Back to Journals » Clinical Ophthalmology » Volume 16
Outcomes of a Refractive Segmented Bifocal Intraocular Lens with a Lower Near Addition
Authors Venter JA, Collins BM, Hannan SJ, Teenan D , Schallhorn JM
Received 26 May 2022
Accepted for publication 29 July 2022
Published 10 August 2022 Volume 2022:16 Pages 2531—2543
DOI https://doi.org/10.2147/OPTH.S376323
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jan A Venter,1,2 Barrie M Collins,1 Stephen J Hannan,1 David Teenan,1 Julie M Schallhorn3,4
1Optical Express, Glasgow, UK; 2Eye and Laser Institute, Port Elizabeth, South Africa; 3University of California, San Francisco, Department of Ophthalmology, San Francisco, CA, USA; 4F.I. Proctor Foundation, University of California, San Francisco, CA, USA
Correspondence: Jan A Venter, Eye and Laser Institute, 205 Cape Road, Newton Park, Port Elizabeth, South Africa, Tel +44 7713 480975, Email [email protected]
Purpose: To evaluate clinical and subjective outcomes of a segmented bifocal IOL with a 2.0 D near addition.
Patients and Methods: Retrospective analyses of patients who had undergone refractive lens exchange with bilateral implantation of the SBL-2 IOL (Lenstec, Inc., Christ Church, Barbados) were performed. The number of patients included in the study was 389 (778 eyes). Refractive, visual and patient-reported outcomes were presented for the last available visit (mean follow-up 2.05 ± 1.33 months).
Results: The percentage of eyes within ± 0.50D and ± 1.00D of emmetropia was 82.5% (642/778) and 97.8% (761/778), respectively. The mean uncorrected intermediate visual acuity (66 cm) of the last available visit was 0.08 ± 0.15 logMAR monocularly and 0.04 ± 0.14 logMAR binocularly. The mean monocular and binocular uncorrected near visual acuity (40 cm) were 0.30 ± 0.15 logMAR and 0.24 ± 0.14 logMAR, respectively. Of all patients, 97.2% (378/389) claimed never to use any correction for distance vision, while 93.1% (362/389) of patients did not require any correction for near vision. The mean scores for visual phenomena (on the scale from 1 – no difficulty to 7 – severe difficulty) were 1.8 ± 1.3, 1.7 ± 1.2, 1.7 ± 1.2 and 1.6 ± 1.2 for glare, halo, starburst, and ghosting/double vision, respectively.
Conclusion: Despite the lower near addition of SBL-2 segmented bifocal IOL, patients achieved reasonable rates of spectacle independence and a low incidence of visual phenomena.
Keywords: segmented bifocal intraocular lenses, low near addition intraocular lenses, presbyopia, refractive lens exchange
Introduction
Multifocal intraocular lenses (IOLs) enjoy increasing popularity among presbyopic patients.1 It is important that premium IOLs provide good vision for distance and a wide range of intermediate and near distances, while maintaining high contrast sensitivity and low visual phenomena. Achieving this goal is the biggest challenge IOL manufacturers currently face. Various multifocal lenses are currently available on the market and can be divided into three distinct types – diffractive, refractive, or a combination of the two.2 Furthermore, the optics of an IOL can be rotationally symmetric (consisting of concentric rings) or rotationally asymmetric (segmented zones with a superior segment for distance vision and an inferior segment with near addition). All diffractive IOLs and most refractive IOLs are rotationally symmetric, while some refractive IOLs are rotationally asymmetric.2 The IOLs of different manufacturers vary in many other features, such as the asphericity, strength of the near addition, or the number of focal points (bifocal or trifocal IOLs).
Changing lifestyles have generated different expectations and an emphasis upon good intermediate vision (desktop computers, tablets, mobile phones for example). In clinical practice, this has been achieved by extending the depth of focus (eg EDOF technology),3,4 the introduction of trifocal lenses,5,6 or simply by lowering the near addition of existing high-add multifocal IOLs.7 The availability of lenses with various reading strengths allows surgeons to customize the lens selection to suit patient’s lifestyle needs.
