Back to Journals » Clinical Ophthalmology » Volume 14
Oral Spironolactone versus Conservative Treatment for Non-Resolving Central Serous Chorioretinopathy in Real-Life Practice
Authors Sinawat S, Thongmee W , Sanguansak T , Laovirojjanakul W , Sinawat S, Yospaiboon Y
Received 2 May 2020
Accepted for publication 9 June 2020
Published 24 June 2020 Volume 2020:14 Pages 1725—1734
DOI https://doi.org/10.2147/OPTH.S260998
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Suthasinee Sinawat,1 Watcharaporn Thongmee,1 Thuss Sanguansak,1 Wipada Laovirojjanakul,1 Supat Sinawat,2 Yosanan Yospaiboon1
1KKU Eye Center, Department of Ophthalmology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; 2Department of Physiology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
Correspondence: Yosanan Yospaiboon; Suthasinee Sinawat
KKU Eye Center, Department of Ophthalmology, Faculty of Medicine, Khon Kaen University, 123 Mitraparb Highway, Khon Kaen 40002, Thailand
Tel +66 43 348383
Email [email protected]; [email protected]
Objective: To compare the efficacy of oral spironolactone treatment versus conservative treatment for patients with persistent central serous chorioretinopathy (CSC) in real-life practice.
Design: Retrospective comparative study.
Patients and Methods: Medical records and retinal images of 62 patients with non-resolving CSC were reviewed. Twenty-one patients received oral spironolactone (50 mg/day) while 41 patients received conservative treatment. Primary outcome was proportion of eyes with complete resolution of subretinal fluid (SRF) within 6 months. Secondary outcome measures included changes in SRF height, central macular thickness (CMT), lesion size and best-corrected visual acuity (BCVA). The occurrence of drug side effect was also assessed.
Results: There was no significant difference in demographic data, clinical characteristics, optical coherence tomography parameters and leaking patterns in fluorescein fundus angiography between two groups. Complete resolution of SRF was significantly higher and faster in the spironolactone group than the conservative treatment group (p=0.03). Although significant anatomical improvement in SRF height, CMT and lesion size were observed in both groups (p < 0.001), final BCVA was improved significantly in only the spironolactone group (p < 0.05). The recurrence of SRF after complete resolution was observed in 4/12 eyes (33.33%) in the treatment group. None of the patients experienced any side effects of spironolactone.
Conclusion: Oral spironolactone (50 mg/day) could achieve both significant anatomical and visual improvement, while the significant visual gain could not be provided with the conservative treatment. Spironolactone should be considered as an alternative treatment option in non-resolving CSC patients who cannot afford the PDT treatment.
Keywords: spironolactone, chronic, non-resolving, persistent, central serous chorioretinopathy
Introduction
Although Central serous chorioretinopathy (CSC) has been associated with various risk factors such as type A behavior, continuous stress, sleeping disturbance, smoking and steroid use; the exact pathogenesis of the disease is still not well ascertained.1–4 Most cases are acute CSC that usually resolves within 3–4 months. The chronic form, duration threshold varies from 3 to 6 months among previous studies, occurs in approximately 5% of cases.2,5 The chronicity often causes irreversible retinal pigment epithelium damage and deterioration of vision.
