Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 10
Oral hyaluronan relieves wrinkles: a double-blinded, placebo-controlled study over a 12-week period
Authors Oe M, Sakai S, Yoshida H, Okado N, Kaneda H, Masuda Y, Urushibata O
Received 15 May 2017
Accepted for publication 12 June 2017
Published 18 July 2017 Volume 2017:10 Pages 267—273
DOI https://doi.org/10.2147/CCID.S141845
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Mariko Oe,1 Seigo Sakai,1 Hideto Yoshida,1 Nao Okado,1 Haruna Kaneda,1 Yasunobu Masuda,1 Osamu Urushibata2
1R&D Division, Kewpie Corporation, Sengawa-cho, Chofu-shi, 2Department of Dermatology, Toho University Ohashi Medical Center, Ohashi, Meguro-ku, Tokyo, Japan
Background: Hyaluronan (HA) has critical moisturizing property and high water retention capacity especially for human skin. This study aimed to evaluate the effect of oral intake of HA.
Methods: The mean molecular weight (MW) of HA is 2 k and 300 k. Sixty Japanese male and female subjects aged 22–59 years who presented with crow’s feet wrinkles were randomly assigned to the HA 2 k or HA 300 k at 120 mg/day or the placebo group. The subjects were administered HA at a rate of 120 mg/day or a placebo for 12 weeks. The skin wrinkles were evaluated by image analysis of skin wrinkle replicas, and their skin condition was evaluated using a questionnaire survey.
Results: During the study period, the HA groups showed better level of the whole sulcus volume ratio, wrinkle area ratio, and wrinkle volume ratio than the placebo group. After 8 weeks of ingestion, the HA 300 k group showed significantly diminished wrinkles compared with the placebo group. Skin luster and suppleness significantly improved after 12 weeks in all groups compared with the baseline.
Conclusion: The results suggest that oral HA (both HA 2 k and HA 300 k) inhibits skin wrinkles and improves skin condition.
Keywords: hyaluronic acid, dietary supplement, skin, wrinkle volume, molecular weight
Background
Hyaluronan (HA) is a body component that is present in every connective tissue and organ, such as skin, synovial fluid, blood vessels, serum, the brain, cartilage, heart valves, and the umbilical cord. In particular, the skin has the largest quantity of HA in the body, with 50% of total body HA present in the skin.1
Wrinkles of the skin are formed under the influence of various factors, such as aging, ultraviolet (UV) light, and dryness. In particular, the degradation of collagen and HA by UV damage causes wrinkles.2,3 It is considered that the cause of crow’s feet is the same.
HA content in the skin is considered to be related to the factors that cause wrinkles. Kawada et al4 treated hairless mice at a dose of 200 mg/kg body weight per day for 6 weeks with exposure to UV irradiation and measured their skin condition. The HA group displayed a significant decrease in UV damage to the skin compared with the control group (p < 0.05). The effect on skin wrinkles through the use of oral HA is expected because the decrease of skin damage leads to relieving of skin wrinkles. In addition, dry skin was improved by oral ingestion of HA.5–8 The quantity of HA in the skin gradually decreases due to aging. For example, a 75-year-old person has only one quarter of the amount of HA in their skin compared with a 19-year-old person.9
With respect to skin wrinkles, there are some surgical techniques that use fillers as a symptomatic treatment. However, the cost of treatment is high, and there are risks of pain and swelling.10 Although topical treatment such as injection of HA takes effect quickly, the effect will be diminished gradually.11 On the other hand, it takes time to relieve wrinkles by taking supplements; however, the effect will be maintained by taking them continuously. Accordingly, the demand for dietary supplements grows because supplements are easy to take continuously and support the inner body. Several studies reported some of the effects that dietary supplements have on keeping skin healthy.12
Two clinical trials of oral HA have been performed. Kim et al treated 52 Korean female subjects aged over 30 years who had crow’s feet wrinkles (placebo group n = 26, HA group n = 26) with HA (MW, 75 k, 240 mg/day) for 8 weeks. The HA group presented with significantly less wrinkles compared with the placebo group (p < 0.05).13
Watanabe et al treated 28 Japanese female subjects aged 30–49 years who had crow’s feet wrinkles (placebo group n = 14, HA group n = 14) with HA (MW, 38 k, 240 mg/day) for 8 weeks. The HA group presented with a significantly decreased maximum wrinkle mean depth compared with the placebo group (p < 0.01).14
It is not confirmed whether the anti-wrinkle effect of oral HA is different depending on its MW or amount of intake. However, it is known that the bioavailability of HA differs depending on the molecular weight.15 Hisada et al reported that the lower molecular weight of HA indicates the more HA permeated through human intestinal Caco-2 cell monolayers.16
Thus, we evaluated anti-wrinkle and MW effects of oral ingestion of HA (MW, 2 k and 300 k, 120 mg/day) in this double-blind, placebo-controlled study for 12 weeks with Japanese male and female subjects aged 22–59 years. Both of the two types of HA (MW, 2 k and 300 k) are composed of simple disaccharide sequences (d-glucuronic acid and d-N-acetylglucosamine, bound through alternating β-1,4- and β-1,3-glycosidic bonds). HA 2 k is slightly more soluble than HA 300 k.
