Back to Journals » Clinical Optometry » Volume 14
OSDI Outcomes Based on Patient Demographic and Wear Patterns in Prosthetic Replacement of the Ocular Surface Ecosystem
Authors Asghari B , Brocks D , Carrasquillo KG, Crowley E
Received 14 September 2021
Accepted for publication 18 December 2021
Published 10 January 2022 Volume 2022:14 Pages 1—12
DOI https://doi.org/10.2147/OPTO.S337920
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Mr Simon Berry
Bita Asghari, Daniel Brocks, Karen G Carrasquillo, Estelle Crowley
BostonSight, Needham, MA, USA
Correspondence: Bita Asghari Tel +1-781-726-7337
Fax +1-781-726-7310
Email [email protected]
Purpose: To evaluate the impact of prosthetic replacement of the ocular surface ecosystem (BostonSight PROSE) treatment on symptom outcomes based on the Ocular Surface Disease Index (OSDI).
Patients and Methods: This was a single-center, retrospective analysis of consecutive patients who initiated PROSE treatment between September 2017 and December 2019 by the same clinician. The primary outcome measure was to compare OSDI survey scores at baseline prior to PROSE treatment and at follow-up, after PROSE treatment. Indication for treatment, sex, age, device diameter, average wear time, preexisting mental illness, duration of PROSE wear, and status of PROSE wear at follow-up were also studied.
Results: A total of 134 patients underwent PROSE treatment and completed a baseline OSDI survey during the study period. Forty-three patients completed a follow-up OSDI survey and were included in the study analysis. The most common treatment indications were keratoconjunctivitis sicca (n=27) and corneal ectasia (n=16). Baseline average OSDI score was 56.9± 23.7 for the 43 subjects who completed a subsequent OSDI survey. The last documented average follow-up OSDI for those 43 subjects was 23.8± 15.6, median (IQR) of 22.9 (10.4 to 32.3), and a statistically significant 54.7± 27.6% average improvement from baseline (p< 0.01). All patients, except for two, showed improvement in OSDI score. Statistically significant improvement occurred regardless of underlying diagnosis with no statistically significant difference based on age, sex, mental illness, or device diameter and no statistical correlation with average wear time, or duration of PROSE wear.
Conclusion: PROSE treatment improves visual function and symptom relief as demonstrated by the OSDI survey. Sex, age, preexisting mental illness, device diameter, average wear time, and duration of wear had no statistically significant impact on OSDI outcomes.
Keywords: PROSE, OSDI survey, scleral lens, ocular surface disease, corneal ectasia, dry eye
Introduction
The role of prosthetic replacement of the ocular surface ecosystem (PROSE) treatment in the management of corneal and ocular surface disorders is well documented in literature.1–5 PROSE treatment involves the use of a custom scleral lens design for the management of a variety of conditions including irregular corneal disorders and ocular surface disease (OSD). Other scleral lens designs have also demonstrated to provide dry eye symptom relief for patients suffering from complex corneal disease.6–9 PROSE treatment is a medical process which includes the customization and dispensing of a PROSE device, or prosthetic device (PD), application and removal training for patients, continued monitoring to assess fit and function of the PD, as well as short and long-term optometry and ophthalmology co-management of a patient’s underlying ocular disease. Improvement in patient symptoms with PROSE treatment has been quantified with the use of the Ocular Surface Disease Index (OSDI; Allergan Inc., Irvine, CA, USA) in literature in the past;1–5,10 however, no literature to our knowledge has studied the impact of sex, age, preexisting mental illness, design diameter, average daily wear time, or duration of lens wear on OSDI outcomes.
Symptom surveys like the OSDI serve as tools to efficiently assess patient symptoms in a standardized manner. The use of questionnaires like the OSDI serve to assess patient visual function and quality of life.11–15 The OSDI survey is a validated tool which is used clinically and in research to assess the symptomatic outcomes of therapeutics from the functional standpoint of a patient.16
The association between mental health and its negative effect on dry eye symptoms and pain has been demonstrated in literature.17–23 Individuals with underlying mental illness such as anxiety, depression, or post-traumatic stress disorder (PTSD) have been shown to have increased dry eye symptoms.21 Siedlecki et al showed patients with greater ocular pain intensity had a higher likelihood of reporting a history of fibromyalgia, depression, anxiety, and migraines.23 In that study, the dry eye patients with greater pain severity were less responsive to treatment. Because of this reported correlation, we investigated mental illness as a variable for OSDI outcomes following PROSE treatment.
This study assesses the OSDI outcomes of PROSE treatment while accounting for such variables as sex, age, mental illness, design diameter, hours of daily wear, and duration of wear. It also reviews the results of PROSE treatment compared to other OSDI outcomes from various previously studied ocular surface and dry eye therapies in literature.
