Back to Journals » Journal of Pain Research » Volume 10
Outcomes of C1–2 joint injections
Authors Aiudi CM, Hooten WM , Sanders RA, Watson JC, Moeschler SM , Gazelka HM , Hoelzer BC, Eldrige JS, Qu W, Lamer TJ
Received 18 June 2017
Accepted for publication 15 August 2017
Published 18 September 2017 Volume 2017:10 Pages 2263—2269
DOI https://doi.org/10.2147/JPR.S144255
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Christopher M Aiudi,1 W Michael Hooten,2 Rebecca A Sanders,2 James C Watson,2 Susan M Moeschler,2 Halena M Gazelka,2 Bryan C Hoelzer,2 Jason S Eldrige,2 Wenchun Qu,3 Tim J Lamer2
1Mayo Clinic School of Medicine, 2Division of Pain Medicine, Department of Anesthesia and Perioperative Medicine, Mayo Clinic, 3Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, MN, USA
Objective: Intra-articular injections of the C1–2 joint are an effective therapeutic option for pain generated from degenerative and inflammatory conditions affecting the joint. Limited information exists about the adverse events (AEs) associated with these injections. The primary aim of this study is to describe the frequency and type of AEs associated with C1–2 joint injections. The secondary aim is to identify clinical factors associated with the occurrence of AEs of C1–2 joint injections.
Design/methods: A retrospective chart review was conducted on all C1–2 joint injections performed at the Mayo Pain Medicine Clinic in Rochester, MN, from January 1, 2005 through July 31, 2015. AE data were extracted from procedural and post-procedural clinical notes. Analysis was conducted to determine correlations between any AE and demographic and clinical characteristics. Using univariate and multivariate logistic regression analyses, associations were determined.
Results: From January 1, 2005 to July 31, 2015, 135 C1–2 injections were performed on 72 patients. Overall, at least 1 AE was reported in 18.5% of the injections. The most common AEs were post-procedural increase in pain and procedural vascular contrast uptake. There was a significant association between AE occurrence and greater pre-procedural maximum pain score.
Conclusions: AEs from C1–2 joint injections occurred commonly, but there were no persistent or serious AEs associated with these injections. The data also demonstrate that patients with higher pre-procedural maximum pain scores are more likely to experience an AE.
Keywords: C1–2 joint, facet, injection, adverse event
Introduction
The atlantoaxial joint, also known as the C1–2 joint, is the major joint that allows for cephalic rotation. The articulation between the atlas and axis is composed of 3 individual joints – 2 lateral atlantoaxial joints and 1 median atlantoaxial joint.1 Being synovial joints, the 2 lateral atlantoaxial joints are subject to degenerative and inflammatory conditions, which may result in neck pain, decrease in range of motion, headaches (i.e., cervicogenic headache), neurogenic pain, or altered sensation from adjacent C2 dorsal root ganglion compression.2–7 Currently, there are limited therapeutic strategies for treating pain originating from the lateral C1–2 joints. Therapeutic options include pharmacological agents, physical therapy, joint injections, and surgery.2–3 Unfortunately, medications and physical therapy are often incompletely effective and surgery is associated with major risks.2–3,8 Intra-articular joint injections have been shown to be beneficial;4–5.9–12 however, the safety of these injections has been questioned.13–16
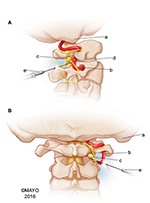
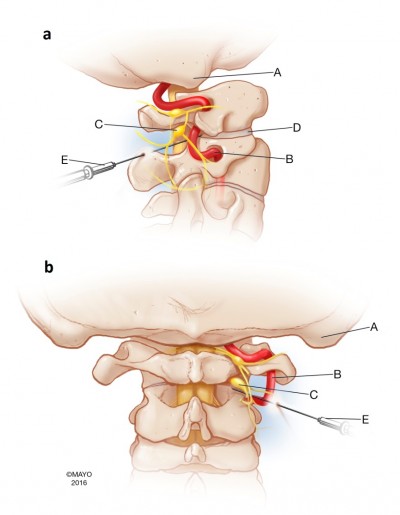
The lateral atlantoaxial joint injection, which will be referred to as simply the C1–2 joint injection, is a highly specialized procedure that should only be performed by a trained interventional spine physician.17 The joint injection is associated with unique risks compared with other vertebral injections due to the proximity of adjacent structures, including the vertebral artery, cervical spinal cord, and the C2 dorsal root ganglion (Figure 1). In addition, there is often variability in the location and trajectories of these structures around the joint.18–19 As a result, these structures can be injured by both the needle used during the procedure as well as the medications that are injected. In fact, reports of significant adverse events (AEs), including stroke leading to coma and death, have been reported with regard to this procedure, causing many to question its safety.13,16 To date, limited information exists about the adverse events associated with these injections. The primary aim of this study is to describe the frequency and types of AEs associated with C1–2 joint injections. The secondary aim is to identify clinical factors associated with the occurrence of AEs of C1–2 joint injections.