Photodynamic therapy (PDT) with half-dose of verteporfin has been reported to be the effective and safe treatment of acute and chronic CSC.6–9 It is superior to placebo, laser photocoagulation, subthreshold micropulse laser treatment and intravitreal anti-vascular endothelial growth factor (anti-VEGF) injection.6,10 In real-life situation, however, many patients could not access to this half-dose PDT because of economic concern and unavailable medical instrument. Since the duration of persistent subretinal fluid (SRF) is one of the impact-poor prognostic factors; therefore, non-resolving and chronic CSC remains to be a therapeutic challenge in the issues of alternative treatment and optimal timing for intervention.11,12 Recently, there are many treatment studies on shortening the duration of subretinal fluid including oral acetazolamide,13 rifampicin,14 intravitreal anti- VEGF,15,16 and anti-glucocorticoid.18–23
Inappropriate activation of the mineralocorticoid pathway could involve in the pathogenesis and have been reported to trigger or aggravate CSC.17,18,24 According to Preference and Trends (PAT) Survey 2016,25 the two commonly used mineralocorticoid receptor (MR) antagonists, spironolactone and eplerenone, have been proposed as promising treatment options for non-resolving CSC.26–33 Although eplerenone is a more selective MR antagonist, it has less potency than spironolactone in vivo, a 50-fold reduced efficacy on MR blockage.34 Furthermore, outcome of a recent randomized, double-blind clinical trial on eplerenone versus placebo treatment in CSC revealed that there was no role for the treatment.35 Spironolactone, however, is cheaper and more available. Many previous studies confirmed the effectiveness of spironolactone in persistent CSC; moreover, well tolerance with few side effects. Oral spironolactone significantly achieved reduced SRF and subfoveal choroidal thickness, as well as an improvement in BCVA.27–32 From literature review, few publications studied the effect of spironolactone in non-resolving and chronic CSC,27,29,30 and there have been only two prospective randomized controlled clinical trials that compared the efficacy between spironolactone and placebo. Although spironolactone showed a significant reduction of SRF more than placebo in persistent CSC, duration of treatment was nevertheless very short term in both studies.27,29 Therefore, this study aims to evaluate the efficacy and safety of spironolactone for the treatment of non-resolving CSC, compared with conservative treatment in a 6-month real-life practice.
Patients and Methods
Medical records, optical coherence tomography (OCT) and fluorescein fundus angiography (FFA) images of patients with persistent CSC from 2012 to 2018 at KKU Eye Center, Srinagarind Hospital, Khon Kaen University, Thailand were reviewed. This study followed the tenets of the Declaration of Helsinki and was approved by the Khon Kaen University Ethics Committee for Human Research (HE611220). Patient consents to review their medical records were not required due to retrospective study design, but the data in case report forms had no linkage to the patient identities and the researcher respected the confidentiality of the patient data. The patients were informed about the off-label use of spironolactone. Inclusion criteria included: (1) patients older than 18 years old; (2) persistent of SRF more than 3 months by history and clinical finding; (3) unaffordable for half-dose verteporfin PDT; (4) patients could adhere to the treatment and follow-up for 6 months. Exclusion criteria included: (1) presence of vitreoretinal or optic nerve diseases; (2) history of previous treatment such as oral acetazolamide, PDT, focal laser application or intravitreal anti-VEGF; (3) contraindicated to spironolactone therapy e.g. hypotension, hyperkalemia, kidney disease and pregnancy; (4) media opacities that cause poor OCT and FFA image quality.
Patients were informed about the off-label use of oral spironolactone. Those who refused to use the drug were treated with conservative treatment. The patients in treatment group received oral spironolactone (Aldactone, Pfizer Inc., USA) 25 mg twice daily. The patients in control group received conservative treatment including oral vitamin B supplement and/or minor tranquilizer medication. At baseline, all patients underwent an ophthalmologic examination including assessment of Snellen best-corrected visual acuity (BCVA), FFA and spectral-domain OCT (Spectralis HRA – OCT, Heidelberg Engineering, Heidelberg, Germany). All patients were counseled on steroid avoidance and acknowledged the side effect of spironolactone. The serum electrolytes were checked before taking the spironolactone and then followed by the primary care physician every 1–2 months. The outcomes were evaluated at 1 month, 3 months and 6 months after treatment.
The following data were collected from the medical records: age, gender, occupation, medical condition, duration of symptoms, prior use of corticosteroids, Snellen BCVA, FFA and OCT images. The SRF height, central macular thickness (CMT), lesion size and serous pigment epithelial detachment (PED) were evaluated on OCT at baseline, 1 month, 3 months and 6 months after treatment. The SRF height was measured at the maximal distance of subretinal detachment between the outer part of external limiting membrane and the inner part of the RPE. Central macular thickness was scaled by Spectralis software. Lesion size was defined by using a built-in caliper to measure at the greatest linear dimension of the entire CSC lesion (Figure 1). The SRF height and lesion size were measured by two independent graders (SS, WT) and the average results were used in the analysis. The pattern of dye leakage on FFA images was also identified and recorded.