Methods
Subjects
The subjects were healthy Japanese male and female volunteers aged 22–59 years who presented with crow’s feet wrinkles. Informed consent was obtained in writing from each subject prior to enrollment in the study. Sixty volunteers were selected. Our exclusion criteria are shown in Table 1. The selected subjects were assigned randomly to three groups, i.e., the placebo, HA 2 k, and HA 300 k treatment groups. After grouping, a double-blind, placebo-controlled study was conducted.
  | Table 1 Exclusion criteria Abbreviation: HA, hyaluronan. |
Supplements and dosage
The two types of HA, Hyabest® (A) and Hyabest® (S) LF-P, used in this study were manufactured by Kewpie Corporation (Tokyo, Japan) and had a 95% purity according to a high-performance liquid chromatography (HPLC) analysis. The mean MWs were ~2 k and 300 k, respectively. Microcrystalline cellulose was obtained from the Asahi Kasei Chemicals Corporation (Tokyo, Japan). The placebo group received two capsules (210 mg microcrystalline per capsule), and the HA 2 k group received two capsules (60 mg HA with microcrystalline cellulose, total 210 mg per capsule) each day. The HA 300 k group received two capsules (60 mg HA with microcrystalline cellulose, total 210 mg per capsule) each day. There were no differences in the appearance and taste of the HA and placebo capsules. The capsules were manufactured by Aliment Industry Co. Ltd (Yamanashi, Japan).
Clinical trial design
This study was conducted by Kewpie Corporation, who carried out a randomized, double-blind, placebo-controlled trial. Subjects were randomly assigned to either the HA 2 k, HA 300 k, or placebo group before starting the study according to stratified randomization based on age by the study coordinator, MO. Allocation was documented in an electronic file accessible only to the study coordinator. The study investigators, NO and HK, who did not know the allocation, handed the samples which are no difference in appearance to the subjects. The subjects consumed two capsules each day for 12 consecutive weeks. The skin evaluation was conducted prior to ingestion and after 4, 8, and 12 weeks of treatment. The evaluation of skin conditions was conducted using the image analysis of skin wrinkle replicas and a questionnaire survey.
Clinical trial register
This study is registered with the Center for Clinical Trials, Japan Medical Association. The clinical trial registration number is the JMA-IIA00234.
This clinical trial was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the institutional review board (Shinkohkai Medical Corporation Institutional Review Board, Tokyo, Japan).
Evaluation of skin
Wrinkle replica image analysis
Collection and analysis of the replica were carried out as follows, according to the Guideline for Evaluation of Anti-wrinkle Products (Japanese Cosmetic Science Society).17 To keep a fixed measurement condition as much as possible, subjects washed their face and rested for 20 minutes in a waiting room with mild environmental conditions (room temperature: 22 ± 2°C, relative humidity: 50% ± 15%). On evaluation day, the subjects applied the skin replica agent (Asahi Techno Lab Corporation, Kanagawa, Japan) on the corner of their eye. After it had sufficiently dried, the participants peeled the replicas off the skin and allowed the replica to dry again. The replicas were evaluated using a three-dimensional (3D) skin analyzing software of digital reflection type ASA–03RXD Ver3.18 (Asahi Techno Lab Corporation). The amount of change relative to the initial value of the mean value in each item was compared among the groups with respect to the placebo group.
