Back to Journals » OncoTargets and Therapy » Volume 12
Overexpression of shugoshin1 predicts a poor prognosis for prostate cancer and promotes metastasis by affecting epithelial–mesenchymal transition
Authors Mu J, Fan L, Liu D, Zhu D
Received 17 October 2018
Accepted for publication 17 January 2019
Published 8 February 2019 Volume 2019:12 Pages 1111—1118
DOI https://doi.org/10.2147/OTT.S191157
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Jiagui Mu,1 Li Fan,1 Duo Liu,1 Dongsheng Zhu2
1Department of Urology, The Second People’s Hospital of Lianyungang, Lianyungang Tumor Hospital, Lianyungang Hospital Affiliated to Bengbu Medical University, Haizhou District, Lianyungang 22200, China; 2Department of Graduate School Urology, Tianjin Medical University, Heping District, Tianjin 300000, China
Objective: The aim of the study was to investigate the role of shugoshinl (SGO1) in human prostate cancer (PCa).
Materials and methods: Quantitative real-time PCR (qRT-PCR) was used to determine the expression of SGO1 in PCa tissues and cell lines. The correlation between SGO1 expression and clinicopathological characteristics of PCa patients was analyzed using Kaplan–Meier analysis. SGO1 siRNA was successfully constructed and transfected into PCa cell lines (LNCaP and PC3). The knockdown efficacy was assessed by qRT-PCR. MTT assay and Transwell assay were conducted to observe the effect of SGO1 on the proliferation and invasion of PCa cell lines.
Results: SGO1-expression levels were found to be higher in the PCa tissues and cell lines. Correlation was identified between the expression of SGO1 and preoperative prostate-specific antigen (P=0.017), lymph-node metastasis (P=0.044), and Gleason score (P=0.041). Patients with higher SGO1 expression displayed more advanced clinicopathological characteristics in addition to a shorter biochemical recurrence-free survival time. Additionally, SGO1 knockdown resulted in the inhibition of PCa cell proliferation, migration, and invasion.
Conclusion: Taken together, the findings of the current study present evidence suggesting that SGO1 could inhibit the growth and invasion of PCa cells, highlighting its potential as a novel therapeutic target for the treatment of PCa.
Keywords: shugoshinl, prostate cancer, RNAi
Background
Prostate cancer (PCa) continues to plague male health around the world, representing the most common malignant tumor among male patients, accompanied by the second highest mortality rate in male patients with malignant tumors.1–3 Studies have revealed that, in 2016, 180,890 male patients in the USA were diagnosed with PCa, and another 85,920 patients succumbed to PCa.4 Early detection based on prostate-specific antigen (PSA) has more recently led to significantly improved clinical treatment outcomes and reduced tumor-related mortality in patients with PCa.5 Surgical treatment is often reserved for patients at an early stage of PCa, with androgen deprivation therapy (ADT) being the more common therapeutic approach for patients with metastasis. However, most patients will enter the castration-resistant prostate cancer (CRPC) phase eventually. The occurrence of CRPC in PCa patients indicates that the median survival time of patients is likely to be <2 years. In addition, once the disease metastasizes, the median survival time of PCa is commonly <5 years.6,7 Therefore, investigating the molecular mechanism of PCa and seeking effective therapeutic targets remain pivotal in the hope of solving the current clinical issues in the treatment of PCa.
Multiple studies8,9 have highlighted genetic instability as a causative factor in the occurrence of abnormal chromosome segregation in humans that can evolve into tumors. In the process of mitosis, the precise separation of sister chromatids is significant for maintaining the stability of the genome and the survival of the cells. If it is abnormally separated, it will lead to the formation of aneuploidy, thus contributing to tumorigenesis. Recently, the research on shugoshinl (SGO1) mainly focuses on the cell embryology, and a few studies demonstrated the role of SGO1 in tumorigenesis and cancer development, with only a few tumors having been reported, such as intestinal cancer10 and liver cancer.11
No studies which evaluate the expression of SGO1 in PCa can be retrieved currently. Therefore, the aim of the present study was to evaluate the effect of SGO1 on the development of PCa. In this study, our objective was to demonstrate that SGO1 could act to promote PCa cell proliferation and invasion, highlighting the potential SGO1 as a novel therapeutic target for PCa.