Methods
This study was approved by the Mayo Foundation Institutional Review Board. All patients provided prior written consent for use of their medical records for research purposes.
Study participants
A retrospective chart review was conducted on all C1–2 joint injections performed at the Mayo Pain Medicine Clinic in Rochester, MN from January 1, 2005 through July 31, 2015. Inclusion criteria included all patients with head or neck pain who underwent a fluoroscopically guided C1–2 joint injection. Exclusion criteria included head or neck pain attributed to cancer (e.g., metastatic bone disease, multiple myeloma), acute trauma, including C1 or C2 fracture, and coagulopathy. Seventy-two unique patients met the inclusion criteria, and 135 individual C1–2 joint injections were performed on these patients.
Study setting
All injections were performed at the Mayo Pain Medicine Clinic in Rochester, MN. The pain medicine clinic utilizes a multidisciplinary, team-based approach to evaluate, diagnose, and treat a broad range of pain disorders. The physician staff were board certified in pain medicine and represented a broad range specialties, including anesthesiology, physical medicine and rehabilitation, neurology, and psychiatry.
Data collection
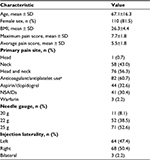
Baseline patient demographic and clinical characteristics were abstracted from electronic medical records and included age, sex, body mass index (BMI), maximum and average daily pain scores during the month prior to the injection, primary location of pain (e.g., head, neck, or both), current use of antiplatelet or anticoagulant medications, and radiographic evidence of morphological changes involving the C1–2 joint (e.g., joint hypertrophy, fluid within the joint, reduced joint space). Procedural details about the C1–2 injections were collected, including laterality (e.g., left, right, bilateral), needle gauge, number of attempts needed to achieve intra-articular needle placement, placement of an intravenous catheter, and use of sedation. Chart review and data abstraction were completed by a single researcher who received training prior to initiation of the review.
AEs were defined a priori and categorized as 1) procedural AEs occurring during or immediately after the injection but prior to patient dismissal from the procedure suite (e.g., paresthesia, seizure, vasovagal response, blood return with needle aspiration, vascular contrast uptake, loss of consciousness, loss of motor or sensory function) or 2) post-procedural AEs occurring after dismissal from the procedure suite (e.g., paresthesia, loss of motor or sensory function, worsening neck or head pain, signs of infection, signs of current or previous bleeding at procedure site, dizziness, vertigo, disturbance of balance). This predefined list of AEs was created by a team of staff interventional pain specialists prior to data collection. The electronic medical record of all patients who sustained any AE was reviewed in an attempt to ascertain the duration of the AE and if further medical evaluation was rendered.
C1–2 Injection description
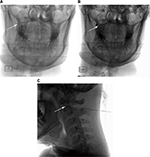
The C1–2 joint injections were performed by pain clinic staff physicians or pain medicine fellows personally supervised by a staff physician. All patients had advanced cervical imaging (CT scan, MRI), which was reviewed by the proceduralist. All injections were performed on the patient in the prone position using a posterior approach with fluoroscopic guidance. The optimal target point, unless individual anatomy dictated otherwise, was the junction of the lateral one-third and the medial two-third of the C1–2 joint. This minimized the likelihood of being too lateral, approaching the territory of the vertebral artery, or being too medial, increasing the risk of epidural or dural entry. Final needle position was confirmed with posteroanterior and lateral fluoroscopic images (Figure 2). Radiopaque contrast was administered under live fluoroscopic imaging and in some cases, digital subtraction angiography was used to enhance contrast visibility. This allowed confirmation of intra-articular needle placement, and the identification of unintentional vascular or neuraxial contrast spread. The injectate consisted of a 1–1.5 mL mixture of local anesthetic and corticosteroid. The local anesthetic consisted of either 1% lidocaine or 0.25% bupivacaine and the volume used was as per the discretion of the procedural physician. Early in the review period, until late 2008, the most common corticosteroid used was triamcinolone. However, as reports of particulate steroid embolization during cervical spine injections accumulated in the literature, procedural recommendations evolved and the corticosteroid was changed to the non-particulate corticosteroid, dexamethasone.20–25
Data analysis
The means and standard deviations were reported for continuous variables, and counts and proportions were recorded for categorical variables. The correlations between any AE, demographic, and clinical characteristics were assessed using Spearman rank correlation. Significant correlations (P<0.05) were then subjected to logistic regression analysis. First, univariate logistic regression analyses were performed using any AE as the dependent variable and the demographic and clinical characteristics as independent variables. Second, multiple variable logistic regression analyses were performed using the same set of dependent and independent variables. The level of significance for all statistical tests was set at P<0.05, and all analyses were completed using SPSS (Version 21.0 IBM Inc.; Chicago, IL, USA).
