cao Z, Bao M, Miele L, Sarkar FH, Wang Z, Zhou Q. Tumour vasculogenic mimicry is associated with poor prognosis of human cancer patients: a systemic review and meta-analysis. Eur J Cancer. 2013;49(18):3914–3923. | ||
Liu Z, Sun B, Qi L, Li H, Gao J, Leng X. Zinc finger E-box binding homeobox 1 promotes vasculogenic mimicry in colorectal cancer through induction of epithelial-to-mesenchymal transition. Cancer Sci. 2012;103(4):813–820. | ||
Shih IeM. Trophoblastic vasculogenic mimicry in gestational choriocarcinoma. Mod Pathol. 2011;24(5):646–652. | ||
Jones RA, Wang Z, Dookie S, Griffin M. The role of TG2 in ECV304-related vasculogenic mimicry. Amino Acids. 2013;44(1):89–101. | ||
Valyi-Nagy K, Kormos B, Ali M, Shukla D, Valyi-Nagy T. Stem cell marker CD271 is expressed by vasculogenic mimicry-forming uveal melanoma cells in three-dimensional cultures. Mol Vis. 2012;18:588–592. | ||
Lin AY, Maniotis AJ, Valyi-Nagy K, et al. Distinguishing fibrovascular septa from vasculogenic mimicry patterns. Arch Pathol Lab Med. 2005;129(7):884–892. | ||
Frenkel S, Barzel I, Levy J, et al. Demonstrating circulation in vasculogenic mimicry patterns of uveal melanoma by confocal indocyanine green angiography. Eye (Lond). 2008;22(7):948–952. | ||
Hillen F, Kaijzel EL, Castermans K, oude Egbrink MG, Löwik CW, Griffioen AW. A transgenic Tie2-GFP athymic mouse model; a tool for vascular biology in xenograft tumors. Biochem Biophys Res Commun. 2008;368(2):364–367. | ||
Clemente M, Pérez-Alenza MD, Illera JC, Peña L. Histological, immunohistological, and ultrastructural description of vasculogenic mimicry in canine mammary cancer. Vet Pathol. 2010;47(2):265–274. | ||
Pulford E, Hocking A, Griggs K, et al. Vasculogenic mimicry in malignant mesothelioma: an experimental and immunohistochemical analysis. Pathology. 2016;48(7):650–659. | ||
Nico B, Mangieri D, Crivellato E, Vacca A, Ribatti D. Mast cells contribute to vasculogenic mimicry in multiple myeloma. Stem Cells Dev. 2008;17(1):19–22. | ||
Vartanian A, Karshieva S, Dombrovsky V, Belyavsky A. Melanoma educates mesenchymal stromal cells towards vasculogenic mimicry. Oncol Lett. 2016;11(6):4264–4268. | ||
El Hallani S, Boisselier B, Peglion F, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133(Pt 4):973–982. | ||
Liu WB, Xu GL, Jia WD, et al. Prognostic significance and mechanisms of patterned matrix vasculogenic mimicry in hepatocellular carcinoma. Med Oncol. 2011;28 Suppl 1:S228–S238. | ||
Seftor RE, Hess AR, Seftor EA, et al. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol. 2012;181(4):1115–1125. | ||
Jue C, Zhifeng W, Zhisheng Z, et al. Vasculogenic mimicry in hepatocellular carcinoma contributes to portal vein invasion. Oncotarget. 2016;7(47):77987–77997. | ||
Tong M, Han BB, Holpuch AS, Pei P, He L, Mallery SR. Inherent phenotypic plasticity facilitates progression of head and neck cancer: endotheliod characteristics enable angiogenesis and invasion. Exp Cell Res. 2013;319(7):1028–1042. | ||
Yao L, Zhang D, Zhao X, et al. Dickkopf-1-promoted vasculogenic mimicry in non-small cell lung cancer is associated with EMT and development of a cancer stem-like cell phenotype. J Cell Mol Med. 2016;20(9):1673–1685. | ||