Materials and methods
Clinical specimen collection
A total of 52 paired PCa tissues and adjacent non-PCa tissues were collected from patients who had undergone radical prostatectomy at The Second People’s Hospital of Lianyungang (Lianyungang, Jiangsu, China) between 2011 and 2014. None of the enrolled patients had received radiotherapy or ADT prior to radical prostatectomy. All the collected clinical specimens were snap frozen in liquid nitrogen and promptly stored at −80°C until RNA extraction. The pathological examination results were confirmed by two professional urology pathologists. The staging of specimens was classified according to the 2002 TNM classification. All the adjacent non-PCa tissues were confirmed as benign prostatic hyperplasia based on pathological findings.
Ethical statement
The study was performed with the approval of the Ethics Committees of The Second People’s Hospital of Lianyungang, with strict adherence to the principles of the Declaration of Helsinki. Prior to the operation, signed written informed consent documents were obtained from each participant.
Cell lines
LNCaP and PC3 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). Both LNCaP and PC3 cells were cultured in RPMI 1640 medium (Corning, NY, USA) supplemented with 10% FBS (Gibco BRL, Gaithersburg, MD, USA), and were incubated in a humidified incubator with 5% CO2 at 37°C.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Trizol reagent (Invitrogen, Waltham, MA, USA) was used to extract total RNA following the manufacturer’s instruction, after which the first strand cDNA was acquired using the Reverse EasyScript One Step gDNA Removal and cDNA Synthesis SuperMix (Trangene, Beijing, China). Next, qRT-PCR was employed by using SYBR Green Master Mixture (Roche, Basel, Switzerland) reagent in an ABI7500 real-time PCAR instrument. Expression levels of SGO1 were calculated based on the comparative 2−ΔΔCt method by using StepOne Software. β-Actin was used as an internal control. qRT-PCR primers were as follows: 1) SGO1: forward: 5′-TGACTTCAACAGCGACACCCA-3′ and reverse: 5′-CACCCTGTTGTTGCTGTAGCCAAA-3′; and 2) β-actin: forward: 5′-TCACCCACACTGTGCCCATCTACGA-3′ reverse: 5′-CAGCGGAACCGCTCATTGCCAATGG-3′. Triplicate wells were set in each group.
siRNA transfection
The siRNA specifically targeting SGO1 (si-SGO1) and the scrambled negative control siRNA were commercially constructed by GenePharma (Shanghai, China). The si-SGO1: 5′-CCGGCACAGCCAGCGAACTATAATTCAAGAGATTATAGTTCACGCTGGCTGTGTTTTTG-3′ was the target sequence for si-SGO1. The cells were plated and cultured in growth media until cell confluence in the six-well plate reached 70% before siRNA transfection using Lipofectamine 3,000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The cells were harvested for qRT-PCR for 48 hours after transfection.
Cell proliferation assay
After a 48-hour period of siRNA transfection, the PCa cells were seeded at a density of 1,000 cells per well in 96-well plates with six replicate wells. At 4, 24, 48, 72, and 96 hours after transfection, 10 μL of MTT Cell Proliferation/Viability Assay kit was added to the plates in accordance with the instructions. Finally, the absorbance was measured at a wavelength of 490 nm using an enzyme-labeled reader. All experiments were performed in triplicates.
Cell migration and invasion assays
Cell migration and invasion assays were adopted using transwell chambers. Next, 200 μL of cell suspension with a density of 1×105 cells was plated into the apical chamber in a serum-free medium. The basolateral chamber was filled with 500 μL of the medium, containing 10% FBS as a chemoattractant. After a 48-hour period of incubation, the cells that failed to migrate through the pores were swabbed with a cotton swab. The lower surface of the membranes was fixed and stained with 0.5% crystal violet, and the cells on the membranes were dyed. An inverted microscope was used to observe and count the number of cells that invaded through the membrane. Five randomly selected fields were counted per chamber. Each experiment was conducted in triplicate.
