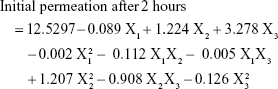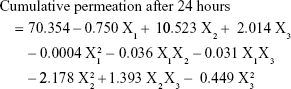Back to Journals » International Journal of Nanomedicine » Volume 14
Optimized vinpocetine-loaded vitamin E D-α-tocopherol polyethylene glycol 1000 succinate-alpha lipoic acid micelles as a potential transdermal drug delivery system: in vitro and ex vivo studies
Authors Ahmed OAA , El-Say KM , Aljaeid BM, Badr-Eldin SM, Ahmed TA
Received 13 September 2018
Accepted for publication 27 November 2018
Published 18 December 2018 Volume 2019:14 Pages 33—43
DOI https://doi.org/10.2147/IJN.S187470
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Osama AA Ahmed,1,2 Khalid M El-Say,1,3 Bader M Aljaeid,1 Shaimaa M Badr-Eldin,1,4 Tarek A Ahmed1,3
1Department of Pharmaceutics, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia; 2Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Minia University, Minia, Egypt; 3Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt; 4Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt
Background: Vinpocetine (VNP), a semisynthetic natural product, is used as a vasodilator for cerebrovascular and age-related memory disorders. VNP suffers from low oral bioavailability owing to its low water solubility and extensive first-pass metabolism. This work aimed at utilizing D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) and alpha lipoic acid (ALA) to develop efficient micellar system for transdermal delivery of VNP.
Materials and methods: VNP-TPGS-ALA micelles were prepared, characterized for particle size using particle size analyzer, and investigated for structure using transmission electron microscope. Optimization of VNP-TPGS-ALA micelles-loaded transdermal films was performed using Box–Behnken experimental design. The investigated factors were percentage of ALA in TPGS (X1), citral concentration (X2), and propylene glycol concentration (X3). Elongation percent (Y1), initial permeation after 2 hours (Y2), and cumulative permeation after 24 hours (Y3) were studied as responses.
Results: Statistical analysis revealed optimum levels of 16.62%, 3%, and 2.18% for X1, X2, and X3, respectively. Fluorescent laser microscopic visualization of skin penetration of the optimized transdermal film revealed marked widespread fluorescence intensity in skin tissue after 0.5, 2, and 4 hours compared with raw VNP transdermal film formulation, which indicated enhancement of VNP skin penetration.
Conclusion: The obtained results highlighted the potentiality of VNP nanostructure-based films for controlling the transdermal permeation of the drug and improving its effectiveness.
Keywords: bioavailability, box behnken design, citral, fluorescent laser microscope, nanostructured-based films, permeation
Introduction
Vinpocetine (VNP), derived from the natural vinca alkaloid vincamine, is widely utilized as a vasodilator and a nootropic agent.1,2 VNP is used for improving memory and cerebral metabolism in patients with cerebrovascular and age-related memory disorders.3,4 In addition, it has shown potential in the treatment of Parkinson’s and Alzheimer’s diseases. The low oral bioavailability due to extensive first-pass metabolism and low aqueous solubility hinders the full utilization of VNP oral dose.5 These challenges of low solubility and bioavailability attracts the attention of researchers to design a more efficient dosage form to deliver VNP.5–9
D-α-Tocopherol polyethylene glycol 1000 succinate (TPGS) is a nonionic surfactant, which is a mixed ester of vitamin E succinate and polyethylene glycol 1000.10 TPGS forms stable micelles in aqueous vehicles due to its amphiphilic structure at a concentration as low as 0.02 wt% with a hydrophile/lipophile balance (HLB) value of 13.2.10,11 TPGS has been widely investigated for its emulsifying and solubilizing effects on poorly water-soluble drugs.11–13 It can also act as a P-glycoprotein inhibitor and has been used as an excipient for overcoming multidrug resistance and for increasing the oral bioavailability of anticancer drugs.12,14 It has been approved as a pharmaceutical excipient by the US Food and Drug Administration (FDA).11 TPGS has also been utilized in the preparation of functionalized nanoparticles with poly lactic-co-glycolic acid for drug delivery.15
Alpha lipoic acid (ALA) is an antioxidant that protects membranes through recycling vitamin E.16 ALA can function as a redox regulator and has shown effects in various oxidative stress models such as ischemia–reperfusion injury and diabetes models.17–20 It is clinically used for the treatment of diabetes complications and for the cure of alcohol, mushroom, and heavy metal poisoning.21,22 In addition, ALA has been proven to enhance cognitive functioning in patients with Alzheimer’s disease.23,24 ALA has also been utilized as a part of nanostructure matrix for improving drug delivery.25,26
Bioavailability problems during oral delivery have attracted the attention of formulators to look for alternative and effective formulations. This work presents the utilization of TPGS and ALA as a novel combination to form micellar structure for transdermal delivery of VNP utilizing a mathematical experimental design. This approach to improve the diffusion of VNP using nanostructure-based transdermal film system could be useful for improving treatment effectiveness of the delivered therapeutic agents and represents a promising alternative for the oral route. In this regard, this work aims to utilize an experimental design to study the effect of formulation parameters of VNP-TPGS-ALA micelles-loaded transdermal films on elongation percent, initial permeation after 2 hours, and cumulative permeation after 24 hours. The in vitro characteristics, ex vivo skin permeation, and skin layers penetration of the optimized formulation were also investigated.