Patients and Methods
Study Design and Subjects
The New England Institutional Review Board ethics committee approval was obtained for the study under BFS-KC-Retrospective-01 for research involving the collection and analysis of existing data or records for patients who underwent PROSE treatment at BostonSight. All guidelines were followed to ensure HIPAA compliance, and we adhered to the Declaration of Helsinki and applicable federal and state laws. This was a retrospective chart review involving patients who initiated PROSE treatment with the same PROSE practitioner at BostonSight (Needham, MA, USA) between September 2017 and December 2019, and who had completed a baseline OSDI survey at the time of PROSE treatment. Patients undergoing PROSE treatment included novel PROSE wearers, habitual scleral lens wearers who were transitioning to PROSE treatment and habitual PROSE wearers who were undergoing retreatment, meaning re-fitting of their PROSE device(s). Indications for retreatment included adjusting the design to improve fit and function, as well as updating the device optics, if necessary, to improve vision. For inclusion in the study, patients must have been at least 18 years old at the time of starting PROSE treatment and completed an OSDI survey at the time of starting treatment and at least one follow-up OSDI. Patients were excluded from the study if they were younger than 18 years old at the time of starting treatment or had not completed both a baseline and follow-up OSDI survey. On retrospective chart screening, 134 patients (234 eyes) underwent PROSE treatment and had a baseline OSDI recorded under the care of the same practitioner during the studied timeframe. Forty-three patients (81 eyes) who continued with PROSE wear and completed a follow-up OSDI survey are in the analyzed cohort described below. There was not any exclusion related to other ocular surface treatments used at the time of starting PROSE treatment or at follow-up. Patients were continued on their other ocular surface treatments as prescribed by their primary ophthalmologist or optometrist. Each patient’s age, sex, and history of mental illness was recorded from examination data as reported by the patient at the time of starting treatment.
PROSE device diameter data was retrieved from device manufacturing records. Baseline habitual scleral lens data for a lens which was fitted by an outside provider was obtained from the fitting provider’s notes on file. If scleral lens data was not available to the practitioner, it was not included as part of our analysis, as no additional access to outside records was requested for the purposes of data collection for this study.
After initiating PROSE treatment, each patient returned to clinic at the appropriate time frame based on the clinical decision making of the practitioner. During a follow-up visit, an OSDI survey may have been administered. If a patient had completed more than one follow-up OSDI survey, the most recent OSDI score was included in this review for analysis. The duration of PROSE wear (number of days) from initial pretreatment baseline OSDI until administration of follow-up OSDI survey was calculated.
Minimum and maximum hours of PROSE daily wear were obtained from exam notes based on patient responses to this standard question which is routinely asked and documented at each office visit. The average hours of daily device wear were calculated using minimum and maximum values reported by each patient. If a patient was a habitual scleral lens or PROSE wearer at the time of starting treatment, they were also routinely asked their minimum and maximum hours of daily wear.
PROSE Treatment
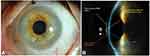
PROSE treatment is a process by which visual function, comfort and ocular health can be restored to a patient with complex corneal disease.1–5,9,24,25 The PD used in PROSE treatment is a transparent dome of gas permeable plastic, ranging from 13–23 mm in diameter. The diameter of each design is varied by the fitting practitioner to achieve optimal physiologic function. The PD fits under the eyelids, rests on the conjunctiva overlying the sclera and creates a clear, smooth surface over the cornea (Figure 1A). Each PD is filled with preservative-free normal saline at the time of application; this pool of fluid remains against the cornea (Figure 1B). The constant exposure of the ocular surface to the fluid can potentiate corneal epithelialization and prevent desiccation while the PD itself serves as a physical barrier. Each PD is customized to accommodate the shape of the eye and is designed with proprietary web-based computer software (FitConnect, BostonSight, Needham, MA, USA) that drives a manufacturing lathe. The Federal Food and Drug Administration (FDA)-approved fabricated PD used in PROSE treatment helps restore vision, support healing, reduce symptoms, and improve quality of life in patients with complex corneal disease including distorted corneal surface and ocular surface disorders.
Each patient referred for PROSE treatment from local, national, and international primary eye care providers undergo a medical chart review prior to being considered for a consultation. Patients who are referred for treatment have failed other treatment modalities for the management of their ocular disease and, therefore, generally have a greater disease severity. The initial consultation visit is used to determine patient candidacy for treatment. Once a patient is deemed a candidate, they then return to clinic to start PROSE treatment. Determining the need for repeat PROSE treatment or refitting of a PD is based on clinical examination, patient symptoms, and decision-making of the practitioner. The PROSE treatment window is a six-month period starting from the date of initiating treatment. During the six-month treatment window, adjustments to the PD can be made until an optimized fit and design and adequate physiological function is achieved for ongoing daily use. Treatment includes PD fitting, intensive training to apply and remove a device, instruction on proper care and hygiene of the device, and monitoring of ocular physiologic response and tolerance of wear. There is typically variability in follow-up time course. Variables which may impact PROSE follow-up frequency and interval include ocular condition or state of the disease, means of travel and distance from home, financial limitations, and access to local eye care for collaboration.
Once a patient has completed treatment, they continue to be monitored at least once a year to assess PROSE device customization and function. All eye care unrelated to PROSE treatment is continued by their local primary eye care provider.