Western blot analysis
The cells were washed twice with cold PBS, and then lysed in RIPA buffer with protease and phosphatase inhibitors (Roche, Complete Mini). Cell debris and insoluble material were removed by centrifugation at 14,000× g at 4°C for 25 minutes. Protein quantitation was conducted using the Bradford protein assay (Bio-Rad, Hercules, CA, USA), and 30 μg of the protein was loaded per lane. Electrophoresis was conducted with SDS-PAGE, and the separated proteins were transferred onto nitrocellulose membranes. The membrane was blocked with 5% skim milk at room temperature for 1 hour, followed by incubation with primary antibodies (mouse anti-E cadherin with 1:50 dilution, ab1416; mouse anti-vimentin antibody with 1:1,000 dilution, ab8978; mouse anti-β-actin with 1:1,000 dilution, ab8226; all the above antibodies were obtained from Abcam plc, Cambridge, UK) at 4°C overnight. After three TBST washes for 10 minutes each, a 1-hour period of incubation was performed with horseradish peroxidase conjugate secondary antibodies at room temperature. The bound antibodies were visualized using enhanced chemiluminescence reagent (32109, Thermo Fisher Scientific).
Statistical analysis
Quantitative data were expressed as mean ± SD. Comparisons of continuous data were performed using an unpaired two-tailed t-test. Kaplan–Meier method and log-rank tests were used to estimate the prognostic value of SGO1 in patients’ survival. All data were analyzed by GraphPad Prism 5.0. Values of P<0.05 were considered statistically significant.
Results
Upregulation of SGO1 expression in PCa tissues and cell lines
Initially, qRT-PCR methods were employed in order to examine the expression of SGO1 in 52 pairs of PCa tissues and adjacent normal tissues. Additionally, the expression of SGO1 was significantly upregulated in PCa tissues, compared with paired adjacent normal tissues (Figure 1A). SGO1 expression in a non-cancer human cell line (BPH1) with two PCa cell lines (LNCaP and PC3) was further detected by qRT-PCR. The result suggested that SGO1 was increased in PCa cell lines, but not in BPH1 (Figure 1B).
Correlation of SGO1 expression with clinicopathological characteristics of PCa patients
The PCa patients were classified into two groups based on SGO1 mRNA expression: a high expression group (n=12) and a low expression group (n=40). Clinicopathological characteristics of two groups were compared (Table 1). Statistical results demonstrated that the high SGO1-expression group was significantly correlated with PSA (P=0.017), lymph-node metastasis (P=0.044), Gleason score (P=0.041), and biochemical recurrence (P=0.045), as compared with those in the low SGO1-expression group. However, it was found to not be correlated with age (P=0.679), prostate volume (P=0.517), or tumor stage (P=0.104).
  | Table 1 The association between SGO1 expression and clinicopathological features of prostate cancer |
Effect of SGO1 expression on biochemical recurrence-free survival
The correlation between the expression of SGO1 with the prognosis of PCa patients after radical prostatectomy was further explored. Kaplan–Meier analysis revealed that a higher SGO1 expression was associated with poor prognosis, in addition to significantly shorter biochemical recurrence-free survival time compared with a low SGO1 expression (P=0.043, Figure 2). These findings collectively indicated that SGO1 was implicated in the degree of aggression associated with the progression of PCa.
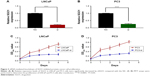
Knockdown of SGO1 inhibited cell proliferation in vitro
The SGO1 expression was downregulated in two PCa cell lines (LNCaP and PC3) by means of transfecting siSGO1 or a NC siRNA into the two cell lines in an attempt to verify the role of SGO1 in the progression of PCa. The qRT-PCR results confirmed the inhibition efficiency of SGO1 expression (P<0.05, Figure 3A and B). MTT assays were employed to explore the impact of SGO1 knockdown on proliferation of PCa cells. The results obtained demonstrated that the knockdown of SGO1 notably inhibited the proliferation of the two cell lines (P<0.05, Figure 3C and D).