Questionnaire survey
A questionnaire survey was completed by the subjects to assess the luster, suppleness, and wrinkles of the facial skin using the five-point evaluation scale (Table 2) at the following times: prior to ingestion and after 4, 8, and 12 weeks of ingestion. For the skin luster and suppleness, one is the worst and five is the best. For the wrinkles, one is least wrinkles; not worry, and five is most wrinkles; most care. The amount of change relative to the initial value of the score mean value in each item was compared among the groups with respect to the placebo group.
  | Table 2 Methods used to evaluate the skin conditions in the questionnaire |
A questionnaire survey on adverse effects, such as allergic reactions, diarrhea, and abdominal pain, was conducted during the study.
Image of microscope camera
The skin surface of the corner of the eye was observed by Digital microscope VHX-2000 (Keyence Corporation, Osaka, Japan).
Statistical analysis
Repeated measures analysis of variance (ANOVA) and Dunnett’s test were used to compare a replica image analysis between the initial value and each measurement point. A one-way ANOVA and multiple comparison tests of Tukey were used to compare the placebo group and the HA group. For the questionnaire survey, a Wilcoxon signed-rank sum test was used to compare the initial value and each measurement point. A one-way ANOVA and the multiple comparison test of Bonferroni were used to compare the placebo group and the HA group. All values obtained are expressed as the mean ± standard errors (SEs). The test results are expressed as mean ± SE. A p-value <0.05 was considered statistically significant, and a p-value of <0.1 was a significant trend. Statistical analysis computer software SPSS version 20 (IBM Corporation, Armonk, NY, USA) was used.
Results
Wrinkle replica image analysis
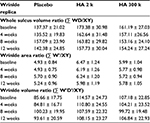
For the evaluation of the wrinkle replica, two people from the placebo group and four people from the HA 2 k group and the HA 300 k group (which originally had 60 people) did not complete the trial due to personal reasons. A total of 50 people were assessed as shown in Table 3. Temporal changes in the whole sulcus volume ratio, wrinkle area ratio, and wrinkle volume ratio during the study period are shown in Table 4. In the placebo group, the whole sulcus volume ratio, wrinkle area ratio, and wrinkle volume ratio had a higher value but were not significant compared with that prior to ingestion. In the HA 2 k group and HA 300 k group, the whole sulcus volume ratio, wrinkle area ratio, and wrinkle volume ratio showed a consistently low value compared with that prior to ingestion.
  | Table 3 Baseline characteristics of subjects Abbreviation: HA, hyaluronan. |
The amount of change in the whole volume ratio, wrinkle area ratio, and wrinkle volume ratio during the test period is shown in Figure 1. In the wrinkle volume ratio after 8 weeks ingestion (Figure 1C), the HA 300 k group showed significantly lower values than the placebo group (p < 0.05). The value before ingestion did not affect the results, therefore a covariance analysis (analysis of covariance [ANCOVA]) was not performed.
Questionnaire survey
Subjective symptoms about the luster, suppleness, and wrinkles were evaluated using a questionnaire. In the questionnaire evaluation, one person from the placebo group and two from both the HA 2 k group and the HA 300 k group (of the original 60 people) did not complete the questionnaire because of personal reasons. A total of 55 people participated. In the luster and suppleness, all groups significantly increased the score after 12 weeks (p < 0.05) compared with that prior to ingestion. For the luster, the HA 2 k group and the HA 300 k group showed an increase of scores compared with the placebo group at all measured points after 4, 8, and 12 weeks. For wrinkles, the placebo group, HA 2 k group, and HA 300 k group showed a significantly decreased score after 8 and 12 weeks compared to prior to ingestion (Figure 2).
In this study, a questionnaire survey and observation were conducted, and there were no adverse events.