OSDI Survey
The OSDI survey was created to quantify symptoms of dry eye disease (DED) and vision-related function in the past week of a patient’s life. The use of questionnaires like the OSDI survey to assess patient quality of life aligns with the FDA’s emphasis on utilizing patient-reported outcomes as a tool to better support medicinal and clinical drug trial outcomes.16 The 12-item validated questionnaire is conducted in-office and has three subcategories: ocular symptoms, vision-related function, and environmental triggers.12–15 Each question is presented to the patient, who will then respond based on a 0 to 4 scale, with 0 corresponding to “none of the time” and 4 corresponding to “all of the time”. A patient can also elect to skip a question if it does not pertain to them. The sum of all the patient’s responses to the questions answered are used in the calculation to determine the final OSDI score. The final score of the OSDI survey ranges from 0 to 100; 0–12 representing normal, 13–22 representing mild DED, 23–32 representing moderate DED, and 33 and greater representing severe DED.12–15
Statistical Analysis
Statistical analysis was performed using Microsoft Excel (Microsoft Corp., Redmond, WA, USA) and Statistics Kingdom online statistics calculator (https://www.statskingdom.com) unless otherwise noted. Normality was determined utilizing an online Shapiro–Wilk test calculator and Q-Q plots. Continuous normally distributed variables are represented as mean ±standard deviation, while continuous variables with non-normal distribution are reported with mean ±standard deviation along with median and interquartile range (IQR). Any comparison of independent data sets which includes variables with non-normal distribution are analyzed for statistical significance with non-parametric testing utilizing a Mann–Whitney U-test calculator. Comparison of paired data sets which includes variables with non-normal distribution are analyzed for statistical significance with non-parametric testing utilizing a Wilcoxon signed-rank test calculator. Comparison of data sets with confirmed normality are analyzed via the applicable t-test (paired t-test or two sample t-test— Welch’s). Nominal and ordinal categorical variables were examined for relationships utilizing the chi-squared test for independence (Social Science Statistics, https://www.socscistatistics.com/tests/chisquare2/default2.aspx). Correlation of normally distributed continuous variables are analyzed utilizing Pearson's correlation coefficient, while Spearman's rank correlation coefficient is utilized to assess correlation if either of the variables are not normally distributed. Significance was set to a p-value of ≤0.05 in all instances of analysis.
Sample size calculations were completed using Clincalc.com (https://clincalc.com/stats/SampleSize.aspx) and utilizing comparable population data from Miller et al12 mean severe OSDI 52.6; SD 16.4, minimally clinically important difference (MCDI) 25.4% to determine a minimum number of 20 subjects were needed to be included in this retrospective analysis to detect a minimally clinically important difference in OSDI following treatment to yield a 95% power at the α=0.05 level.
Results
Indication for PROSE Treatment
Indications for PROSE treatment in the studied population included the broad categories of distorted corneal shape, OSD and high refractive error. Patient diagnoses were further sub-categorized by ocular graft v host disease (oGVHD), keratoconjunctivitis sicca (KCS), filamentary keratitis (FK), exposure keratopathy (EK), atopic keratoconjunctivitis (AK), limbal stem cell deficiency (LSCD), corneal ectasia, Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis syndrome (TENS), postpenetrating keratoplasty (PKP), persistent epithelial defect (PED), corneal neuralgia, corneal scar/opacity, pathologic myopia and other. Corneal ectasia diagnoses included keratoconus, postastigmatic keratotomy and/or postradial keratotomy, pellucid marginal degeneration, and post-LASIK ectasia. The most common indication for treatment was KCS, or DED (Table 1).
 |
Table 1 Analysis of OSDI Outcomes with PROSE Wear, Based on Condition |
Baseline OSDI
The average baseline OSDI for the entire analyzed cohort of 43 subjects who completed a follow-up OSDI survey was 56.9±23.7, which is categorized as severe based on the OSDI scale.
Overall OSDI Outcomes
Of the 43 patients who completed a follow-up OSDI, all but two showed improvements in OSDI score. One patient had a baseline OSDI score of zero and follow-up OSDI score of zero. Another patient who proceeded with PROSE treatment had an increase in OSDI from baseline (45.83) to follow up (60.42). All diagnosis groups showed average improvement in OSDI with treatment (Table 1). The average follow-up OSDI was 23.8±15.6, median (IQR) of 22.9 (10.4 to 32.3), a statistically significant average reduction of 33.14±22.0, p<0.01, equivalent to an average reduction of 54.7±27.6%. The most common diagnosis and indication for PROSE treatment was KCS, with 27 patients completing a follow-up OSDI. Patients with KCS had a statistically significant average OSDI reduction of 36.8±22.9, p<0.01, equivalent to an average OSDI reduction of 55.6±28.6%.
Age
The 43 patients who completed a follow-up OSDI survey had an average age of 50.9±12.0 years. Age was not found to have a statistically significant correlation with OSDI change (r= −0.04, p=0.80).
Sex
Of the 43 patients who completed a follow-up OSDI, 18 were male and 25 were female. The average baseline OSDI was 42.9±23.8 for the 18 males and 67.0±17.8 for the 25 females. There was a statistically significant 52.4±17.0% (p<0.01) and 56.4±20.4% (p<0.01) average reduction in OSDI score for males and females, respectively, demonstrating no statistical difference in OSDI outcomes with PROSE treatment based on sex (p=0.103). Chi-squared test for independence confirmed that there was no significant association between sex and OSDI improvement (with OSDI improvement grouped into categories: ≤9, 10–19, 20–29, 30–39, and ≥40), χ2 (4, n=43)=5.6, p=0.23.
Preexisting Mental Illness
To control for potential impact of preexisting mental illness on OSDI outcomes, preexisting mental illness at the time of the start treatment was assessed as part of each patient’s reported personal medical history. Fifteen of the 43 patients in the analyzed cohort had a psychiatric diagnosis at baseline which included diagnoses of depression, anxiety, bipolar disorder, PTSD, or obsessive-compulsive disorder (OCD). Baseline OSDI average of the 15 patients with preexisting mental illness was 66.0±22.9. Those 28 patients without mental illness had baseline OSDI average of 52.1±22.7. Follow-up OSDI improved by a statistically significant average of 52.0±32.0% (p<0.01) and 56.2±24.9% (p<0.01) for those with psychiatric diagnosis and for those without, respectively. There was no statistically significant difference in improvement in OSDI based on mental illness status (p=0.48). Chi-squared test for independence confirmed that there was no significant association between mental illness and OSDI improvement (with OSDI improvement grouped into categories: ≤9, 10–19, 20–29, 30–39, and ≥40), χ2 (4, n=43)=5.7, p=0.22.