Materials and methods
Materials
VNP, TPGS, citral, propylene glycol (PG), ethanol, hydroxypropyl methylcellulose (viscosity 4,000 cP, 2% [w/v] solution), and fluorescein isothiocyanate (FITC)-dextran (average molecular weight 20,000 Da) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Preparation of VNP-TPGS-ALA micelles
VNP-loaded TPGS-ALA micelles were prepared by dissolving VNP, TPGS, and ALA in ethanol using a magnetic stirrer for 5 minutes.27 Distilled water was then added to the prepared solution. After dispersion of the components, ethanol was removed using a rotary evaporator (BÜCHI Labortechnik AG, Flawil, Switzerland). The prepared dispersion was then centrifuged at 30,000 rpm for 45 minutes at 4°C. The residue was lyophilized using a freeze dryer (alpha 1-2 LD plus lyophilizer; Christ, Osterode am Harz, Germany) for 48 hours and stored for further characterization.
Evaluation of VNP-TPGS-ALA micelles
Particle size
The prepared VNP-TPGS-ALA dispersion was investigated for mean particle size by light scattering technique using a Zetatrac particle size analyzer (Microtrac Inc., Montgomeryville, PA, USA).28
Transmission electron microscopy
A sample of VNP-TPGS-ALA micelles preparation was exposed to a transmission electron microscope (100CX; JEOL, Tokyo, Japan). A drop of diluted micelles was added onto a microscope grid and then stained with 2% uranyl acid. The grid was dried before investigation.29
Box–Behnken experimental design for VNP-TPGS-ALA transdermal films
Based on the preliminary investigation of VNP-TPGS-ALA transdermal formulations, a three-factor three-level Box–Behnken experimental design (Statgraphics Centurion XV version 15.2.05 software; StatPoint Technologies Inc., Warrenton, VA, USA) was used to investigate the effect of the studied variables.30 A total of 15 runs was constructed with a fully randomized order of experiments. The investigated independent variables (factors) were percentage of ALA in TPGS (X1), citral concentration (X2), and PG concentration (X3). The studied dependent variables (responses) were elongation percent (Y1), initial permeation after 2 hours (Y2), and cumulative permeation after 24 hours (Y3) of the prepared VNP-TPGS-ALA films. The levels of the investigated independent variables and the constraints for the dependent variables are shown in Table 1. The design was constructed to maximize the elongation percent and to achieve sustained permeation profile of drug from the transdermal films with VNP initial permeation of 20% after 2 hours and cumulative permeation of 75% after 24 hours.
Preparation of VNP-TPGS-ALA transdermal films
According to the formulation composition depicted in the experimental design (Table 2), VNP-TPGS-ALA transdermal films were prepared by the dispersion of the prepared micelles containing VNP equivalent to 40 mg in 50 mL distilled water. Hydroxypropyl methylcellulose (2%, w/v) and specified amounts of the penetration enhancer (citral) and PG, as plasticizer, were added.31 The dispersion was stirred using a magnetic stirrer and then kept for 24 hours in the fridge. After that, the preparation was poured in a glass petri dish (9 cm diameter) and stored at 40°C until complete evaporation of water and formation of the films.