Knockdown of SGO1 inhibited cell migration and invasion in vitro
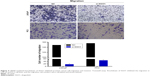
Transwell assay indicted that the knockdown of SGO1 inhibited PCa cell migration and invasion ability (P<0.05, Figure 4). Therefore, SGO1 was considered to act as an oncogene in the promotion of PCa cell proliferation.
SGO1 facilitated epithelial–mesenchymal transition (EMT) in PCa cells
EMT is an important mechanism in tumor cell metastasis. In order to ascertain as to whether EMT markers were changed in the PCa cells in the presence of SGO1 knockdown, the two EMT markers, E-cadherin and vimentin, were selected for Western blot analysis. The results obtained revealed that the expression of E-cadherin was increased while vimentin expression was decreased when SGO1 was knocked down in LNCaP cells and PC3 cells (Figure 5). These findings suggested that SGO1 is a promoter of PCa cell migration and invasion and the underlying mechanism is involved with the EMT pathway.
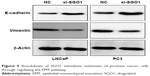  | Figure 5 Knockdown of SGO1 stimulates metastasis of prostate cancer cells through regulating the EMT pathway. |
Discussion
PCa remains a common male malignant tumor accompanied by high rates of mortality. In recent years, basic treatment of PCa has remained focusing on surgical approaches or ADT. However, PCa often inevitably progresses into a more advanced stage, which is commonly referred to as CRPC. The early detection of PSA, which represents an early diagnosis of PCa, can significantly improve patient outcome and treatment effectiveness. However, regarding early diagnosis markers, PSA has limited sensitivity and specificity. Accumulating reports have suggested that PCa is the result of a combination of aberrant expressed multiple genes and multiple factors; however, at present, no key genes or new therapeutic targets for PCa have been found.12 These have brought enormous challenges to modern medicine. Therefore, it is urgently needed to identify PCa-specific expression genes or proteins for the diagnosis and treatment of PCa.
As a conserved protein, SGO1 plays a critical role in ensuring the correct dissociation of mitotic sister chromatids. A previous study13 concluded that, in the process of mitosis, SGO1 is located on the centromere when DNA replication is completed. Prior to the formation of the spindle, the adhesion proteins were attached to the centromeres of the sister chromatids. Until the middle of mitosis, a series of monitoring mechanisms, including spindle detection points, act to inhibit the activation of anaphase-promoting complex/cyclosome (APC/C) complexes by inhibiting the activation of separase. APC/C is a multifunctional E3 ubiquitin ligase that takes part in biological processes of cell cycle, metabolism, DNA damage and repair, autophagy, apoptosis, senescence and tumorigenesis. The activation of APC/C complex resulted in the end of mitosis, activation of the separase, and the dissociation of centromeric adhesion protein. Wong et al14 concluded in their study that SGO1 is the substrate of APC/C, and proteomics studies found that SGO1 is a component of the mitotic spindle. Meanwhile, certain reports have suggested that the loss of SGO1 function activates the spindle checkpoint, and then activates APC/C and separase. Resultantly, the adhesion protein is dissociated from the centromere and mitosis stop.15 These all indicate that, after SGO1 is down-regulated, mitosis of cells is blocked, resulting in slower cell proliferation. All the above-mentioned studies are consistent with our findings.
At present, very few investigations on SGO1 have been conducted, all of which are sporadic in nature and generally pertain to the relationship between SGO1 and tumorigenesis. SGO1 is mainly related to neuroblastoma,16 liver cancer,11,17 lung cancer,18 and colon tumor.19 In neuroblastoma, SGO1 exerts its effect in the DNA damage response and represents a potential molecular target for treatment of MYCN-amplified neuroblastoma.16 In liver tumorigenesis, animal experiments have indicated that SGO1 can affect the stability of chromosomes and play a carcinogenic role.17 Similar findings were reported in the human body.11 Regarding lung cancer, an animal investigation on mice revealed that SGO1 could cause defects in the lung immune system by activating Wnt signaling, which ultimately led to tumorigenesis.18 Interestingly, in relation to colon tumors, a study suggested that the down-regulation of SGO1 contributed to tumor proliferation.19 This may be attributed to different carcinogenic mechanisms in different tumors, which require further research.