Average Daily Wear Time (AWT)
The AWT in the 43 patients who completed a follow-up OSDI survey was 12.0±3.4 hours per day. Average hours of daily PROSE wear was not found to have a statistically significant correlation with OSDI change (r= −0.03, p=0.84). When analyzing AWT based on 12 hours or less of daily wear (n=22, AWT 9.4±2.4 hours) and greater than 12 hours of daily wear (n=21, AWT 14.8±1.5 hours), we again affirmed no statistically significant difference in the average amount of change in OSDI outcomes when comparing group to group (p=0.71). There was a statistically significant OSDI improvement in each group (p<0.01 in both groups).
Duration of Wear
The duration of PROSE wear, measured in days, until the follow-up OSDI was administered varied between the 43 patients who completed a follow-up survey. The average duration of wear from baseline until the administration of the final follow-up OSDI survey was 459.9±244.5, median (IQR) of 515.0 (221.5 to 659.5) days. The overall range for length of time from baseline pre-PROSE treatment OSDI until final OSDI for the entire cohort of analyzed patients was 42 to 874 days. Duration of wear was not found to have a statistically significant correlation with OSDI change (r= −0.09, p=0.54).
Final PROSE Device Diameter
The average final optimized PD diameter for all 43 patients was 18.8±0.87, median (IQR) of 18.75 (18.5 to 19.5) mm. Four of the 43 subjects were habitual scleral lens or habitual PROSE wearers at baseline, with an average baseline diameter of 17.2±1.2, median (IQR) of 18.0 (16.3 to 18.1) mm. Those four subjects had an average final PD diameter of 19.1±0.4, median (IQR) of 19.0 (19.0 to 19.5) mm, which was an average increase of 1.9±1.2, median (IQR) of 1.5 (1.2 to 2.5) mm in overall diameter from baseline. Final PROSE device diameter was not found to have a statistically significant correlation with OSDI change (r= −0.09, p=0.55).
Habitual vs Novel Wearers
Of the 43 patients who completed a follow-up OSDI, the average baseline OSDI score for habitual scleral lens or habitual PROSE wearers (n=4) was 30.7±22.2. Three of these four subjects were habitual scleral lens wearers. The fourth habitual wearer wore a scleral lens in one eye and a PD for the fellow eye at baseline. These four habitual wearers had an average final OSDI score of 10.9±8.1, median (IQR) of 10.42 (7.8 to 13.5), equating to an overall average reduction of symptoms by 44.0±31.4%. The average baseline OSDI for novel PROSE wearers (n=39) was 59.6±22.2. Novel PROSE wearers had a final average OSDI score of 25.1±15.6, median (IQR) of 25.0 (14.6 to 33.3), equating to an overall reduction of symptoms by 55.8±27.0%. There was no statistically significant difference in the reduction in OSDI when comparing habitual scleral lens and habitual PROSE wearers versus novel PROSE wearers (p=0.23).
OSDI Outcome—per Question
When analyzing the results of the OSDI survey by individual question, an overall average improvement was seen for every question which was answered at baseline and at follow-up (Table 2). The greatest improvement in symptoms was noted for question 5 which asked the patient how often they experienced poor vision. There was a statistically significant average reduction from 2.70±1.44 at baseline to 0.79±0.94 at follow up, an overall 1.91±1.73 average improvement in quality of vision (p<0.01). The least improvement in symptoms was noted for question 12 which asked the patient how often their eyes felt uncomfortable in areas that are air conditioned. There was a statistically significant average reduction from 1.58±1.69 at baseline to 0.86±1.32 at follow-up, an overall average improvement of 0.72±1.83 (p<0.01).
 |
Table 2 Analysis of the Average Response Scores to the OSDI Surveya, by Question, at Follow-up |
Adverse Events
Of the 43 patients who had baseline and follow-up OSDI, no adverse events were observed, including but not limited to no incidents of infectious keratitis, corneal abrasion, corneal graft rejection or failure.
Discussion
This study retrospectively reports the OSDI outcomes for a population of 43 subjects that not only underwent PROSE treatment, but also had recorded baseline and follow-up OSDI survey scores. It is the first study to evaluate for associations of sex, age, mental illness, hours of daily wear or AWT, and duration of wear with PROSE treatment OSDI outcomes. We report that PROSE treatment resulted in a significant improvement in average OSDI outcomes regardless of these variables in the study group. In addition there was an average improvement for every single item on the OSDI questionnaire. Device diameter showed no significant direct effect on OSDI outcomes. All categories of indication and underlying conditions showed significant improvement in OSDI outcomes.