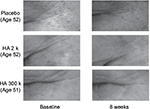
Image of microscope camera
We could not confirm the change of the skin surface after ingestion for the subjects who ingested placebo; however, the skin surface of the subjects who ingested HA tended to improve. As an example of each group, microscopic images of the outer corner of the eye are shown prior to ingestion and after 8 weeks of ingestion (Figure 3).
Discussion
This was a double-blind, randomized placebo-controlled study of people aged 22–59 years who were healthy Japanese men and women and who presented with crow’s feet wrinkles. They were given the 120 mg HA of MW 2 k or 300 k per day for 12 weeks. As a result, in the replica image analysis evaluation of wrinkles and the questionnaire survey of subjective symptoms in the skin, it was confirmed that ingestion of HA improved the luster and suppleness of the skin and caused a decrease in skin wrinkles.
Temporal changes in the whole sulcus volume ratio, wrinkle area ratio, and wrinkle volume ratio during the study period are shown in Table 4. In the placebo group, the whole sulcus volume ratio, wrinkle area ratio, and wrinkle volume ratio increased at 8 weeks probably because this study was conducted in dry season from January to April. In the replica image analysis of both the HA 2 k group and HA 300 k group, the transition of the amount of change in the whole volume ratio, wrinkle area ratio, and wrinkle volume ratio maintained low values compared with the placebo group and shown suppressed wrinkles compared with the time prior to ingestion. The amount of change in the wrinkle volume ratio significantly decreased at 8 weeks after ingestion in the HA 300 k group compared to the placebo group (Figure 1). The HA 300 k group and the HA 2 k group shown suppressed wrinkles compared with the placebo group (HA 300 k, p = 0.046; HA 2 k, p = 0.052). A previous randomized controlled trial (RCT) study showed ingestion of the HA of MW 38 k suppressed wrinkles significantly compared with the placebo group.14 In a previous oral administration study using labeled HA to confirm its localization by autoradiography in rats, it is suggested that HA was decomposed to low MW by intestinal bacteria, absorbed into the body, and some of decomposed HA was migrated to the skin.18 For this reason, the efficacy of oral HA on wrinkles could not be different depending on the MW of HA.
Considering the role of HA in the skin, it is suggested that migration of HA to the skin acts on the wrinkled skin as follows:
There are fibroblasts in the dermis of the skin. In these fibroblasts, collagen fibers, elastin fibers, and HA are synthesized. In vitro, HA prompts the proliferation of fibroblasts of the dermis, and it was confirmed that intake of HA promoted HA synthesis in fibroblasts.19,20 It is presumed that part of the orally ingested HAs promote HA synthesis in fibroblasts of the dermis, maintain normal skin, and are involved in the suppression of wrinkles. In addition, because the fibroblast cells are growing, it is possible to suppress wrinkles by promoting collagen synthesis.
The epidermis above the dermis consists of the stratum corneum, the granular layer, spinous layer, and the basal layer beginning from the upper layer. The necessary HA in the epidermis is synthesized in the keratinocytes of the basal layer.21,22 It is reported that HA binds to receptors (CD44) present on the keratinocyte surface and normalizes the skin functions through signaling.23 In addition, it is known that HA has a high water retention.24 In the skin, HAs are thought to work in the suppression of the wrinkle formation by their normal skin function and high moisture retention. In addition to this, it is presumed that the suppression of wrinkle formation is involved in several factors HA causes.
From these mechanisms, it is believed to have led to an efficacy against wrinkles, the improvement of subjective symptoms in the skin, and the improvement of skin conditions through visual observation in this study.
Because the turnover for the skin is said to be 28 days, functionality through oral ingestion of the HA will require long-term continuous intake.25 It was confirmed that HA is safe in humans when 200 mg/day HA was ingested for 12 months; a long-term ingestion.26 In addition, various safety studies were also conducted, and safety was confirmed.27–37 Therefore, HA can be said to be a food material, which is suitable for long-term continuous intake.
The previous study of the MW 38 k and 75 k HA showed the effect on wrinkles after ingestion of 4 and 8 weeks compared with placebo group. There is no study about the effect of HA under 38 k or over 75 k on wrinkles. This study clarified that the MW 2 k and 300 k HA had effects on wrinkles. Therefore, we confirmed that HA between the MW 2 k and 300 k had effects on wrinkles.