Results
Demographic and clinical characteristics
The clinical characteristics of the injections are summarized in Table 1. The majority of injections were performed on female patients and the mean BMI was 26.3. The mean maximum and average daily pain scores prior to the injections were 7.7 and 5.5, respectively. The majority of injections (60.7%) were performed on patients using an antiplatelet or anticoagulant medication at the time of the procedure.
Adverse events
Overall, twenty-five injections (18.5%) were associated with an AE. These AEs were categorized into either procedural or post-procedural AEs as previously described (Table 2). Thirteen (9.6%) AEs occurred during or immediately after the injection. The most frequent procedural AE was vascular contrast uptake (n=5; 3.7%) followed by paresthesia (n=3; 2.2%). Four of the 13 injections with procedural AEs were aborted for patient safety. These 4 procedural AEs included recurrent vascular contrast uptake (n=2), extravasation of contrast (n=1), and blood return on aspiration with needle insertion (n=1). All procedural AEs resolved and no long-term patient sequelae were identified. Twelve (8.9%) post-procedural AEs occurred. The most frequently occurring post-procedural AE was increased pain (n=8; 5.9%) No infections or signs of post-procedural bleeding were identified. All post-procedural AEs resolved within 3 months without further medical intervention, and no long-term sequelae were identified.
  | Table 2 Procedural and post-procedural AEs (N=135 injections) Abbreviation: AEs, adverse events. |
Associations between any AE and demographic and clinical characteristics
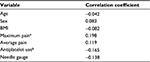
The correlations between any AE and demographic and clinical characteristics are listed in Table 3. A significant positive correlation was observed between the occurrence of any AE and pre-procedural maximum pain score (r=0.198, n=135, P<0.05). In the univariate logistic regression analysis with AE as the dependent variable, greater pre-procedural maximum pain scores were significantly associated (odds ratio [OR], 1.65; 95% CI, 1.04–2.62; P=0.034) with the occurrence of any AE (Table 4). The significant association between AE and greater pre-procedural maximum pain scores was retained (OR, 1.90; 95% CI, 1.05–3.45; P=0.034) in a multiple variable logistic regression model adjusted for age, sex, BMI, average pain score, needle gauge, and the use of antiplatelet medications. Final analysis showed that for every 0.64 increase on the maximum pre-procedure VAS the patient’s odds ratio for experiencing an AE was increased by 1.9.
Discussion
The overall prevalence of AEs in this retrospective study was 18.5%. The most common procedural AE was vascular uptake of contrast and the most common post-procedural AE was increased pain. All post-procedural AEs resolved within 3 months without further medical intervention. In a multivariable logistic regression model with the occurrence of any AE as the dependent variable, the maximum pain score within 1 month prior to the procedure was associated with the occurrence of any AE after adjusting for age, sex, BMI, mean daily pain score, needle gauge, and use of antiplatelet medications.
Although the safety of C1–2 joint injections has been highly scrutinized, limited information exists about AEs associated with the injection.13,16 The major concern with the C1–2 injection is the risk of significant AEs. A previous report regarding an AE of the C1–2 injection described the unfortunate occurrence of a posterior circulation stroke resulting in death.13 This report raised concern regarding the safety of this procedure, even to the extent of calling for the procedure to be abandoned.16 As with all decisions in clinical practice, an evaluation of the risks and benefits associated with this procedure is vital. There have been several studies evaluating the efficacy of the C1–2 joint injections.4–12 In a retrospective study of 32 C1–2 injections, 40.6% of patients had an exacerbation of their headache symptoms during the injection, but 81.2% of patients had a >50% reduction in their pain score after the injection.9 This supports the efficacy of these injections for patients with pain from the C1–2 joint, but the study did not focus on AEs as a primary outcome. Furthermore, the small sample size may not have been large enough to accurately describe any characteristics of the AEs associated with this procedure. In this current study, the occurrence of AEs was the primary outcome. The results support the relative low risk and safe use of injections when performed by trained interventional spine physicians for the treatment of pain from the C1–2 joint.