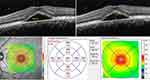 |
Figure 1 Measurement of subretinal fluid height (upper left), lesion size (upper right) and central macular thickness (lower). |
The primary outcome of the study was the proportion of eyes with complete resolution of SRF in each group during the follow-up period. The secondary outcomes were the changes in SRF height, CMT, lesion size and mean BCVA from baseline to 6 months. Recurrence rate and adverse drug reaction of the treatment were also evaluated and analyzed.
Statistical Analyses
Categorical data were summarized using proportion, difference of proportion with 95% confidence intervals and percentage. Continuous data were summarized using means and standard deviation. Categorical variables were analyzed using the chi-square test or Fisher’s exact test. Continuous variables were compared between the groups, and pre- and post-treatment values were compared within the groups using the parametric independent t-test. The proportion of eyes with complete resolution of SRF in both groups was compared by Kaplan–Meier survival estimate and analyzed using Cox regression test. Using multivariate analysis, the prognostic factors associated with the success of treatment were also analyzed using Cox regression test and expressed in terms of hazard ratio and 95% confidence intervals. Snellen visual acuity records were converted to logarithm of the minimum angle of resolution (logMAR) for statistical analysis. The statistical evaluation was performed using STATA software, version 10.0 (Statacorp LLC, Texas, USA). A p-value of less than 0.05 was considered to be statistically significant.
Results
Patient Demographics
Sixty-two eyes of 62 patients (38 men and 24 women) were included in the study. Mean age was 44.96±8.62 years old. Twenty-one eyes (12 men and 9 women) received spironolactone therapy (treatment group) and 41 eyes (26 men and 15 women) received conservative treatment including oral vitamin B supplement and/or minor tranquilizer medication (control group). Mean duration of the symptom was 5.28±1.10 months in the treatment group and 4.24±1.92 months in the control group. Previous corticosteroid use was reported in 4 cases (2 cases in the treatment group due to herbal use and steroid inhaler in asthma treatment and 2 cases in the control group due to topical steroid use in psoriasis treatment and oral steroid use in scleroderma treatment). Mean duration of spironolactone treatment was 4.85±1.42 months (range 2–7 months). One patient in treatment group received spironolactone for 7 months due to recurrent and persistent SRF beyond 6 months. All baseline data of patients in both groups were not significantly different (Table 1).
 |
Table 1 Demographic Data and Clinical Characteristics |
Baseline OCT Parameters and FFA Patterns
The mean baseline SRF height, CMT and lesion size on OCT was 222, 380 and 2977 μm in the treatment group and 255, 416 and 3481 μm in the control group, respectively. Nine patients in the treatment group and 10 patients in the control group had accompanied serous PED. Most of fluorescein angiography patterns were non-specific, followed by expansile dot pattern and smokestack. The expansile dot pattern occurred in 9 patients (42.86%) in the treatment group and 19 patients (46.34%) in the control group. The smokestack dye leakage was found in only one patient (4.76%) in the treatment group and two patients (4.88%) in the control group. There were no statistically significant differences in baseline OCT parameters and FFA pattern between both groups (Table 1).
Complete Resolution of Subretinal Fluid
The proportion of eyes with complete resolution of SRF was statistically significantly higher in the treatment group (57.14%) than those in the control group (31.71%) (p = 0.032) during the study; while undetectable SRF was demonstrated in 9 (42.86%) patients since the first month after spironolactone treatment (Table 2). Kaplan–Meier survival analysis of complete SRF regression over the 6-month follow-up of patients in both groups is demonstrated in Figure 2.