Based on the aforementioned previously performed studies, we subsequently asserted the notion that SGO1 may play a role in promoting cancer. However, the relationship between PCa and SGO1 is not fully understood. In the current study, a key finding revealed that the expression level of SGO1 in PCa tissues was significantly higher than that in normal prostate tissues. The Gleason score, tumor stage, and overall survival of PCa patients with high expression of SGO1 were significantly worse than those with low expression. This result suggests that SGO1 may be a indicator predicting poor prognosis for patients with PCa. This relationship between expression of SGO1 and PCa prognosis among patients undergoing radical prostatectomy represents a novel finding. During the current study, there were 52 cases of patients with high SGO1 expression which was often accompanied by higher tumor stage and Gleason score. These findings were all consistent with the results previously observed in lung and liver cancer, with our results demonstrating that SGO1 acts as an oncogene that elevates the risk of cell proliferation and metastasis in PCa.
Additionally, to further verify the oncogene capacity of SGO1, SGO1 knockdown in PCa cell lines LNCaP and PC3 was performed. The results obtained showed that SGO1 knockdown significantly inhibited cell growth and invasion in PCa cell lines in vitro.
To further investigate the molecular mechanism of SGO1 in promoting the metastasis of PCa cell lines, we examined the pertinent protein markers of the EMT pathway, with the results obtained indicating that SGO1 could promote PCa metastasis by regulating EMT-related proteins expression.
SGO1 variant has been previously reported as a novel tumor marker in recent years. In cases of non-small-cell lung cancers, a study using 82 frozen human tissue samples found that SGO1 variant B mRNA expression was related to taxane resistance, and that down-regulation of SGO1 variant B increased the sensitivity to taxane.20 A similar finding was also found in human colon cancer, indicating that SGO1 transcript variant P1 could cause abnormal mitosis and unstable chromatid cohesion.21 The similar phenomenon, we will further study in prostate cancer.
There were some limitations faced in the current study. The finding that high expression of SGO1 leads to worse clinical outcome in PCa patients is only based on the different levels of SGO1 using clinical tissue samples. First, the clinical data were collected using a retrospective approach; second, the sample size is relatively small; third, the verifications were in vitro. Therefore, the other potential molecular mechanism that SGO1 enhances the carcinogenesis of PCa requires further investigation.
Conclusion
Taken together, the key findings of our study present evidence indicating that increased SGO1 expression is a common event in PCa, which suggests that SGO1 may act as an indicator predicting an unfavorable prognosis for PCa patients. SGO1 was ultimately found to play a role in PCa by enhancing proliferation, migration, and invasion, suggesting that it promotes tumorigenesis in PCa, thus highlighting its promise as a prognostic factor and new therapeutic target for the treatment of PCa.
Ethics approval and consent to participate
The study was performed with the approval of the Ethics Committees of The Second People’s Hospital of Lianyungang, with strict adherence to the principles of the Declaration of Helsinki. Prior to the operation, signed written informed consent forms were obtained from each participant.
Acknowledgment
Jiagui Mu and Li Fan are co-first authors.
Disclosure
The authors report no conflicts of interest in this work.