From the wide choice of multifocal IOL designs, asymmetric segmented bifocal IOLs have undergone tremendous development over the last decade.8 In our initial experience with the segmented bifocal SBL-3 IOL (Lenstec, Inc., Christ Church, Barbados), we reported good restoration of near vision with a relatively smooth defocus curve.9 Further studies corroborated our findings,10–14 and the IOL is currently undergoing an FDA clinical trial in the United States. Recently, the manufacturer released a new IOL based on the same platform with a lower near addition (SBL-2). To our knowledge, there are no published studies reporting the outcomes and spectacle independence achieved with this IOL. The aim of this study is to present early clinical and patient-reported outcomes of this novel intraocular lens.
Patients and Methods
A retrospective review of patients who had undergone a refractive lens exchange with the Lenstec SBL-2 (Lenstec, Inc., Christ Church, Barbados) segmented bifocal intraocular lens was conducted. The study was deemed exempt from full review by the Committee on Human Research at the University of California, San Francisco, because it used only retrospective, de-identified patient data. Patients were well informed about the procedure, the type of implanted IOL and signed a consent form. All participants also consented to the use of their de-identified and anonymized data for statistical analysis and research purposes.
The study patients were treated between September 2020 and June 2021 by four surgeons in 12 surgical centers. Inclusion criteria were the presence of presbyopia and/or mild cataract (with corrected distance visual acuity 20/40 or better), bilateral implantation of Lenstec SBL-2 IOL, attendance of at least one-month postoperative exam, and a completed patient experience questionnaire (PEQ). Patients with significant ocular pathology or previous laser vision correction were excluded. The data from the last available visit of each patient was used in all analyses.
Prior to surgery, patients underwent a detailed ophthalmic examination consisting of visual acuities, manifest refraction, slit lamp examination, dilated fundus examination, biometry, wavefront aberrometry, endothelial cell count, corneal topography and retinal optical coherence tomography.
Visual acuity measurements consisted of corrected distance (CDVA), uncorrected distance (UDVA), uncorrected near (UNVA), and uncorrected intermediate (UIVA) visual acuities. Near and intermediate visual acuities were measured with an early treatment diabetic retinopathy study (ETDRS) chart at 40 cm and 66 cm, respectively.
On post-operative visits, patients were asked to complete a patient experience questionnaire. The questionnaire assessed the overall satisfaction with vision, as well as patient’s functional vision when performing a variety of tasks. The difficulty with visual phenomena (glare, halo, starburst, and ghosting/double vision) was rated on a 7-point discrete scale from no difficulty to severe difficulty. The frequency of glasses or contact lens use was assessed on a 5-point scale (never use glasses or contact lenses, up to 25% of the time, 25% to 50% of the time, 50% to 75% of the time, and 75% to 100% of waking hours).
Intraocular Lens and Surgical Technique
The SBL-2 is a segmented bifocal intraocular lens (Figure 1). The IOL combines a distance sector and a surface-embedded 2.00 D near addition sector positioned in the anterior optic of the IOL. It is a hydrophilic acrylic IOL (26% water content) with a neutral aberration profile, 5.75 mm optic diameter, and an 11.00 mm overall length.
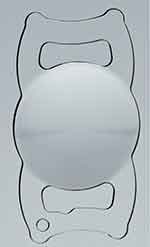 |
Figure 1 SBL-2 intraocular lens. |
All surgeries were performed under topical anesthesia using the standard phacoemulsification technique. A 2.75 mm clear corneal incision was created and typically placed on the steepest meridian to neutralize existing corneal astigmatism. In patients with corneal astigmatism less than 0.50, the incision was placed either superiorly or temporally, according to the surgeon’s preference. The corneal astigmatism used in surgical planning was determined by IOLMaster (IOLMaster 700, Carl Zeiss Meditec AG, Jena, Germany).
The typical size of anterior capsulorhexis was 5.00 to 6.00 mm. The SBL-2 IOL was inserted into the capsular bag with the near addition segment positioned inferiorly and slightly nasally. Postoperative medications included topical antibiotics (levofloxacin 5 mg/mL, four times/day) and steroidal anti-inflammatory drugs (dexamethasone 0.1%, four times/day) for 2 weeks.
All lens calculations were performed with IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany) utilizing the Barrett Universal II formula.
Statistical Analysis
The outcomes were presented with the standard graphs required to report the outcomes of refractive surgery with an addition of cumulative UIVA and UNVA. The normality of the data was assessed with the Kolmogorov–Smirnov test. Preoperative to postoperative change in continuous variables was calculated with the paired t-test for normally distributed data and with the Wilcoxon signed-rank test for non-parametric data. The responses to questionnaire items were presented as percentages. Statistical analysis was performed with Microsoft Excel software (Microsoft Corp.).