Wu S, Yu L, Cheng Z, Song W, Zhou L, Tao Y. Expression of maspin in non-small cell lung cancer and its relationship to vasculogenic mimicry. J Huazhong Univ Sci Technolog Med Sci. 2012;32(3):346–352. | ||
Comito G, Calvani M, Giannoni E, et al. HIF-1α stabilization by mitochondrial ROS promotes Met-dependent invasive growth and vasculogenic mimicry in melanoma cells. Free Radic Biol Med. 2011;51(4):893–904. | ||
Wang M, Zhao X, Zhu D, et al. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36(1):60. | ||
Wan HY, Li QQ, Zhang Y, et al. MiR-124 represses vasculogenic mimicry and cell motility by targeting amotL1 in cervical cancer cells. Cancer Lett. 2014;355(1):148–158. | ||
Witkiewicz H, Oh P, Schnitzer JE. II. Capsular vaso-mimicry formed by transgenic mammary tumor spheroids implanted ectopically into mouse dorsal skin fold: implications for cellular mechanisms of metastasis. F1000Res. 2013;2:9. | ||
Wang F, Li XK, Xu HY, et al. N-cadherin participated in invasion and metastasis of human esophageal squamous cell carcinoma via taking part in the formation of vasculogenic mimicry. Med Oncol. 2015;32(2):480. | ||
Folberg R, Arbieva Z, Moses J, et al. Tumor cell plasticity in uveal melanoma: microenvironment directed dampening of the invasive and metastatic genotype and phenotype accompanies the generation of vasculogenic mimicry patterns. Am J Pathol. 2006;169(4):1376–1389. | ||
Mueller AJ, Bartsch DU, Folberg R, et al. Imaging the microvasculature of choroidal melanomas with confocal indocyanine green scanning laser ophthalmoscopy. Arch Ophthalmol. 1998;116(1):31–39. | ||
Liu J, Li J, Rosol TJ, Pan X, Voorhees JL. Biodegradable nanoparticles for targeted ultrasound imaging of breast cancer cells in vitro. Phys Med Biol. 2007;52(16):4739–4747. | ||
Shirakawa K, Kobayashi H, Sobajima J, Hashimoto D, Shimizu A, Wakasugi H. Inflammatory breast cancer: vasculogenic mimicry and its hemodynamics of an inflammatory breast cancer xenograft model. Breast Cancer Res. 2003;5(3):136–139. | ||
Kobayashi H, Shirakawa K, Kawamoto S, et al. Rapid accumulation and internalization of radiolabeled herceptin in an inflammatory breast cancer xenograft with vasculogenic mimicry predicted by the contrast-enhanced dynamic MRI with the macromolecular contrast agent G6-(1B4M-Gd)(256). Cancer Res. 2002;62(3):860–866. | ||
Shin HJ, Kim EK, Moon HJ, Yoon JH, Han KH, Kwak JY. Can increased tumoral vascularity be a quantitative predicting factor of lymph node metastasis in papillary thyroid microcarcinoma? Endocrine. 2014;47(1):273–282. | ||
Iakovlev VV, Gabril M, Dubinski W, et al. Microvascular density as an independent predictor of clinical outcome in renal cell carcinoma: an automated image analysis study. Lab Invest. 2012;92(1):46–56. | ||
Liu XM, Zhang QP, Mu YG, et al. Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol. 2011;105(2):173–179. | ||
Ahmadi SA, Moinfar M, Gohari Moghaddam K, Bahadori M. Practical application of angiogenesis and vasculogenic mimicry in prostatic adenocarcinoma. Arch Iran Med. 2010;13(6):498–503. | ||
Wu S, Yu L, Wang D, et al. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer. 2012;12:535. | ||
Liang J, Yang B, Cao Q, Wu X. Association of vasculogenic mimicry formation and CD133 expression with poor prognosis in ovarian cancer. Gynecol Obstet Invest. 2016;81(6):529–536. | ||