All but two of the 43 subjects individually showed improvement in OSDI. One of these two patients had an OSDI score of zero both at baseline and at follow-up. This bilateral ectasia patient presented wearing a soft contact lens in the right eye and a scleral lens in the left eye. At baseline, the patient reported poor quality vision particularly in the right eye, with intermittent redness and discomfort while wearing the scleral lens in the left eye. The patient’s reported symptoms correlated with clinical exam findings. The patient was motivated to pursue PROSE treatment for both eyes to improve vision for the right eye and comfort for the left eye. Despite the patient’s reported symptoms at baseline, they answered zero (“none of the time”) for all questions on the OSDI survey. A total OSDI score of zero would indicate an asymptomatic, comfortable patient with optimized visual function, which most certainly was not the case. At follow-up, the patient was happy with the improved vision and comfort obtained for both eyes with PROSE, with clinical findings correlating with these subjective reports. Again, at follow-up, the patient answered zero for all OSDI survey questions. The disconnect between the significant presenting symptoms and the presenting OSDI score of zero could be most likely explained either by the patient not properly understanding the survey instructions or less likely, the OSDI survey not capturing the specific type of symptoms the patient felt were problematic.
The one patient who had a higher OSDI score at follow-up than baseline had SJS/TENS. A chart review revealed improvement in symptoms reported by the patient following PROSE treatment, continued comfortable daily wear with AWT of 12 hours, however, this was not reflected in the final OSDI score.
Interestingly, in both cases there was no improvement in OSDI at follow-up, however, the clinical findings and reported mitigation of symptoms suggested an improvement in visual function. Notably, these patients continued with PROSE wear, which they both felt was a successful treatment and had significantly addressed their presenting concerns. Cases such as these could be further analyzed in future studies, which could investigate whether clearer instructions are necessary for patients to properly understand the OSDI survey or whether question updates are necessary to better capture certain symptomatic or visual function experiences which may be missed in the current iteration of the survey.
Of note, the baseline OSDI score for patients included in this study were similar to what has been reported in the past for other studies assessing PROSE treatment OSDI outcomes based on ocular condition and indication for wear.1–5,26
Age and Sex
OSDI outcomes with PROSE treatment were not statistically different by sex, indicating that males and females over 18 years of age were equally likely to have similar improvement in OSDI outcome with treatment. In the population studied, the average baseline OSDI was notably more severe in the female (67.0±17.8) vs the male groups (42.9±23.8). Despite this discrepancy in severity, the two groups had average OSDI improvement following PROSE treatment that was not statistically different (52.4±17.0% for males and 56.4±20.4% for females).
No correlation was found between age and the amount of improvement in OSDI following PROSE treatment. The presumption can therefore be made that we need not expect a greater or lesser efficacy in improving visual function and symptoms in different adult age groups treated with PROSE. Although there is existing literature to support the benefit for PROSE treatment in the pediatric population,27–31 additional studies of a similar nature with a pediatric cohort are necessary to help elucidate whether any different effect of this treatment in the pediatric population is apparent when assessing visual function via a symptom survey. A limitation in pursuing a study of this kind may be the repeatability and reliability of responses from pediatric patients. Notably, the landmark study of OSDI validity only included patients that were 18 years of age or older.14
Visual Function
Patients with corneal disease including irregular corneal shape and OSD can experience poor quality of vision. PROSE treatment has been demonstrated in literature to improve vision in patients by providing a smooth refractive surface for a multitude of conditions ranging from corneal ectasia or scarring, to severe OSD.2,9,10,26,29,31 Although visual acuity at baseline and follow-up was not assessed in this study, the OSDI survey questionnaire revealed the greatest average improvement for question 5 which asked the patient how often they experienced poor vision. This finding is consistent with literature and supports that PROSE treatment can improve quality of vision in patients suffering from a variety of corneal and ocular surface diseases.
Preexisting Mental Illness
Following PROSE treatment there was a statistically significant improvement in OSDI in the group of patients with mental illness and in the group of patients without mental illness (p<0.01). Prior literature has shown not only that patients with mental illness are more likely to experience symptoms like dry eye and pain, but also that the dry eye patients with greater pain severity were less likely to respond to treatment.18,22,23 Despite this propensity to be more symptomatic and recalcitrant, we found no statistically significant difference in improvement of OSDI regardless of preexisting mental illness. The combination of mental illness and OSD can present some of the most challenging cases. To date, to our knowledge, no prior literature has explored the efficacy of specific ocular surface treatments for this subset population with mental illness and OSD. Our findings suggest that these challenging cases may have significant improvement of symptomatology and visual function with the utilization of PROSE treatment. Further studies in this mental illness cohort comparing various treatment modalities and efficacy in a controlled trial would be advantageous to prioritize treatment options for this group. The current TFOS DEWS II Report “Recommendations for the staged management and treatment of DED”32 may or may not apply to this subgroup with mental illness and DED and a different staged approach may be applicable. In this study, we notably report that patients with mental illness (OSDI average reduction of 52.0±32.0%) improved just as much as patients without mental illness (OSDI average reduction of 56.2±24.9%) with PROSE treatment, as no statistically significant difference in OSDI improvement was present between the groups (p=0.48).
Average Daily Wear Time (AWT) and Duration of Wear
Average daily wear time of PROSE for each patient can vary based on visual function, ocular surface health and respective underlying ocular condition. A patient wears their PROSE device(s) for an appropriate number of hours to meet their needs of improving vision, comfort, and/or supporting the ocular surface.
It is important to recognize that AWT may be restricted as advised by the prescribing practitioner in patients who warrant limited number of hours of wear for proper physiologic function with PROSE wear. For instance, for an eye with compromised endothelial pump function such as related to an aged weakened corneal graft, the practitioner may limit wear time to five hours or less to prevent the onset of corneal edema and subsequent visual blurring. Whether wear time was limited by the practitioner or the reasons for limiting wear time were not studied in this retrospective review. Regardless of the reason for varying average daily wear time amongst the studied population, it is worth noting that there was no correlation between average hours of daily PROSE wear and reduction in OSDI. One might assume that more hours of wear would equate to more improvement in visual symptoms. However, our results suggest that it is not “more” hours of wear each day that is of importance, but rather, the “proper” number of hours of wear as deemed appropriate by the prescribing clinician.