References
Huang H, Muscatelli S, Naslund M, Badiyan SN, Kaiser A, Siddiqui MM. Evaluation of cancer-specific mortality with surgery versus radiation as primary therapy for localized high-grade prostate cancer in men younger than 60 years old. J Urol. 2019;201(1):120–128. | ||
Pervaiz R, Tulay P, Faisal F, Serakinci N. Incidence of cancer in the Turkish Republic of northern Cyprus. Turk J Med Sci. 2017;47(2):523–530. | ||
Kapoor A, Hotte SJ. Localized prostate cancer. Can Urol Assoc J. 2016;10(7S–8S):S138–S139. | ||
Benedetti M, Zona A, Beccaloni E, Carere M, Comba P. Incidence of breast, prostate, testicular, and thyroid cancer in Italian contaminated sites with presence of substances with endocrine disrupting properties. Int J Environ Res Public Health. 2017;14(4):E355. | ||
Cabarkapa S, Perera M, Mcgrath S, Lawrentschuk N. Prostate cancer screening with prostate-specific antigen: a guide to the guidelines. Prostate Int. 2016;4(4):125–129. | ||
Basourakos SP, Hoffman K, Kim J. Active surveillance in prostate cancer: new efforts, new voices, new hope. BJU Int. 2017;120(1):4–5. | ||
Golla V, Kaplan AL. Testosterone therapy on active surveillance and following definitive treatment for prostate cancer. Curr Urol Rep. 2017;18(7):49. | ||
Babu JR, Jeganathan KB, Baker DJ, Wu X, Kang-Decker N, van Deursen JM. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J Cell Biol. 2003;160(3):341–353. | ||
Jeganathan KB, Malureanu L, van Deursen JM. The Rae1-Nup98 complex prevents aneuploidy by inhibiting securin degradation. Nature. 2005;438(7070):1036–1039. | ||
Rao CV, Sanghera S, Zhang Y, et al. Systemic chromosome instability resulted in colonic transcriptomic changes in metabolic, proliferation, and stem cell regulators in Sgo1−/+ mice. Cancer Res. 2016;76(3):630–642. | ||
Wang LH, Yen CJ, Li TN, Elowe S, Wang WC, Wang LH. Sgo1 is a potential therapeutic target for hepatocellular carcinoma. Oncotarget. 2015;6(4):2023–2033. | ||
Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2016;387(10013):70–82. | ||
Verzijlbergen KF, Nerusheva OO, Kelly D, et al. Shugoshin biases chromosomes for biorientation through condensin recruitment to the pericentromere. Elife. 2014;3:e01374. | ||
Wong WK, Kelly T, Li J, Ma HT, Poon RY. SGO1C is a non-functional isoform of Shugoshin and can disrupt sister chromatid cohesion by interacting with PP2A-B56. Cell Cycle. 2015;14(24):3965–3977. | ||
Pallai R, Bhaskar A, Barnett-Bernodat N, Gallo-Ebert C, Nickels JT Jr, Rice LM. Cancerous inhibitor of protein phosphatase 2A promotes premature chromosome segregation and aneuploidy in prostate cancer cells through association with shugoshin. Tumour Biol. 2015;36(8):6067–6074. | ||
Murakami-Tonami Y, Ikeda H, Yamagishi R, et al. Sgo1 is involved in the DNA damage response in MYCN-amplified neuroblastoma cells. Sci Rep. 2016;6:31615. | ||
Yamada HY, Zhang Y, Reddy A, et al. Tumor-promoting/progressing role of additional chromosome instability in hepatic carcinogenesis in Sgo1 (Shugoshin 1) haploinsufficient mice. Carcinogenesis. 2015;36(4):429–440. | ||
Yamada HY, Kumar G, Zhang Y, et al. Systemic chromosome instability in Shugoshin-1 mice resulted in compromised glutathione pathway, activation of Wnt signaling and defects in immune system in the lung. Oncogenesis. 2016;5(8):e256. | ||
Iwaizumi M, Shinmura K, Mori H, et al. Human Sgo1 downregulation leads to chromosomal instability in colorectal cancer. Gut. 2009;58(2):249–260. | ||
Matsuura S, Kahyo T, Shinmura K, et al. SGOL1 variant B induces abnormal mitosis and resistance to taxane in non-small cell lung cancers. Sci Rep. 2013;3:3012. | ||
Kahyo T, Iwaizumi M, Shinmura K, et al. A novel tumor-derived SGOL1 variant causes abnormal mitosis and unstable chromatid cohesion. Oncogene. 2011;30(44):4453–4463. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.