 |
Table 2 Comparison of Eyes with Complete Resolution of SRF in Both Groups |
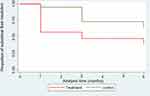 |
Figure 2 Kaplan–Meier survival estimates over 6-month follow up of patients in both groups. Cox regression test: p = 0.032, HR = 0.42, 95% CI (0.19, 0.93). |
Subretinal Fluid Height
The mean SRF height was significantly decreased in each group (p < 0.001), from 222.4 um at baseline to 103.2 um at 6 months in the treatment group and from 255.2 µm to 140.5 µm in the control group (Table 3 and Figure 3). The mean reduction of SRF was 119.1 and 114.7 µm in the treatment group and control group, respectively. The mean % change from baseline in the treatment group was 54.5% as compared with 35.6% in the control group. Although patients with 100% reduction of SRF height in the treatment group were much more than those in the control group, the overall SRF height reduction from baseline to 6 months between both groups did not reach statistical significance (p=0.06) (Table 4).
 |
Table 3 Secondary Outcomes Measured at Baseline, 1 Month, 3 Months and 6 Months |
 |
Table 4 Secondary Outcome Changes from Baseline to 6 Months in Both Groups |
Central Macular Thickness
The mean CMT was significantly decreased in each group (p < 0.001), from 380.3 µm at baseline to 257.3 µm at 6 months in the treatment group and from 416.4 µm to 309.4 in the control group (Table 3 and Figure 3). The CMT reduction of more than 10% from baseline was noted in 80.95% of patients in the treatment group and 70.73% in the control group. Nevertheless, there was no statistically significant difference in overall CMT reduction between both groups (p = 0.72) (Table 4).
Lesion Size
The mean lesion size was significantly decreased in each group, from 2977.6 µm at baseline to 1966.2 um at 6 months in the treatment group (p = 0.02) and from 3481.6 µm to 2220.6 µm in the control group (p < 0.001) (Table 3 and Figure 3). Although patients with 50–100% reduction of lesion size in the treatment group (76.19%) were much more than those in the control group (39.03%), the overall lesion size reduction from baseline to 6 months between both groups did not demonstrate statistical significance (p=0.27) (Table 4).
Best-Corrected Visual Acuity
In the treatment group, the mean BCVA significantly improved from 0.47 logMAR at baseline to 0.38 logMAR at 6 months (p < 0.05). The mean BCVA improved from 0.27 logMAR at baseline to 0.24 logMAR at 6 months in the control group, but this improvement did not have statistical significance (p = 0.23) (Table 3 and Figure 3). The percentage of eyes with BCVA improvement was observed in 47.62% of the treatment group and 29.27% in the control group. Moreover, the 2-line and 3-line BCVA gain in the treatment group was much more than those in the control group. However, the overall BCVA change did not demonstrate a statistical significance (p =0.39) (Table 4).
Many prognostic factors were analyzed including age, history of steroid use, duration of disease, baseline BCVA, SRF, CMT and lesion size, presence of serous PED, SRF resolved at 3 months and FFA patterns. Table 5 demonstrates that duration of disease less than 6 months, SRF resolved at 3 months and expansile dot pattern of dye leakage on FFA were associated with the success of treatment at 6 months with statistically significant difference. During the 6-month follow-up period, moreover, the recurrence of SRF after complete resolution was observed in 4/12 eyes (33.33%) in the treatment group and 4/13 eyes (30.76%) in the control group. No patient experienced any side effects including the anti-androgenic effect such as gynecomastia, which might be adverse drug reactions from using spironolactone.