The length of time from baseline pre-PROSE treatment OSDI until final OSDI ranged from 42 to 874 days, with no statistical indication in this time frame that more days of wear results in a trend toward further improved OSDI outcomes. Moon et al studied OSDI outcomes of OSD patients fitted with scleral lenses and showed a 30–40 score reduction in OSDI in the first week, with statistically similar results at weeks 4, 8, and 12. This corroborates the findings assessed in our study that increased duration of wear does not statistically correlate with further improved OSDI outcomes.33 Further prospective studies with set time points for evaluating OSDI could investigate the average number of days to the “plateau” effect and steady state achievement for the OSDI score with PROSE treatment. Subgroup analysis could provide powerful data to set proper patient timeframe expectations for improvement in signs and symptoms in specific pathologies undergoing PROSE treatment.
Habitual vs Novel Wearers
Habitual scleral and habitual PROSE wearers had improvement in OSDI outcomes following PROSE treatment. This group included patients that presented at baseline wearing scleral lenses or PROSE devices and proceeded with initial PROSE treatment or PROSE retreatment, respectively. Although only four patients were in this subgroup, this data suggests scleral lens design and fitting endpoint adjustments positively affect OSDI outcome. Although the indication for transition from their current habitual lens/device was not recorded in this study, it is likely that this indication and the necessary adjustments and customizations in the newly dispensed PROSE device resulted in OSDI improvement. Moreover, it is important to note that all patients were receiving a newly manufactured device. It is possible that simply transitioning to a new device free of scratches and/or deposits also contributed to improved OSDI outcomes. The integrity of the habitual PD or habitual scleral lens was not assessed as part of this study. Further studies are needed to assess OSDI outcomes based on condition/age of a lens as well as evaluating associations to specific fitting adjustments.
Device Diameter in Novel PROSE Wearers
In the studied population, we did not find a significant correlation between final PD diameter and OSDI outcome. An optimally fitting PROSE device endpoint in the opinion of the clinician, which included a finalized lens diameter, was reached for each patient at the time when the final follow-up OSDI survey was administered. It is important to note that the average PD diameter of patients who completed a follow-up OSDI was 18.8mm±0.87, median (IQR) of 18.75 (18.5 to 19.5), which is notably greater than the average scleral lens diameter of 15–17mm which two-thirds of practitioners fit.34 As such, our results may not be translated to smaller diameter designs.
PROSE Treatment Outcomes vs Other Therapies
Management of OSD, specifically DED, includes lubrication, lid hygiene, manual gland expression, use of anti-inflammatory medications, immunosuppressant therapies, and tear retention.35 The therapeutic use of contact lenses including soft and scleral lenses or PROSE devices for the treatment of DED or KCS is well-documented in literature.3,4,35,36 The use of scleral lenses has grown exponentially in recent years, with scleral lenses or PROSE devices now considered as primary or secondary treatment of choice for intractable ocular surface diseases.37,38
The OSDI outcomes analyzed in this study were similar to what has been previously reported in literature with PROSE treatment and scleral lens wear.1–3,33 Other studies have used the OSDI survey to assess treatment outcomes in KCS or dry eye patients. When comparing PROSE treatment OSDI outcomes in patients with KCS to other therapies used to treat patients with KCS or DED, PROSE treatment reported the greatest percentage OSDI reduction (Table 3).39–43
 |
Table 3 Comparison of OSDI Outcomes Between PROSE Treatment vs Other Therapies for the Treatment of Keratoconjunctivitis Sicca, or Dry Eye Disease |
One limitation of the retrospective framework of this study is that there was no restriction placed on the other ocular treatment modalities patients used during the studied timeframe. At the time of PROSE treatment, patients were continued on the other ocular surface treatments prescribed by their primary ophthalmologist or optometrist and there were no specific concomitant treatment modalities that prompted exclusion from the analyzed cohort. Future studies, particularly prospective in nature, could specifically investigate whether any ocular treatments may negatively or positively effect OSDI improvement when used in conjunction with PROSE treatment.
In addition, other ocular surface variables that may affect the efficacy of various treatments, including PROSE, is of high importance. Of great significance, tear film quality and tear film performance can impact the utility and success of ocular surface interventions. In a prospective study, a standardized assessment of aqueous and evaporative tear film issues would provide data to determine the correlation and effect of such issues on treatment success. Recent studies have elucidated the utility of OCT to evaluate tear film quality and performance, which could be a valuable assessment to include as part of a future prospective study protocol.44–46
Patient Population
Consideration should be given to the patient population included in this study. Patients who are referred for PROSE treatment generally suffer from more severe complex corneal disease and often have attempted but failed other prior therapies for management of ocular surface diseases and irregular corneal shape. The average baseline OSDI score for all patients included in this study is classified as severe based on the OSDI.