Results
The number of eyes included in this study was 778 (389 patients). The mean follow-up of the study group was 2.05 ± 1.33 months. Of all patients, 51.4% (200/389) were female, and 48.6% (189/389) were male. The preoperative age ranged between 36 and 85 years (median 55 years, mean 55.6 ± 6.9 years). Table 1 summarizes the main preoperative and postoperative clinical variables of the study cohort.
 |
Table 1 Preoperative and Postoperative Clinical Data (778 Eyes of 389 Patients) |
Refractive Outcomes
There was a wide range or refractive errors included in the cohort, with the preoperative sphere ranging between −10.5D and +7.50D (Table 1). Figure 2 depicts the distribution of postoperative manifest spherical equivalent (MSE). The percentage of eyes within ±0.50D and ±1.00D of the intended target was 82.5% (642 eyes of 778) and 97.8% (761/778), respectively. The mean manifest spherical equivalent of the last available visit was −0.13 ± 0.42D (range: −2.25D, +1.38D). A scatter plot of attempted vs achieved manifest spherical equivalent is shown in Figure 3.
 |
Figure 2 Refractive outcomes (D – diopter). |
 |
Figure 3 Predictability of manifest spherical equivalent (D – diopter). |
The mean refractive cylinder changed from the preoperative value of −0.53 ± 0.38D (range: 0.00D to −2.50) to −0.36 ± 0.37D (range: 0.00D to −2.50) at the last available visit (p<0.01). Figure 4 depicts the distribution of preoperative and postoperative refractive astigmatism. At the last available visit, 78.8% (613/778), 91.3% (710/778) and 96.4% (750/778) of eyes had the absolute postoperative refractive astigmatism ≤0.50D, ≤0.75D and ≤1.00D, respectively.
 |
Figure 4 The distribution of preoperative and postoperative refractive astigmatism (D – diopter). |
Visual Outcomes
Figure 5 depicts postoperative monocular and binocular UDVA compared to preoperative monocular CDVA. Of all eyes, 70.6% (549/778) and 98.2% (764/778) achieved postoperative monocular UDVA of 20/20 and 20/40 or better, respectively. The percentage of patients with binocular UDVA 20/20 and 20/40 or better was 89.7% (349/389) and 99.7% (388/389).
 |
Figure 5 Postoperative monocular and binocular uncorrected distance visual acuity (UDVA) compared to preoperative monocular corrected distance visual acuity (CDVA). |
Figure 6 displays the difference between preoperative CDVA and postoperative UDVA. Of all eyes, 75.3% (586/778) had postoperative monocular UDVA within one line of their preoperative CDVA.
 |
Figure 6 The difference between preoperative corrected distance visual acuity (CDVA) and postoperative uncorrected distance visual acuity (UDVA). |
Intermediate visual acuity is shown in Figure 7. Monocular UDVA of 20/32 or better was achieved in 88.6% (689/778) of eyes, while the same level of binocular UIVA was achieved in 93.8% (365/389) of patients. The mean UIVA of the last available visit was 0.08 ± 0.15 logMAR (≈20/25+1) monocularly and 0.04 ± 0.14 logMAR (≈20/20−2) binocularly.
 |
Figure 7 Postoperative monocular and binocular uncorrected intermediate visual acuity (UIVA). |
Figure 8 depicts cumulative uncorrected near visual acuity. UNVA of 20/40 or better was recorded in 67.2% (523/778) of eyes monocularly and 79.9% (311/389) of patients binocularly. The mean monocular and binocular UNVA were 0.30 ± 0.15 logMAR (≈20/40) and 0.24 ± 0.14 logMAR (≈20/30−2), respectively.
 |
Figure 8 Postoperative monocular and binocular uncorrected near visual acuity (UNVA). |
Safety (the change between preoperative and postoperative CDVA) is shown in Figure 9. Of all eyes, only one eye (0.1%) had CDVA reduced by more than 2 lines at the last available visit, which was due to cystoid macular edema.
 |
Figure 9 Change in preoperative to postoperative corrected distance visual acuity (CDVA). |
Patient Experience Questionnaire
Table 2 shows a summary of outcomes from the postoperative questionnaire. Of all patients, 89.5% (348/389) were “very satisfied” or “satisfied” with their postoperative vision, and 96.4% (375/389) would recommend the procedure to their friends or family. Furthermore, 91.3% (355/389) of patients reported they were more satisfied with their vision post-surgery compared to their vision with contact lenses or glasses before surgery.
 |
Table 2 Patient Experience Questionnaire Outcomes |
The percentage of patients having a lot of difficulty or unable to drive because of their vision was 3.3% (13/389), while 83.3% (324/389) had no difficulty or only a little difficulty with night driving.