Shirakawa K, Wakasugi H, Heike Y, et al. Vasculogenic mimicry and pseudo-comedo formation in breast cancer. Int J Cancer. 2002;99(6):821–828. | ||
Li M, Gu Y, Zhang Z, et al. Vasculogenic mimicry: a new prognostic sign of gastric adenocarcinoma. Pathol Oncol Res. 2010;16(2):259–266. | ||
Guzman G, Cotler SJ, Lin AY, Maniotis AJ, Folberg R. A pilot study of vasculogenic mimicry immunohistochemical expression in hepatocellular carcinoma. Arch Pathol Lab Med. 2007;131(12):1776–1781. | ||
Wang W, Lin P, Han C, Cai W, Zhao X, Sun B. Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma. J Exp Clin Cancer Res. 2010;29:60. | ||
Meir T, Zeschnigk M, Masshöfer L, Pe’er J, Chowers I. The spatial distribution of monosomy 3 and network vasculogenic mimicry patterns in uveal melanoma. Invest Ophthalmol Vis Sci. 2007;48(5):1918–1922. | ||
Liu T, Sun B, Zhao X, et al. HER2/neu expression correlates with vasculogenic mimicry in invasive breast carcinoma. J Cell Mol Med. 2013;17(1):116–122. | ||
Massi D, Franchi A, Paglierani M, et al. Vasculogenic mimicry has no prognostic significance in pT3 and pT4 cutaneous melanoma. Hum Pathol. 2004;35(4):496–502. | ||
Spiliopoulos K, Peschos D, Batistatou A, et al. Immunohistochemical study of vasculogenic mimicry and angiogenesis in melanocytic tumors of the eye and the periocular area. Anticancer Res. 2017;37(3):1113–1120. | ||
Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. | ||
Lezcano C, Kleffel S, Lee N, et al. Merkel cell carcinoma expresses vasculogenic mimicry: demonstration in patients and experimental manipulation in xenografts. Lab Invest. 2014;94(10):1092–1102. | ||
van der Schaft DW, Seftor RE, Seftor EA, et al. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst. 2004;96(19):1473–1477. | ||
Xia Y, Cai XY, Fan JQ, et al. Rho kinase inhibitor fasudil suppresses the vasculogenic mimicry of B16 mouse melanoma cells both in vitro and in vivo. Mol Cancer Ther. 2015;14(7):1582–1590. | ||
Itzhaki O, Greenberg E, Shalmon B, et al. Nicotinamide inhibits vasculogenic mimicry, an alternative vascularization pathway observed in highly aggressive melanoma. PLoS One. 2013;8(2):e57160. | ||
Seftor RE, Seftor EA, Kirschmann DA, Hendrix MJ. Targeting the tumor microenvironment with chemically modified tetracyclines: inhibition of laminin 5 gamma2 chain promigratory fragments and vasculogenic mimicry. Mol Cancer Ther. 2002;1(13):1173–1179. | ||
Wei H, Wang F, Wang Y, et al. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 2017;108(3):478–487. | ||
Ju RJ, Li XT, Shi JF, et al. Liposomes, modified with PTD(HIV-1) peptide, containing epirubicin and celecoxib, to target vasculogenic mimicry channels in invasive breast cancer. Biomaterials. 2014;35(26):7610–7621. | ||
Liu Y, Mei L, Yu Q, et al. Multifunctional tandem peptide modified paclitaxel-loaded liposomes for the treatment of vasculogenic mimicry and cancer stem cells in malignant glioma. ACS Appl Mater Interfaces. 2015;7(30):16792–16801. | ||
Meng J, Sun B, Zhao X, et al. Doxycycline as an inhibitor of the epithelial-to-mesenchymal transition and vasculogenic mimicry in hepatocellular carcinoma. Mol Cancer Ther. 2014;13(12):3107–3122. | ||
Serova M, Tijeras-Raballand A, Dos Santos C, et al. Everolimus affects vasculogenic mimicry in renal carcinoma resistant to sunitinib. Oncotarget. 2016;7(25):38467–38486. | ||