The multivariable multinomial logistic regression analysis demonstrated a significant association between pre-procedural maximum pain score and the occurrence of any AE. The potential mechanism(s) of this finding are unknown but it may be that greater pre-procedural maximum pain score could represent worse C1–2 joint pathology thus increasing the complexity and risks associated with needle placement and/or medication injection. Another possible explanation for increased procedural and post-procedural pain-related AEs in patients with higher pre-procedural pain scores is that chronic C1–2 joint pain may lead to the development of central sensitization in some patients. Further studies on C1–2 joint injections are needed to evaluate the impact of pain sensitivity and psychological variables as potential predictors for AEs. Finally, patients with higher pre-procedural pain scores may have more procedure-related anxiety/apprehension, increasing the risk to experience an AE such as a vasovagal response.
The results of this study have several clinical implications. First, relatively few AEs resulted in patient harm. Most procedural AEs had no untoward patient effects. For example, patients were unaware of procedural blood return during needle aspiration since this procedural occurrence did not cause symptoms or patient discomfort. While many would consider this a near-AE since no patient harm occurred, it was still considered an AE in this study since there was potential for patient harm and the occurrence of these procedural AEs identified times when a significant AE could have occurred if not properly managed. Some procedural AEs did have negative effects on patients, such as temporary paresthesia, vasovagal response, and increased pain. While these AEs did occur, they did not result in significant patient harm due to close patient monitoring in the immediate post-procedural period and appropriate response of trained providers who quickly ameliorated these effects. This demonstrates the importance of protocols for providers and ancillary staff when dealing with peri-procedural events. For example, an IV line was placed in 92.6% (n=125) of patients. This was done as a safety measure in case of a major AE. While most post-procedural AEs spontaneously resolved, some required conservative interventions to alleviate the symptoms. These interventions included physical therapy, heat/ice therapy, massage therapy, and further injections. Notably, all post-procedural AEs resolved within 3 months of the initial injection. All of these findings support the idea that a multimodality treatment plan is necessary to optimally treat pain from the C1–2 joint.2,3
Limitations
This study had several limitations, including the retrospective observational nature of the study design, the study being conducted at a single institution, and an inability to control for external confounders. The retrospective nature of this study relied on accurate recordkeeping of AE assessments and treatment plans. At the time of AE reporting, there was no standardized protocol for what constituted an AE. To account for this, a predefined list of AEs was created to evaluate during the review. The lists were created by a team of interventional pain specialists prior to data collection. While the lists captured all AEs found in the study, there is a possibility that an AE happened in a patient that data analysts did not consider since it was not part of the predefined list. To account for this, a category of “other” was included in the predefined list. Because this study was conducted at a single tertiary center with institution-specific protocols, the outcomes may not represent the broader scope of procedural locations. Finally, this study was unable to control for other interventions patients may have sought prior to follow-up appointments, such as manual maneuvers or physical therapy, which could have treated or caused a post-procedural AE.
Conclusion
This retrospective chart review is the largest study evaluating the frequency and characteristics of AEs associated with C1–2 joint injections. The results demonstrate that AEs from C1–2 joint injections occurred commonly during and after the procedure, but there were no persistent or serious health/life-threatening AEs associated with these injections. As such, the data demonstrate the relative safety of these injections. The data also demonstrate an association between pre-procedural maximum pain scores and the occurrence of AEs. This study should provide clinicians with more guidance as to the risks associated with this procedure. Although this study was foundational in describing the AEs of the C1–2 joint injection procedure, further prospective studies are needed to more accurately analyze risk factors associated with and occurrence of the AEs.