When questioned about the difficulty with a variety of tasks that include near or intermediate vision (eg, cooking, fixing things around the house, sewing, using hand tools, reading or working with a computer), 11 patients reported a lot of difficulty (incidence 2.8%, 11/389) and only 1 patient (incidence 0.3%, 1/389) claimed to be unable to do these tasks because of the vision. The majority of patients (92.5%, 360/389) had no difficulty or only a little difficulty performing near vision tasks.
Overall, the patients scored very well in the tasks requiring good distance vision (eg, sport or outdoor activities). As little as 0.6% (2/389) of patients claimed to have a lot of difficulty or were unable to do these tasks because of their vision.
When questioned about the postoperative use of glasses or contact lenses, 97.2% (378/389) of patients claimed not to use any correction for distance vision, while 93.1% (362/389) of patients did not require any correction for near vision tasks. The questions about spectacles/contact lens usage were combined to calculate the percentage of patients who achieved total spectacle independence. Of all patients, 92.0% (358/389) claimed not to use any correction for either distance or near vision.
Figure 10 depicts the distribution of the scores for postoperative visual phenomena, rated on the scale from 1 to 7 (no difficulty to severe difficulty). The percentage of patients reporting no visual phenomena or very mild symptoms (combined score 1 and 2) was 77.4% (301/389), 80.2% (312/389), 78.1% (304/389), 85.3% (332/389) for glare, halo, starburst, and ghosting/double vision, respectively. An equal proportion of patients (1.8% or 7/389) experienced significant glare, halo, or starburst (combined score 6 and 7), while 3.3% (13/389) reported significant ghosting or double vision. The mean scores for visual phenomena were 1.8 ± 1.3, 1.7 ± 1.2, 1.7 ± 1.2 and 1.6 ± 1.2 for glare, halo, starburst and ghosting/double vision, respectively.
 |
Figure 10 Postoperative visual phenomena difficulty. |
Discussion
Segmented refractive bifocal IOLs with an inferior near addition have been successfully used in presbyopic patients undergoing cataract surgery.8 The first models to enter the market were the IOLs from the Lentis Mplus range, followed by the later release of the Lentis Comfort range (Teleon Surgical B.V., Spankeren, Netherlands; formerly manufactured by Oculentis GmbH, Berlin, Germany). These IOLs are available in near additions of 3.00D, 2.00D, and 1.50D. Despite some issues with the biocompatibility of these IOLs,15 the optical principle of rotational asymmetry has been well-tolerated in patients, and their advantages and shortcomings have been described in the literature in detail.8,16
Lenstec (Lenstec, Inc., Christ Church, Barbados) is the second manufacturer to introduce an asymmetric segmented bifocal IOL into the market, and their current portfolio consists of the SBL-3 IOL (3.00D near addition) and SBL-2 IOL (2.00D near addition). Although the intraocular lenses of the two manufacturers seem to have a very similar optic design, there are some distinct differences. Intraocular lenses from the Lentis Mplus range have posterior sector-shaped near vision segment, while the SBL IOLs have the near-vision segment in the anterior optic of the IOL. The near vision segment of SBL IOLs extends closer to the periphery of the IOL optic, a feature that can, in theory, result in better near vision and less visual phenomena.14 The configuration of the near segment of SBL IOLs also seems to be different as the segment covers a larger surface of the IOL and extends closer to the central part of the optic.