 |
Table 5 Multivariate Analysis of Factors Associated with the Complete Resolution of SRF |
Discussion
Acute or chronic CSC is usually classified by considering the duration of serous retinal detachment (SRD) and the presence of diffuse retinal pigment epitheliopathy. Since no consensus exists over the duration threshold and chronic form characterized by extended pigment epitheliopathy, then the terms “non-resolving” or “persistent” CSC are more appropriate to describe CSC with long-lasting SRD after the initial 3 months period.17
In this study, we demonstrated that spironolactone 50 mg per day allowed complete resolution of SRF in 57.14% at 6 months. This finding was similar to previous studies.28,30 Daruich et al showed 50% of studied eyes had a complete resolution of foveal SRF at 6 months.28 In a prospective interventional study,30 spironolactone was prescribed 25 mg daily for the first 6 weeks. If SRF persisted by that time, treatment was continued with the double dosage. The SRF resolved completely in 18.75% of studied eyes at 6 weeks that much lower than 42.85% at 1 month in our study. This difference may be explained by lower dosage of spironolactone during the initial 6 weeks. When the dosage was increased to 50 mg per day, the complete SRF resolution at the final visit was 43.75%, which was comparable to our study.30 However, Bousquet et al reported complete SRF resolution in only 26.56% of treated eyes at 6 months. This discrepancy may be attributed to the fact that most patients (88%) in their retrospective case series were treated with eplerenone, which had less potency than spironolactone.31 Moreover, Zola et al evaluated the outcomes in patients with chronic CSC who maintained oral MR antagonists for at least 6 months. They found complete SRF resolution 31%, 44%, 69% and 81% of eyes at months 6, 12, 18 and 24, respectively.32 This lower rate of success at 6 months may be caused by the retinal pigment epitheliopathy. Their increasing rate of complete SRF resolution with time indicates that the duration of anti-glucocorticoid intakes should be longer in chronic cases.
Up to the present, there have been only two prospective randomized controlled clinical trials that compared the efficacy between spironolactone and placebo, but duration of treatment was 1–2 months in both studies.27,29 Our 6-month study demonstrated that the proportion of eyes with complete resolution of SRF was significantly higher and faster in the treatment group than those in the control group. Interestingly, eyes with conservative treatment also had complete SRF resolution in 31% of patients, as well as significant anatomical improvement. These findings were in harmony with previous studies.36–38 Daruich et al evaluated the factors influencing the duration of acute CSC episode in patients with no treatment, and found that majority of the patients had SRF resolution by 6 months. The results showed that 61.54% of them resolved within 3 months, whereas 38.46% resolved from months 4 to months 6.36 Dang et al evaluated the effect of Helicobacter pylori eradication in patients with acute CSC and reported that 34.6% of the patients in the control group achieved complete SRF resolution.37 Ozkaya et al also reported that 25.9%, 41.56% and 53.25% of the patients with acute CSC achieved complete spontaneous regression at 3, 6 and 12 months, respectively.38 These results indicated that spontaneous SRF resorption by RPE pump continued to work in chronic CSC after 3 months.
Moreover, our study also compared anatomical and functional outcomes between the treatment group and the control group. SRF height, central macular thickness and lesion size in each group improved significantly from baseline to 6-month visit. These results also accorded well with those in previous studies.28,30,31 When compared between the groups, however, there was no statistically significant difference in the improvement of SRF height, CMT and lesion size, though clinical improvement was much more in the treatment group than the control group.
In terms of BCVA improvement, the present study demonstrated that the final BCVA in the spironolactone group improved significantly when compared to the baseline visit. This result was consistent with other previous studies.28,30-32 Pichi et al conducted a prospective placebo-controlled trial in persistent CSC and reported that both spironolactone and eplerenone treatment were effective in improving BCVA and promoting the reabsorption of SRF; while there was no statistical improvement of BCVA in the control group.29 Our study with longer treatment duration and follow-up period also revealed that no significant visual improvement was found in the conservative treatment group, though SRF height, CMT and lesion size decreased significantly. The explanation for this result could be attributed to the structural and functional damage in photoreceptor and RPE cells from long-standing SRF. Furthermore, when compared between the groups, the percentage of eyes with BCVA improvement was more in the treatment group than the control group. The 2-line and 3-line BCVA gain in the treatment group was also much more than those in the control group. However, there was no statistically significant difference in BCVA changes between the groups, though clinical significance was noted.