Characterization of the prepared VNP-TPGS-ALA transdermal films
The physical appearance of the prepared films was investigated using a magnifying glass for observing surface appearance and defects. Film thickness was measured using a micrometer (Mitutoyo Co., Kawasaki, Japan).
Film elongation percent
The prepared films were evaluated for elongation percent as previously described.32 Briefly, rectangular film strips of 1×4 cm were fixed in such a way that the length of the film between the jaws was 2 cm under a weight of 200 g for a minute. The elongation percent was calculated according to equation 1.
|
|
Ex vivo diffusion study of the prepared VNP-TPGS-ALA transdermal films
The study protocol was approved by the Research Ethics Committee, Faculty of Pharmacy, King Abdulaziz University, that ensured the care and use of animals according to the EU Directive 2010/63/EU on the protection of animals used for scientific purposes and Guiding Principle in Care and Use of Animals (DHEW publication NIH 80-23). Full-thickness skin samples of 3×3 cm area were excised from the abdominal region of shaved Wistar rats and freed from any subcutaneous fats. The prepared skin was mounted between the donor and receptor compartments of the diffusion cells with the dermal side in direct contact with the receptor medium. The diffusion of VNP from the prepared transdermal films (15 formulations) was carried out using automated Franz diffusion cell apparatus MicroettePluss™ (automated Franz diffusion cell apparatus; Hanson Research, Chatsworth, CA, USA) with a diffusion area of 1.77 cm2.31 Buffered saline solution (pH 7.4) was used as a receiver medium in the receptor chamber in which the temperature was kept at 37°C and the stirring rate was 400 rpm. VNP samples were analyzed using a previously reported HPLC method validated and adopted to our laboratory.33
Optimization of VNP-TPGS-ALA micelles-loaded transdermal film
The results obtained for the responses (Y1−Y3) of the 15 prepared formulations were subjected to statistical analysis, and the optimum combination of these factors was deduced. The predicted VNP-TPGS-ALA micelles-loaded transdermal film, deduced by the design, utilizing the optimum level of factors was prepared and evaluated for elongation percent and diffusion parameters. The observed data obtained were compared with the predicted values for validation of the optimized formulation.
Visualization of skin penetration for the optimized TPGS-ALA-loaded transdermal film using fluorescence laser microscope
FITC-dextran (permeability tracer, 0.15 μmol/mL) was used instead of VNP in the preparation of the optimized transdermal formulation.34–36 FITC-dextran-loaded micelle transdermal films were added on the rat skin, and the penetration of FITC-dextran across the rat skin was investigated. Transdermal film loaded with raw FITC-dextran (control), that is, no micelles included, was prepared and treated as described for labeled optimized transdermal formulation. The treated skin was removed after 0.5, 2, and 4 hours and kept in 10% buffered formalin as a fixative.37,38 Blocks of skin sample (paraffin wax sections of 4 μm thickness) were prepared using a microtome. The prepared samples were observed using Zeiss Axio Observer D1 Inverted Dic Fluorescence Microscope (Carl Zeiss AG, Oberkochen, Germany). Filter used was 470/40 nm excitation, 495 beam splitter, and 525/50 nm emission. Images were acquired with identical acquisition parameters, with minimum excitation and gain.
Results and discussion
Preparation and characterization of VNP-TPGS-ALA micelles
The main factors that affect the micelles ability to deliver drugs include solubilizing capacity and stability. The choice of TPGS in this study was based on its ability to form stable micelles and to efficiently solubilize poorly soluble drugs owing to its low critical micelle concentration and high HLB. TPGS has also been reported as a skin permeation enhancer.39,40 ALA is a powerful antioxidant that could enhance the cognitive function in patients with Alzheimer’s disease. ALA molecules act as surface active agents, reduce the surface energy, and spontaneously aggregate in aqueous medium to form micelles.26 Li et al proved the ability of ALA to stabilize and enhance the drug loading of polymeric micelles.41 Accordingly, incorporation of ALA could provide combined advantages of micelles stabilization and drug-loading enhancement, in addition to augmenting the cognition-improving action of VNP.