To our knowledge, there are two studies directly comparing the outcomes of segmented refractive bifocal IOLs of these two manufacturers.10,12 A retrospective study by McNeely et al10 compared the asymmetric multifocal IOLs with variations in near segment placements and near additions. Authors concluded that bilateral implantation of a 3.00D near add IOL (Lentis Mplus LS-312 MF30 in one group and SBL-3 in the second group) with the reading segment in inferonasal position resulted in similar outcomes. However, the outcomes were compared with the third group of patients having a low near add IOL in the dominant eye (Lentis Mplus LS-312 MF20) with a supertemporal position of the near segment and an inferonasally-placed higher addition IOL (SBL-3) in the non-dominant eye. The third group had similar visual acuity outcomes but better scores for quality of vision. The same authors later published a prospective study comparing bilateral implantation of Lentis Mplus LS-312 MF30 and bilateral implantation of SBL-3. However, in this second study, authors found slightly superior outcomes for near vision and spectacle independence for the SBL-3 IOL, despite the same near addition of the two IOLs.12
As expected, the UNVA reported in this study was approximately 1 to 2 Snellen lines worse compared with the outcomes achieved with SBL-3 IOL.10–14 Nevertheless, the majority of patients achieved good functional near visual acuity, with 79.9% of participants having 20/40 or better binocular UNVA. On the other hand, the achieved binocular intermediate visual acuity was excellent with the mean value close to 20/20 (0.04 ± 0.14 logMAR). This outcome is difficult to compare with the performance of the SBL-3 IOL, because the intermediate vision reported in the literature has typically been measured further away (70 to 80 cm,9–14 as opposed to 66 cm in the current study). Also, there is a large variation of reported UIVA outcomes (between 0.10 and 0.41logMAR9–14) for the SBL-3 IOL, possibly attributed to the differences in testing methodology and reading charts. Defocus curve testing would provide more information about intermediate VA performance and should be pursued in further studies.
The total spectacle independence (92.0%) was above the rate generally expected with premium multifocal IOLs.17,18 This outcome is surprising for an IOL with a lower near addition. A possible explanation is that SBL-2 IOL has been specifically selected by patients who have a stronger preference for intermediate vision. Additionally, the patients included in this study had very good preoperative corrected vision and little or no ocular pathology. This could result in better overall visual and refractive outcomes and subsequent increased spectacle independence.
It is claimed that segmented bifocal intraocular lenses reduce the incidence of optical side effects by incorporating only two refractive zones into their design. Fewer transition zones between different powers should lead to less energy loss and improved vision quality and contrast sensitivity.8 On the other hand, the asymmetric sectorial design of these lenses is more sensitive to tilt or decentration, which means that malpositioning of the IOL results in poorer visual quality.19,20 In the current study, very few patients reported significant optical side effects. The mean scores for visual phenomena (measured on a scale from 1 to 7), were on average 1 to 1.5 score better than those of our initial experience with the SBL-3 IOL.9 This suggests that lower near addition might result in less photic phenomena, however, this claim would need to be verified in a matched comparison of the two IOL types. Interestingly, of all questioned side effects, ghosting or double vision had the lowest score, a notable finding given the widespread perception of increased coma aberrations for segmented multifocal IOLs.13,14 However, it has been postulated that the amount of measured coma may be due the limitations of commercially available wavefront sensors in patients with asymmetric IOLs.21
Our study has some limitations that need to be acknowledged. The most important is the retrospective design and a relatively short follow-up. A longer follow-up is needed to assess refractive stability and the impact of neuroadaptation on subjective outcomes. Furthermore, given the retrospective nature of this study, selection bias could not be excluded. A low near addition IOL could have been implanted specifically in patients with certain visual requirements, which could have affected some of the patient-reported outcomes. In addition, some aspects that might affect the performance of the IOL need to be evaluated further, including the role of pupil size or angle kappa. Consideration of angle kappa in asymmetric segmented bifocal IOLs is a frequently discussed topic.22–24 Yet, customized angle kappa implantation showed little impact on outcomes of the SBL-3 IOL,25 the predecessor of the IOL evaluated in the current study. However, another small study found that pupil size might be of some relevance for this type of intraocular lens, and these findings deserve further investigation.26 Another limitation of the study is the use of a non-validated questionnaire. However, the questions are derived from a well-established PROWL27 questionnaire and have been successfully used in other large population analyses of refractive surgery outcomes.28,29
In conclusion, the evaluated segmented bifocal IOL successfully restored distance and near vision in presbyopic patients with a low incidence of severe visual side effects. The reported spectacle independence was surprisingly high for an IOL with a lower near addition, an outcome that possibly highlights the growing importance of good intermediate vision among presbyopic patients. The reported subjective outcomes presented in this study can help to set realistic expectations during the preoperative informed consent process of refractive lens exchange candidates. Due to the limitations of the study, such as its retrospective nature, relatively short follow-up, and the use of a non-validated questionnaire, the outcomes need to be further verified in prospective studies, ideally in direct comparison to other multifocal IOLs on the market. However, the large number of patients included in the analysis and the real-world clinical settings of the study should mitigate some of the limitations of this retrospective cohort.