In this study, we evaluated the effect of HA of 120 mg/day on wrinkles. It was not studied for the minimum effective dose. It was also not verified whether there is similar effectiveness in MWs other than 2 k and 300 k. In addition, it is not certain that changes of HA content in the skin were caused through oral ingestion of HA and what affect HA has regarding the wrinkle-inducing factor. Regarding these issues, further research is required in the future.
Conclusion
This study showed that the oral ingestion of the MW 2 k or 300 k HA for 12 weeks suppresses wrinkles and improves the skin’s luster and suppleness in people aged 59 years or less who were healthy Japanese men and women over 22 years old. From the above, HA consumption is expected to be used as a method to maintain healthy skin.
Acknowledgments
We gratefully acknowledge the individuals who participated in the study. This study was funded by the Kewpie Corporation.
Disclosure
Ms. Mariko Oe, Mr. Seigo Sakai, Mr. Hideto Yoshida, Ms. Haruna Kaneda, Ms. Nao Okado, Dr. Yasunobu Masuda are employees of Kewpie Coroporation. MD Osamu Urushibata is a professor of Toho University. The authors report no other conflicts of interest in this work.
References
Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6(7):2397–2404. | ||
Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. | ||
Dai G, Freudenberger T, Zipper P, et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am J Pathol. 2007;171(5):1451–1461. | ||
Kawada C, Kimura M, Masuda Y, Nomura Y. Oral administration of hyaluronan prevents skin dryness and epidermal thickening in ultraviolet irradiated hairless mice. J Photochem Photobiol B. 2015;153:215–221. | ||
Kajimoto O, Odanaka W, Sakamoto W, Yoshida K, Takahashi T. [Clinical effect of hyaluronic acid diet for dry skin -objective evaluation with microscopic skin surface analyzer]. J New Rem Clin. 2001;50:548–560. Japanese. | ||
Sato T, Sakamoto W, Odanaka W, Yoshida K, Urushibata O. [Clinical effects of hyaluronic acid diet for dry and rough skin]. Aesthe Derma. 2002;12:109–120. Japanese. | ||
Sato T, Yoshida T, Kanemitsu T, Yoshida K, Hasegawa M, Urushibata O. [Clinical effects of hyaluronic acid diet for moisture content of dry skin]. Aesthe Derma. 2007;17:33–39. Japanese. | ||
Yoshida T, Kanemitsu T, Narabe O, Tobita M. [Improvement of dry skin by a food containing hyaluronic acids derived from microbial fermentation]. J New Rem Clin. 2009;58:143–155. Japanese. | ||
Longas MO, Russell CS, He XY. Evidence for structural changes in dermatan sulfate and hyaluronic acid with aging. Carbohydr Res. 1987;159(1):127–136. | ||
Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30(6):599–614. | ||
Mashiko T, Kinoshita K, Kanayama K, Feng J, Yoshimura K. Perpendicular strut injection of hyaluronic acid filler for deep wrinkles. Plast Reconstr Surg Glob Open. 2015;3(11):e567. | ||
Szyszkowska B, Lepecka-Klusek C, Kozłowicz K, Jazienicka I, Krasowska D. The influence of selected ingredients of dietary supplements on skin condition. Postepy Dermatol Alergol. 2014;31(3):174–181. | ||
Kim HK, Moon TK, Kim NS. [Effect of hyaluronan on wrinkle]. Food Style 21. 2007;11:42–46. Japanese. | ||
Watanabe M, Matsui K, Kondo S. [Effects of low molecular weight hyaluronic acid by oral intake to beautify skin -placebo-controlled double-blind comparative study]. Jpn Pharmacol Ther. 2015;43:57–64. Japanese. | ||
Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol. 2015;2015:563818. | ||
Hisada N, Satsu H, Mori A, et al. Low-molecular-weight hyaluronan permeates through human intestinal Caco-2 cell monolayers via the paracellular pathway. Biosci Biotehnol Biochem. 2008;72(4):1111–1114. | ||