The demographic data in our study, mean age of patients and proportion of male, were accordingly comparable to previous studies.27–31 However, the prognostic factors related to the success of treatment were dissimilar. In chronic CSC, the MR antagonists response was delayed when compared with persistent and recurrent cases.28 Age of more than 51 years, subfoveal choroidal thickness and great subfoveal SRF resolution were associated with good treatment response in prior publications.28,31,32 In our study, however, the good prognostic factors related to the complete resolution of SRF were duration of the disease less than 6 months, SRF resolved at 3 months and expansile dot pattern of leakage in FFA. We did not find statistically significant association of age with the treatment success. Up to the present, our study first reported the expansile dot of leakage as the positive prognostic factors in persistent CSC.
The recurrence could occur, despite the patients being on treatment with MR antagonists. In our study, we found recurrence rate of 33.33% in spironolactone group. Zola et al showed recurrence rate of 50% after cessation of treatment at month 6 and 25% in patients under extended MR antagonist treatment (10–24 months).32 This suggested that lower recurrence rate may be the result of the longer duration of drug treatment.
Due to the higher affinity to mineralocorticoid receptor, spironolactone produces more side effects that could appear earlier in the first month after treatment. The incidence of side effect of spironolactone varied widely from 20% to 75%, which was 3–4 times higher than that of eplerenone.20,28,29,31,32 Furthermore, the occurrence of side effect in patients treated with spironolactone was much earlier than those treated with eplerenone. In a long-term study, all side effects of spironolactone appeared within 7 months whereas after 18 months in eplerenone.31 The reported side effects included dizziness, hypotension, hyperkalemia, gynecomastia, sudation, asthenia, arm tingling, gastric pain, urinary retention and constipation. Fortunately, all could be resolved after discontinuing or switching to eplerenone.28,29,31,32 In this study, none of the patients in the treatment group experienced any side effect. This may be attributed to the relatively short treatment duration and careful monitoring of serum potassium in the patients. Since longer treatment duration would be beneficial in patients with chronic CSC, so careful screening and monitoring were important during the treatment.
The strength of our study is the first report to compare the efficacy between spironolactone and conservative treatment in treatment-naïve non-resolving CSC. The limitation is the retrospective design that there may be some missing data especially choroidal thickness measurement with enhanced depth imaging (EDI) OCT. In addition, patients who could not access to or refuse to receive PDT treatment and have rather good vision might refuse to try oral spironolactone treatment. Baseline BCVA in the treatment group was therefore worse than the control group though it did not demonstrate a statistical significance. This may introduce a potential selection bias. Further prospective randomized controlled trial in a large series of patients and a longer period of follow-up is therefore warranted and would provide more conclusive information on the long-term efficacy, recurrence rate, prognostic factors and safety of spironolactone treatment in non-resolving and chronic CSC.
Conclusion
Spironolactone 50 mg per day has shown promising anatomical and functional effects without any tissue damage from laser and photodynamic therapy in patients with non-resolving CSC. The complete resolution of SRF in the treatment group was significantly higher and faster than those in the control group. Most spironolactone-treated patients had complete resolution of SRF in as early as the first month. Early resolution of SRF decreases the chance and degree of further structural damage and consequent visual impairment. This explains the result that spironolactone significantly improved the final BCVA whereas conservative treatment did not. Since the recurrence usually occurs, longer treatment duration will be advantageous in persistent CSC although the optimal duration of treatment remains to be determined. Despite the off-label use, spironolactone appears to be an effective and safe treatment option in patients with non-resolving CSC.