Disclosure
Julie Schallhorn, MD, is a consultant for Carl Zeiss Meditec, Vanda Pharmaceuticals, and Allergan. She holds stock options in Long Bridge Medical and Octavia. None of the other authors have a financial or proprietary interest in the products and materials presented in this paper.
References
1. Alio JL, Plaza-Puche AB, Fernandez-Buenaga R, Pikkel J, Maldonado M. Multifocal intraocular lenses: an overview. Surv Ophthalmol. 2017;62(5):611–634. doi:10.1016/j.survophthal.2017.03.005
2. Salerno LC, Tiveron MC, Alio JL. Multifocal intraocular lenses: types, outcomes, complications and how to solve them. Taiwan J Ophthalmol. 2017;7(4):179–184. doi:10.4103/tjo.tjo_19_17
3. Rocha KM. Extended depth of focus IOLs: the next chapter in refractive technology? J Refract Surg. 2017;33(3):146–149. doi:10.3928/1081597x-20170217-01
4. Akella SS, Juthani VV. Extended depth of focus intraocular lenses for presbyopia. Curr Opin Ophthalmol. 2018;29(4):318–322. doi:10.1097/icu.0000000000000490
5. Xu Z, Cao D, Chen X, Wu S, Wang X, Wu Q. Comparison of clinical performance between trifocal and bifocal intraocular lenses: a meta-analysis. PLoS One. 2017;12(10):e0186522. doi:10.1371/journal.pone.0186522
6. Jin S, Friedman DS, Cao K, et al. Comparison of postoperative visual performance between bifocal and trifocal intraocular Lens based on randomized controlled trails: a meta-analysis. BMC Ophthalmol. 2019;19(1):78. doi:10.1186/s12886-019-1078-1
7. Kim KH, Kim WS. Visual outcome and patient satisfaction of low-power-added multifocal intraocular lens. Eye Contact Lens. 2018;44(1):60–67. doi:10.1097/ICL.0000000000000314
8. Moore JE, McNeely RN, Pazo EE, Moore TC. Rotationally asymmetric multifocal intraocular lenses: preoperative considerations and postoperative outcomes. Curr Opin Ophthalmol. 2017;28(1):9–15. doi:10.1097/ICU.0000000000000339
9. Venter JA, Barclay D, Pelouskova M, Bull CE. Initial experience with a new refractive rotationally asymmetric multifocal intraocular lens. J Refract Surg. 2014;30(11):770–776. doi:10.3928/1081597X-20141021-09
10. McNeely RN, Pazo E, Spence A, et al. Comparison of the visual performance and quality of vision with combined symmetrical inferonasal near addition versus inferonasal and superotemporal placement of rotationally asymmetric refractive multifocal intraocular lenses. J Cataract Refract Surg. 2016;42(12):1721–1729. doi:10.1016/j.jcrs.2016.10.016
11. McNeely RN, Pazo E, Spence A, et al. Visual outcomes and patient satisfaction 3 and 12 months after implantation of a refractive rotationally asymmetric multifocal intraocular lens. J Cataract Refract Surg. 2017;43(5):633–638. doi:10.1016/j.jcrs.2017.01.025
12. McNeely RN, Pazo E, Spence A, et al. Visual quality and performance comparison between 2 refractive rotationally asymmetric multifocal intraocular lenses. J Cataract Refract Surg. 2017;43(8):1020–1026. doi:10.1016/j.jcrs.2017.05.039
13. Wang X, Tu H, Wang Y. Comparative analysis of visual performance and optical quality with a rotationally asymmetric multifocal intraocular lens and an apodized diffractive multifocal intraocular lens. J Ophthalmol. 2020;2020:7923045. doi:10.1155/2020/7923045
14. Lian H, Ma W, Wei Q, Yuan X. A comparative study on early vision quality after implantation of refractive segmental and diffractive multifocal intraocular lens. Pak J Med Sci. 2020;36(7):1607–1612. doi:10.12669/pjms.36.7.3364