Conclusion
Baseline OSDI in the subject population improved by an average of 54.7±27.6% following PROSE treatment. There was no statistically significant difference in outcomes based on, age, sex, lens diameter, preexisting mental illness, duration of wear or hours of daily wear. Improvement occurred regardless of diagnosis, which included OSD, corneal ectasia and refractive indications. Compared to other therapeutics used traditionally for the treatment of DED or KCS, PROSE treatment showed greater percentage improvement in OSDI outcomes. Overall, the findings in this report support that PROSE treatment can be considered for a multitude of corneal and ocular surface diseases with the expectation of improved OSDI symptoms.
Funding
There is no funding to report.
Disclosure
All authors are salaried employees of BostonSight, Needham, MA, USA where the BostonSight PROSE treatment was developed. None of the authors have a proprietary interest in PROSE treatment or the prosthetic devices used in PROSE treatment. The authors report no other potential conflicts of interest in this work.
References
1. Chiu GB, Bach D, Theophanous C, Heur M. Prosthetic replacement of the ocular surface ecosystem (PROSE) scleral lens for Salzmann’s nodular degeneration. Saudi J Ophthalmol. 2014;28(3):203–206. doi:10.1016/j.sjopt.2014.06.001
2. Heur M, Bach D, Theophanous C, Chiu GB. Prosthetic replacement of the ocular surface ecosystem scleral lens therapy for patients with ocular symptoms of chronic Stevens-Johnson syndrome. Am J Ophthalmol. 2014;158(1):49–54. doi:10.1016/j.ajo.2014.03.012
3. Chahal JS, Heur M, Chiu GB. Prosthetic replacement of the ocular surface ecosystem scleral lens therapy for exposure keratopathy. Eye Contact Lens. 2017;43(4):240–244. doi:10.1097/ICL.0000000000000265
4. Dimit R, Gire A, Pflugfelder SC, Bergmanson JPG. Patient ocular conditions and clinical outcomes using a PROSE scleral device. Contact Lens Anterior Eye. 2013;36(4):159–163. doi:10.1016/j.clae.2013.02.004
5. Theophanous C, Irvine JA, Parker P, Chiu GB. Use of prosthetic replacement of the ocular surface ecosystem scleral lenses in patients with ocular chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(12):2180–2184. doi:10.1016/j.bbmt.2015.07.027
6. Segal O, Barkana Y, Hourovitz D, et al. Scleral contact lenses may help where other modalities fail. Cornea. 2003;22(4):308–310. doi:10.1097/00003226-200305000-00006
7. La Porta Weber S, Becco De Souza R, Gomes JÁP, Hofling-Lima AL. The use of the esclera scleral contact lens in the treatment of moderate to severe dry eye disease. Am J Ophthalmol. 2016;163:167–173.e1. doi:10.1016/j.ajo.2015.11.034
8. Schornack MM. Scleral lenses: a literature review. Eye Contact Lens. 2015;41:3–11. doi:10.1097/ICL.0000000000000083
9. Baran I, Bradley JA, Alipour F, Rosenthal P, Le HG, Jacobs DS. PROSE treatment of corneal ectasia. Contact Lens Anterior Eye. 2012;35:222–227. doi:10.1016/j.clae.2012.04.003
10. Bhattacharya P, Mahadevan R. Quality of life and handling experience with the PROSE device: an Indian scenario. Clin Exp Optom. 2017;100(6):710–717. doi:10.1111/cxo.12519
11. Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21:310–316. doi:10.1097/ICU.0b013e32833a8c15
12. Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128:94. doi:10.1001/archophthalmol.2009.356
13. Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–1419. doi:10.1016/S0161-6420(03)00462-7
14. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615
15. Walt JG, Rowe SK. Evaluating the functional impact of dry eye: the Ocular Surface Disease Index. Drug Inf J. 1997;31:b5.
16. Bottomley A, Jones D, Claassens L. Patient-reported outcomes: assessment and current perspectives of the guidelines of the Food and Drug Administration and the reflection paper of the European Medicines Agency. Eur J Cancer. 2009;45:347–353. doi:10.1016/j.ejca.2008.09.032
17. Laad S, Singh H, Singh S, et al. Magnitude of dry eye among medical students and its impact on their mental health: a questionnaire based survey. J Evid Based Med Healthc. 2019;6(35):2393–2396. doi:10.18410/jebmh/2019/489
18. Keogh E, Cochrane M. Anxiety sensitivity, cognitive biases, and the experience of pain. J Pain. 2002;3:320–329. doi:10.1054/jpai.2002.125182
19. Szakáts I, Sebestyén M, Németh J, Birkás E, Purebl G. The role of health anxiety and depressive symptoms in dry eye disease. Curr Eye Res. 2016;41(8):1044–1049. doi:10.3109/02713683.2015.1088955
20. Kim KW, Han SB, Han ER, et al. Association between depression and dry eye disease in an elderly population. Investig Ophthalmol Vis Sci. 2011;52:7954. doi:10.1167/iovs.11-8050
21. Galor A, Feuer W, Lee DJ, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the National United States veterans affairs administrative database. Am J Ophthalmol. 2012;154:340–346.e2. doi:10.1016/j.ajo.2012.02.009
22. Klauenberg S, Maier C, Assion HJ, et al. Depression and changed pain perception: hints for a central disinhibition mechanism. Pain. 2008;140:332–343. doi:10.1016/j.pain.2008.09.003