Zhang Y, Sun X, Huang M, Ke Y, Wang J, Liu X. A novel bispecific immunotoxin delivered by human bone marrow-derived mesenchymal stem cells to target blood vessels and vasculogenic mimicry of malignant gliomas. Drug Des Devel Ther. 2015;9:2947–2959. | ||
Ruffini F, Graziani G, Levati L, Tentori L, D’Atri S, Lacal PM. Cilengitide downmodulates invasiveness and vasculogenic mimicry of neuropilin 1 expressing melanoma cells through the inhibition of αvβ5 integrin. Int J Cancer. 2015;136(6):E545–E558. | ||
Cong R, Sun Q, Yang L, Gu H, Zeng Y, Wang B. Effect of Genistein on vasculogenic mimicry formation by human uveal melanoma cells. J Exp Clin Cancer Res. 2009;28:124. | ||
Zhang S, Li M, Gu Y, et al. Thalidomide influences growth and vasculogenic mimicry channel formation in melanoma. J Exp Clin Cancer Res. 2008;27:60. | ||
Vartanian AA, Burova OS, Stepanova EV, Baryshnikov AY, Lichinitser MR. Melanoma vasculogenic mimicry is strongly related to reactive oxygen species level. Melanoma Res. 2007;17(6):370–379. | ||
Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3(6):411–421. | ||
Xu Y, Li Q, Li XY, Yang QY, Xu WW, Liu GL. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J Exp Clin Cancer Res. 2012;31:16. | ||
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. | ||
Song Y, Mu L, Han X, et al. MicroRNA-9 inhibits vasculogenic mimicry of glioma cell lines by suppressing Stathmin expression. J Neurooncol. 2013;115(3):381–390. | ||
Liu W, Lv C, Zhang B, Zhou Q, Cao Z. MicroRNA-27b functions as a new inhibitor of ovarian cancer-mediated vasculogenic mimicry through suppression of VE-cadherin expression. RNA. 2017;23(7):1019–1027. | ||
Liu R, Cao Z, Tu J, et al. Lycorine hydrochloride inhibits metastatic melanoma cell-dominant vasculogenic mimicry. Pigment Cell Melanoma Res. 2012;25(5):630–638. | ||
Liu W, Meng M, Zhang B, et al. Dehydroeffusol effectively inhibits human gastric cancer cell-mediated vasculogenic mimicry with low toxicity. Toxicol Appl Pharmacol. 2015;287(2):98–110. | ||
Luan YY, Liu ZM, Zhong JY, Yao RY, Yu HS. Effect of grape seed proanthocyanidins on tumor vasculogenic mimicry in human triple-negative breast cancer cells. Asian Pac J Cancer Prev. 2015;16(2):531–535. | ||
Serwe A, Rudolph K, Anke T, Erkel G. Inhibition of TGF-β signaling, vasculogenic mimicry and proinflammatory gene expression by isoxanthohumol. Invest New Drugs. 2012;30(3):898–915. | ||
Guo JQ, Zheng QH, Chen H, et al. Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int J Oncol. 2014;45(3):1065–1072. | ||
Zhang JT, Sun W, Zhang WZ, et al. Norcantharidin inhibits tumor growth and vasculogenic mimicry of human gallbladder carcinomas by suppression of the PI3-K/MMPs/Ln-5γ2 signaling pathway. BMC Cancer. 2014;14:193. | ||
Yao N, Ren K, Wang Y, et al. Paris polyphylla suppresses proliferation and vasculogenic mimicry of human osteosarcoma cells and inhibits tumor growth in vivo. Am J Chin Med. 2017;45(3):575–598. | ||
Chiablaem K, Lirdprapamongkol K, Keeratichamroen S, Surarit R, Svasti J. Curcumin suppresses vasculogenic mimicry capacity of hepatocellular carcinoma cells through STAT3 and PI3K/AKT inhibition. Anticancer Res. 2014;34(4):1857–1864. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