Task Force Committee for Evaluation of Anti-aging Function. Guideline for evaluation of anti-wrinkle products. J Jpn Cosmet Sci Soc. 2006;30:316–332. | ||
Oe M, Mitsugi K, Odanaka W, et al. Dietary hyaluronic acid migrates into the skin of rats. ScientificWorldJournal. 2014;2014:378024. | ||
Greco RM, Iocono JA, Ehrlich HP. Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix. J Cell Physiol. 1998;177(3):465–473. | ||
Lüke HJ, Prehm P. Synthesis and shedding of hyaluronan from plasma membranes of human fibroblasts and metastatic and non-metastatic melanoma cells. Biochem J. 1999;343(pt 1):71–75. | ||
Tammi R, Ripellino JA, Margolis RU, Maibach HI, Tammi M. Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol. 1988;90(3):412–414. | ||
Sakai S, Yasuda R, Sayo T, Ishikawa O, Inoue S. Hyaluronan exists in the normal stratum corneum. J Invest Dermatol. 2000;114(6):1184–1187. | ||
Bourguignon LY. Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am J Pathol. 2014;184(7):1912–1919. | ||
Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;58(1):255–315. | ||
Halprin KM. Epidermal “turnover time” – a re-examination. Br J Dermatol. 1972;86(1):14–19. | ||
Tashiro T, Seino S, Sato T, Matsuoka R, Masuda Y, Fukui N. Oral administration of polymer hyaluronic acid alleviates symptoms of knee osteoarthritis: a double-blind, placebo-controlled study over a 12-month period. ScientificWorldJournal. 2012;2012:167928. | ||
Morita H, Kawakami Y, Shimomura K, Sunaga M. [Acute toxicity study of sodium hyaluronate(SL-1010) in rats and dogs]. Jpn Pharmacol Ther. 1991;19:13–18. Japanese. | ||
Morita H, Kawakami Y, Suzuki S, Hirata M, Koizumi H. [Thirteen-week subcutoneous toxicity study on sodium hyaluronate(SL-1010) with 4-week recovery test in rats]. Jpn Pharmacol Ther. 1991;19:19–52. Japanese. | ||
Oe M, Yoshida T, Kanemitsu T, Matsuoka R, Masuda Y. [Repeated 28-day oral toxicological study of hyaluronic acid in rats]. Pharmacometrics. 2011;81:11–21. Japanese. | ||
Hasegawa T, Miyoshi K, Nomura A, Nakazawa M. [Subacute toxicity test on sodium hyaluronate(SPH) in rats by intraperitoneal administration for 3 months and recovery test]. Pharmacometrics. 1984;28:1021–1040. Japanese. | ||
Miyoshi K, Hasegawa T, Nakazawa M. [Chronic toxicity test on sodium hyaluronate(SPH) in beagle dogs by intra-articular administration for 6 months and recovery test (1) General findings]. Pharmacometrics. 1985;29:49–81. Japanese. | ||
Ono C, Iwama A, Nakajima Y, Kitsuya A, Nakamura T. [Reproductive and developmental toxicity study on sodium hyaluronate(SH)-(1)Study on subcutaneous administration to rats during the period of organogenesis]. Jpn Pharmacol Ther. 1992;20:11–26. Japanese. | ||
Guo F, Geng G, Wang H, Liu H, Zhi Y. [Teratogenicity test of sodium hyaluronate]. Food Drug. 2010;12:321–323.Chinese. | ||
Onishi M, Nagata T, Saigou K, Sameshima H, Nagata R. [Mutagenicity studies of sodium hyaluronate(SH)]. Jpn Pharm Ther. 1992;20:65–72. Japanese. | ||
Aruga F, Miwa Y, Fuzimura T, Ohta S. [Micronucleus test of sodium hyaluronate(SH) with mice]. Jpn Pharmacol Ther. 1992;20:73–75. Japanese. | ||
Takemoto M, Ohzone Y, Asahi K. [Antigenicity test of sodium hyaluronate(SH)]. Jpn Pharmacol Ther. 1992;20:59–64. Japanese. | ||
Seino S, Takashita F, Asari A, Masuda Y, Kunou M, Ochiya T. No Influence of exogenous hyaluronan on the behavior of human cancer cells or endothelial cell capillary formation. J Food Sci. 2014;79(7):1469–1475. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.