15. Yildirim TM, Labuz G, Khoramnia R, et al. Impact of primary calcification in segmented refractive bifocal intraocular lenses on optical performance including straylight. J Refract Surg. 2020;36(1):20–27. doi:10.3928/1081597X-20191119-01
16. Venter JA, Pelouskova M, Collins BM, Schallhorn SC, Hannan SJ. Visual outcomes and patient satisfaction in 9366 eyes using a refractive segmented multifocal intraocular lens. J Cataract Refract Surg. 2013;39(10):1477–1484. doi:10.1016/j.jcrs.2013.03.035
17. Zvornicanin J, Zvornicanin E. Premium intraocular lenses: the past, present and future. J Current Ophthalmol. 2018;30(4):287–296. doi:10.1016/j.joco.2018.04.003
18. Rosen E, Alio JL, Dick HB, Dell S, Slade S. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: metaanalysis of peer-reviewed publications. J Cataract Refract Surg. 2016;42(2):310–328. doi:10.1016/j.jcrs.2016.01.014
19. Liu X, Xie L, Huang Y. Effects of decentration and tilt at different orientations on the optical performance of a rotationally asymmetric multifocal intraocular lens. J Cataract Refract Surg. 2019;45(4):507–514. doi:10.1016/j.jcrs.2018.10.045
20. Pazo EE, Richoz O, McNeely R, Millar ZA, Moore TC, Moore JE. Optimized visual outcome after asymmetrical multifocal iol rotation. J Refract Surg. 2016;32(7):494–496. doi:10.3928/1081597X-20160503-01
21. Akondi V, Perez-Merino P, Martinez-Enriquez E, et al. Evaluation of the true wavefront aberrations in eyes implanted with a rotationally asymmetric multifocal intraocular lens. J Refract Surg. 2017;33(4):257–265. doi:10.3928/1081597X-20161206-03
22. Bonaque-Gonzalez S, Jaskulski MT, Carmona-Ballester D, Pareja-Rios A, Trujillo-Sevilla JM. Influence of angle Kappa on the optimal intraocular orientation of asymmetric multifocal intraocular lenses. J Optom. 2021;14(1):78–85. doi:10.1016/j.optom.2020.07.004
23. Tchah H, Nam K, Yoo A. Predictive factors for photic phenomena after refractive, rotationally asymmetric, multifocal intraocular lens implantation. Int J Ophthalmol. 2017;10(2):241–245. doi:10.18240/ijo.2017.02.10
24. Kim JW, Eom Y, Chung HW, et al. Factors for good near and distance visual outcomes of multifocal intraocular lens with inferior segmental near add. Graefes Arch Clin Exp Ophthalmol. 2020;258(8):1735–1743. doi:10.1007/s00417-020-04761-1
25. Liu Y, Gao Y, Liu R, et al. Influence of angle kappa-customized implantation of rotationally asymmetric multifocal intraocular lens on visual quality and patient satisfaction. Acta Ophthalmol. 2020;98(6):e734–e742. doi:10.1111/aos.14356
26. Pazo EE, McNeely RN, Richoz O, Nesbit MA, Moore TCB, Moore JE. Pupil influence on the quality of vision in rotationally asymmetric multifocal IOLs with surface-embedded near segment. J Cataract Refract Surg. 2017;43(11):1420–1429. doi:10.1016/j.jcrs.2017.08.013
27. Eydelman M, Hilmantel G, Tarver ME, et al. Symptoms and satisfaction of patients in the patient-reported outcomes with laser in situ keratomileusis (PROWL) studies. JAMA Ophthalmol. 2017;135(1):13–22. doi:10.1001/jamaophthalmol.2016.4587
28. Schallhorn SC, Venter JA, Hannan SJ, Hettinger KA, Teenan D. Effect of postoperative keratometry on quality of vision in the postoperative period after myopic wavefront-guided laser in situ keratomileusis. J Cataract Refract Surg. 2015;41(12):2715–2723. doi:10.1016/j.jcrs.2015.06.034
29. Schallhorn SC, Hannan SJ, Teenan D, Schallhorn JM. Role of the treating surgeon in the consent process for elective refractive surgery. Clin Ophthalmol. 2016;10:2391–2402. doi:10.2147/OPTH.S120345
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