Acknowledgments
There are no funding sources for this research or manuscript. An abstract of this manuscript was presented at the 15th Annual Pain Medicine Meeting, 2016, San Diego, CA, USA.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Bogduk N, Mercer S. Biomechanics of the cervical spine. I: normal kinematics. Clin Biomech (Bristol Avon). 2000;15(9):633–648. | ||
Bogduk N, Govind J. Cerivcogenic headache: as assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 2009;8(10):959–968. | ||
Haldeman S, Dagenais S. Cervicogenic headache: a critical review. Spine J. 2001;1(1):31–46. | ||
Aprill C, Axinn M, Bogduk N. Occipital headaches stemming from the lateral atlanto-axial (C1–2) joint. Cephalalgia. 2002;22(1):15–22. | ||
Busch E, Wilson P. Atlanto-occipital and atlanto-axial injections in the treatment of headache and neck pain. Reg Anesth. 1989;14:45. | ||
Naja Z, El-Rajab M, Al-Tannir M, Ziade F, Tawfik O. Repetitive occipital nerve blockade for cervicogenic headache: expanded case report of 47 adults. Pain Pract. 2006;6(4):278–284. | ||
Dreyfuss P, Michaelsen M, Fletcher D. Atlantooccipital and lateral atlanto-axial joint pain patterns. Spine (Phila Pa 1976). 1994;19(10):1125–1131. | ||
Jansen J, Sjaastad O. Cervicogenic headache: long-term prognosis after neck surgery. Acta Neurol Scand. 2007;115(3):185–191. | ||
Narouze SN, Casanova J, Mekhail N. The longitudinal effectiveness of lateral atlantoaxial intra-articular steroid injection in the treatment of cervicogenic headache. Pain Med. 2007;8(2):184–188. | ||
Zhou L, Hud-Shakoor Z, Hennessey C, Ashkenazi A. Upper cervical facet joint and spinal rami blocks for the treatment of cervicogenic headache. Headache. 2010;50(4):657–663. | ||
Glemarec J, Guillot P, Laborie Y, Berthelot J, Prost A, Maugars Y. Intraarticular glucocorticosteroid injection into the lateral atlantoaxial joint under fluoroscopic control. A retrospective comparative study in patients with mechanical and inflammatory disorders. Joint Bone Spine. 2000;67(1):54–61. | ||
Chevrot A, Cermakova E, Vallee C, et al. C1–2 arthrography. Skelet Radiol. 1995;24(6):425–429. | ||
Edlow BL, Wainger BJ, Frosch MP, Copen WA, Rathmell JP, Rost NS. Posterior circulation stroke after C1–C2 intraarticular facet steroid injection: evidence for diffuse microvascular injury. Anesthesiology. 2010;112(6):1532–1535. | ||
Bush K, Hillier S. Outcome of cervical radiculopathy treated with periradicular/epidural corticosteroid injections: a prospective study with independent clinical review. Eur Spine J. 1996;5(5):319–25. | ||
Scanlon GC, Moeller-Bertram T, Romanowsky SM, Wallace MS. Cervical transforaminal epidural steroid injections: more dangerous than we think? Spine (Phila Pa 1976). 2007;32(11):1249–1256. | ||
Datta S, Manchikanti L. It is time to abandon atlanto-axial joint injections: do no harm. Anesthesiology. 2011;114(1):222–224. | ||
Bogduk, N, editors. Practice Guidelines for Spinal Diagnostic and Treatment Procedures: 2nd Edition. Hinsdale, IL: International Spine Intervention Society (ISIS); 2013. | ||
Lau SW, Sun LK, Lai R, et al. Study of the anatomical variations of vertebral artery in C2 vertebra with magnetic resonance imaging and its application in the C1-C2 transarticular screw fixation. Spine (Phila Pa 1976). 2010;35(11):1136–1143. | ||
Igarashi T, Kikuchi S, Sato K, Kayama S, Otani K. Anatomic study of the axis for surgical planning of transarticular screw fixation. Clin Orthop Relat Res. 2003;408:162–166. | ||
Huntoon MA. Anatomy of the cervical intervertebral foramina: vulnerable arteries and ischemic neurologic injuries after transforaminal epidural injections. Pain. 2005;117(1–2):104–111. | ||
Huntoon M. Cervical spine: case presentation, complications, and their prevention. Pain Med. 2008;9(1):S35–S40. | ||
Tiso RL, Cutler T, Catania JA, Whalen K. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004;4(4):468–474. | ||
Benzon HT, Chew TL, McCarthy RJ, Benzon HA, Walega DR. Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology. 2007;106(2):331–338. | ||
Dreyfuss P, Baker R, Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and non-particulate corticosteroid preparations for cervical radicular pain. Pain Med. 2006;7(3):237–242. | ||
Rathmell JP, Benzon HT, Dreyfuss P, et al. Safeguards to prevent neurologic complications after epidural steroid injections: consensus opinions from a multidisciplinary working group and national organizations. Anesthesiology. 2015;122(5):974–984. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.