Acknowledgments
The authors thank Dr. Kaewjai Thepsuthammarat, Clinical Epidemiology Unit, Faculty of Medicine, Khon Kaen University for statistical analyses. This study was supported by invitation research grant (IN59161) from the Faculty of Medicine, Khon Kaen University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liew G, Quin G, Gillies M, et al. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41(2):201–214. doi:10.1111/j.1442-9071.2012.02848.x
2. Semeraro F, Morescalchi F, Russo A, et al. Central serous chorioretinopathy: pathogenesis and management. Clin Ophthalmol. 2019;13:2341–2352. doi:10.2147/OPTH.S220845
3. Liu B, Deng T, Zhang J. Risk factors for central serous chorioretinopathy: a systematic review and meta-analysis. Retina. 2016;36(1):9–19. doi:10.1097/IAE.0000000000000837
4. Chatziralli I, Kabanarou SA, Parikakis E, et al. Risk factors for central serous chorioretinopathy: multivariate approach in a case-control study. Curr Eye Res. 2017;42(7):1069–1073. doi:10.1080/02713683.2016.1276196
5. Loo RH, Scott IU, Flynn HW, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22(1):19–24. doi:10.1097/00006982-200202000-00004
6. Ma J, Meng N, Xu X, et al. System review and meta-analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmol. 2014;92(8):e594–e601. doi:10.1111/aos.12482
7. Lu HQ, Wang EQ, Zhang T, et al. Photodynamic therapy and anti-vascular endothelial growth factor for acute central serous chorioretinopathy: a systematic review and meta-analysis. Eye (Lond). 2016;30(1):15–22. doi:10.1038/eye.2015.208
8. Salehi M, Wenick AS, Law HA, et al. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015;12:CD011841.
9. Lim JI, Glassman AR, Aiello LP, et al. Collaborative retrospective macula society study of photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology. 2014;121(5):1073–1078. doi:10.1016/j.ophtha.2013.11.040
10. van Dijk EHC, Fauser S, Breukink MB, et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic serous chorioretinopathy. The PLACE trial. Ophthalmology. 2018;125:1547–1555. doi:10.1016/j.ophtha.2018.04.021
11. Gilbert CM, Owens SL, Smith PD, et al. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68(11):815–820. doi:10.1136/bjo.68.11.815
12. Behnia M, Khabazkhoob M, Aliakbari S, et al. Improvement in visual acuity and contrast sensitivity in patients with central serous chorioretinopathy after macular subthreshold laser therapy. Retina. 2013;33(2):324–328. doi:10.1097/IAE.0b013e3182670fa3
13. Pikkel J, Beiran I, Ophir A, et al. Acetazolamide for central serous retinopathy. Ophthalmology. 2002;109:1723–1725. doi:10.1016/S0161-6420(02)01157-0
14. Steinle NC, Gupta N, Yuan A, et al. Oral rifampin utilisation for the treatment of chronic multifocal central serous retinopathy. Br J Ophthalmol. 2012;96(1):10–13. doi:10.1136/bjophthalmol-2011-300183
15. Okamoto M, Matsuura T, Ogata N. Choroidal thickness and choroidal blood flow after intravitreal bevacizumab injection in eyes with central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging Retina. 2015;46:25–32. doi:10.3928/23258160-20150101-04
16. Beger I, Koss MJ, Koch FH. Treatment of central serous chorioretinopathy: microPulse photocoagulation versus bevacizumab. Ophthalmologe. 2012;109:1224–1232. doi:10.1007/s00347-012-2688-7
17. Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi:10.1016/j.preteyeres.2015.05.003
18. Bousquet E, Beydoun T, Zhao M, et al. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy-a pilot study. Retina. 2013;33:2096–2102. doi:10.1097/IAE.0b013e318297a07a
19. Herold TR, Prause K, Wolf A, et al. Spironolactone in the treatment of central serous chorioretinopathy - a case series. Graefes Arch Clin Exp Ophthalmol. 2014;252:1985–1991. doi:10.1007/s00417-014-2780-6
20. Kapoor KG, Wagner AL. Mineralocorticoid antagonists in the treatment of central serous chorioretinopathy: a comparative analysis. Ophthalmic Res. 2016;56:17–22. doi:10.1159/000444058
21. Ghadiali Q, Jung JJ, Yu S, et al. Central serous chorioretinopathy treated with mineralocorticoid antagonists: a one-year pilot study. Retina. 2016;36:611–618. doi:10.1097/IAE.0000000000000748
22. Herold TR, Rist K, Priglinger S, et al. Long-term results and recurrence rates after spironolactone treatment in non-resolving central serous chorio-retinopathy (CSCR). Graefes Arch Clin Exp Ophthalmol. 2017;255(2):221–229. doi:10.1007/s00417-016-3436-5
23. Sun X, Shuai Y, Fang W, et al. Spironolactone versus observation in the treatment of acute central serous chorioretinopathy. Br J Ophthalmol. 2018;102(8):1060–1065. doi:10.1136/bjophthalmol-2017-311096
24. Zhao M, Celerier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122(7):2672–2679. doi:10.1172/JCI61427
25. Stone TW. ASRS 2016 Preferences and Trends (PAT) survey: American Society of Retina Specialists (ASRS).[cited November 5, 2016] Available from: http://www.asrs.org/asrs-community/pat-survey.