23. Siedlecki AN, Smith SD, Siedlecki AR, Hayek SM, Sayegh RR. Ocular pain response to treatment in dry eye patients: ocular pain treatment response. Ocul Surf. 2020;18(2):305–311. doi:10.1016/j.jtos.2019.12.004
24. He X, Donaldson KE, Perez VL, Sotomayor P. Case series: overnight wear of scleral lens for persistent epithelial defects. Optom Vis Sci. 2018;95:70–75. doi:10.1097/OPX.0000000000001162
25. Lim P, Ridges R, Jacobs DS, Rosenthal P. Treatment of persistent corneal epithelial defect with overnight wear of a prosthetic device for the ocular surface. Am J Ophthalmol. 2013;156:1095–1101. doi:10.1016/j.ajo.2013.06.006
26. Rosenthal P, Cotter J. The Boston scleral lens in the management of severe ocular surface disease. Ophthalmol Clin North Am. 2003;16:89–93. doi:10.1016/S0896-1549(02)00067-6
27. Gungor I, Schor K, Rosenthal P, Jacobs DS. The Boston Scleral Lens in the treatment of pediatric patients. J AAPOS. 2008;12:263–267. doi:10.1016/j.jaapos.2007.11.008
28. Kamal SM, Riccobono K, Kwok A, Edmond JC, Pflugfelder SC. Unilateral pediatric neurotrophic keratitis due to congenital left trigeminal nerve aplasia with PROSE (prosthetic replacement of the ocular surface ecosystem) treatment. Am J Ophthalmol Case Rep. 2020;20. doi:10.1016/j.ajoc.2020.100854
29. Wang Y, Rao R, Jacobs DS, Saeed HN. Prosthetic replacement of the ocular surface ecosystem treatment for ocular surface disease in pediatric patients with Stevens-Johnson syndrome. Am J Ophthalmol. 2019;201:1–8. doi:10.1016/j.ajo.2019.01.006
30. Remington CD, Jacobs DS. PROSE treatment for pediatric patients with neurotrophic keratitis. Investig Ophthalmol Vis Sci. 2015;56(7):6076.
31. Rosenthal P, Croteau A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. 2005;31:130–134. doi:10.1097/01.ICL.0000152492.98553.8D
32. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4). doi:10.1016/j.jtos.2017.08.003
33. Moon J, Lee SM, Hyon JY, Kim MK, Oh JY, Choi HJ. Large diameter scleral lens benefits for Asians with intractable ocular surface diseases: a prospective, single-arm clinical trial. Sci Rep. 2021;11(1):1–11. doi:10.1038/s41598-021-82010-z
34. Harthan J, Nau CB, Barr J, et al. Scleral lens prescription and management practices: the scope study. Eye Contact Lens. 2018;44:S228–S232. doi:10.1097/ICL.0000000000000387
35. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3). doi:10.1016/j.jtos.2017.05.006
36. Li J, Zhang X, Zheng Q, et al. Comparative evaluation of silicone hydrogel contact lenses and autologous serum for management of Sjögren syndrome-associated dry eye. Cornea. 2015;34(9):1072–1078. doi:10.1097/ICO.0000000000000515
37. Scanzera AC, Bontu S, Joslin CE, McMahon T, Rosenblatt M, Shorter E. Prevalence of ocular surface disease and corneal irregularity and outcomes in patients using therapeutic scleral lenses at a tertiary care center. Eye Contact Lens. 2020;46(6):364–367. doi:10.1097/ICL.0000000000000679
38. Barnett M, Courey C, Fadel D, et al. CLEAR - Scleral lenses. Contact Lens Anterior Eye. 2021;44(2):270–288. doi:10.1016/j.clae.2021.02.001
39. Yılmaz U, Küçük E, Koç Ç, Gökler E. Comparison of autologous serum versus preservative free artificial tear in patients with dry eyes due to systemic isotretinoin therapy. Curr Eye Res. 2017;42(6):827–831. doi:10.1080/02713683.2016.1255758
40. Latifi G, Banafshe Afshan A, Houshang Beheshtnejad A, et al. Changes in corneal subbasal nerves after punctal occlusion in dry eye disease. Curr Eye Res. 2020;1–7. doi:10.1080/02713683.2020.1833349
41. Yüksel B, Bozdaǧ B, Acar M, Topaloǧlu E. Evaluation of the effect of topical cyclosporine A with impression cytology in dry eye patients. Eur J Ophthalmol. 2010;20(4):675–679. doi:10.1177/112067211002000405
42. Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens. 2014;40(5):289–296. doi:10.1097/ICL.0000000000000049
43. Finis D, Hayajneh J, König C, Borrelli M, Schrader S, Geerling G. Evaluation of an automated thermodynamic treatment (Lipiflow®) system for meibomian gland dysfunction: a prospective, randomized, observer-masked trial. Ocul Surf. 2014;12(2):146–154. doi:10.1016/j.jtos.2013.12.001
44. Napoli PE, Nioi M, d’Aloja E, Fossarello M. The bull’s eye pattern of the tear film in humans during visual fixation on en-face optical coherence tomography. Sci Rep. 2019;9(1). doi:10.1038/s41598-018-38260-5
45. Napoli PE, Coronella F, Satta GM, Fossarello M, Aegerter C. A novel technique of contrast-enhanced optical coherence tomography imaging in evaluation of clearance of lipids in human tears. PLoS One. 2014;9(11):e109843. doi:10.1371/journal.pone.0109843
46. Napoli PE, Coronella F, Satta GM, et al. Evaluation of the adhesive properties of the cornea by means of optical coherence tomography in patients with meibomian gland dysfunction and lacrimal tear deficiency. PLoS One. 2014;9(12):e115762. doi:10.1371/journal.pone.0115762
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.