26. Chin EK, Almeida DR, Roybal CN, et al. Oral mineralocorticoid antagonists for recalcitrant central serous chorioretinopathy. Clin Ophthalmol. 2015;9:1449–1456. doi:10.2147/OPTH.S86778
27. Bousquet E, Beydoun T, Rothschild P-R, et al. Spironolactone for nonresolving central serous chorioretinopathy: a randomized controlled crossover study. Retina. 2015;35(12):2505–2515. doi:10.1097/IAE.0000000000000614
28. Daruich A, Matet A, Dirani A, et al. Oral mineralocorticoid-receptor antagonists: real-life experience in clinical subtypes of nonresolving central serous chorioretinopathy with chronic epitheliopathy. Transl Vis Sci Technol. 2016;5(2):2. doi:10.1167/tvst.5.2.2
29. Pichi F, Carrai P, Ciardella A, et al. Comparison of two mineralcorticosteroids receptor antagonists for the treatment of central serous chorioretinopathy. Int Ophthalmol. 2017;37(5):1115–1125. doi:10.1007/s10792-016-0377-2
30. Falavarjani KG, Amirsardari A, Habibi A, et al. Visual and anatomical outcomes of spironolactone therapy in patients with chronic central serous chorioretinopathy. J Ophthalmic Vis Res. 2017;12(3):281–289. doi:10.4103/jovr.jovr_139_16
31. Bousquet E, Dhundass M, Lejoyeux R, et al. Predictive factors of response to mineralocorticoid receptor antagonists in nonresolving central serous chorioretinopathy. Am J Ophthalmol. 2019;198:80–87. doi:10.1016/j.ajo.2018.09.034
32. Zola M, Daruich A, Matet A, et al. Two-year follow-up of mineralocorticoid receptor antagonists for chronic central serous chorioretinopathy. Br J Ophthalmol. 2019;103(8):1184–1189. doi:10.1136/bjophthalmol-2018-312892
33. Wang SK, Sun P, Tandias RM, et al. Mineralocorticoid receptor antagonists in central serous chorioretinopathy: a meta-analysis of randomized controlled trials. Ophthalmol Retina. 2019;3(2):154–160. doi:10.1016/j.oret.2018.09.003
34. De Gasparo M, Joss U, Ramjoué HP, et al. Three new epoxy-spirolactone derivatives: characterization in vivo and in vitro. J Pharmacol Exp Ther. 1987;240(2):650–656.
35. Lotery A, Sivaprasad S, O’Connell A, et al.; VICI trial investigators. Eplerenone for chronic serous chorioretinopathy in patients with active previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10220):294–303. doi:10.1016/S0140-6736(19)32981-2
36. Daruich A, Matet A, Marchionno L, et al. Acute central serous chorioretinopathy: factors influencing episode duration. Retina. 2017;37(10):1905–1915. doi:10.1097/IAE.0000000000001443
37. Dang Y, Mu Y, Zhao M, et al. The effect of eradicating Helicobacter pylori on idiopathic central serous chorioretinopathy patients. Ther Clin Risk Manag. 2013;9:355–360. doi:10.2147/TCRM.S50407
38. Ozkaya A, Alkin Z, Ozveren M, et al. The time of resolution and the rate of recurrence in acute central serous chorioretinopathy following spontaneous resolution and low-fluence photodynamic therapy: a case-control study. Eye. 2016;30(7):1005–1010. doi:10.1038/eye.2016.79
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