The prepared VNP-TPGS-ALA micelles showed an average particle size, measured by the dynamic light scattering technique, of 129±18 nm with a polydispersity index of 0.59 (Figure 1A). The photomicrographs of VNP-TPGS-ALA micelles preparation examined by TEM, shown in Figure 1B, revealed a wide range of sizes of clusters for VNP-TPGS-ALA micelles with larger clumps that were interpreted as micelle aggregates.
Preparation and characterization of VNP-TPGS-ALA transdermal films
The prepared transdermal films were smooth in appearance and uniform in thickness without visible cracks. The results of the elongation percent (Y1), the initial permeation after 2 hours (Y2), and the cumulative permeation after 24 hours (Y3) of the VNP-TPGS-ALA transdermal films are presented in Table 2 and Figure 2.
Box–Behnken experimental design for VNP-TPGS-ALA transdermal films
The quantitative effects of the independent variables (X1−X3) on the dependent variables (Y1−Y3) were fitted into regression quadratic equations (equations 2–4).
|
|
|
|
|
|
The significance and magnitude of the effects of the investigated dependent variables on the studied responses are represented using Pareto charts and three-dimensional (3D) response surface plots in Figures 3 and 4, respectively. The synergistic and antagonistic effects of the independent variables are indicated by the positive and negative signs in the Pareto charts.
The estimated effects of factors and associated P-values for the dependent variables are presented in Table 3. A P-value less than 0.05 was considered significant. The estimate values in Table 3 reflect the magnitude of the effect of each factor on the response relative to the other factors. The greater the absolute value of the estimate, the more the effect of that factor on the studied response. The estimate’s sign designates the trend’s direction. A positive sign of an estimate denotes a direct correlation of the variable with the studied response, while a negative sign denotes an inverse one.
Effect on the elongation percent (Y1)
Generally, the elongation percent reflects the mechanical properties of the prepared transdermal film. A hard and brittle film is characterized by moderate tensile strength and low elongation, but a soft and tough film is described by high tensile strength and high elongation.42 So, the incorporation of the appropriate plasticizer concentration is important to improve the elasticity, increase toughness, and reduce the brittleness of the prepared film.43 To obtain a tough but flexible film, the current study aimed to investigate the effect of PG at different levels from 1% to 5%. The obtained results in Table 2 showed that elongation percent varied from 5% (F4) to 140% (F9). The statistical analysis revealed that PG (X3) and its quadratic term showed positive significant effects on the elongation percent (Y1) (Figure 3A) with P-values of 0.0001 and 0.0024, respectively (Table 3). These results indicated that the plasticizer percentage (X3) was the most significant factor affecting the elongation percent (Y1). The 3D response surface graph illustrated in Figure 4A and B demonstrated the magnitude of this effect. This finding could be explained by polymer chain interruption by the action of PG that leads to softening and extension of the film matrix. This finding on increasing the elongation percent of films by the action of PG is in accordance with the previously reported data.32,44,45
Effect on initial drug permeation after 2 hours (Y2)
The permeation profiles of VNP-TPGS-ALA transdermal films with no ALA in their content displayed the highest initial VNP permeation after 2 hours ranging from 19.39% (F15) to 30.36% (F10), whereas formulations containing the highest percentage of ALA (30%) displayed the lowest initial VNP permeation after 2 hours ranging from 10.24% to 14.1% for F11 and F12, respectively (Figure 2). At the same levels of X2 and X3, when X1 increased from 0% to 30%, Y2 values decreased from 21.58% (F8) to 10.24% (F11), from 19.39% (F15) to 11.84% (F6), from 30.38% (F10) to 12.3% (F14), and from 22.3% (F2) to 14.1% (F12). As illustrated in Figures 3B and 4C, the percentage of ALA in TPGS (X1) is the only factor that significantly affected the initial permeation after 2 hours (Y2) in a negative trend with a P-value of 0.0015 (Table 3). This finding could be attributed to the poor aqueous solubility of ALA that could result in reduced hydrophilicity of TPGS-ALA micelles, and consequently, reduced permeation rate of VNP from VNP-TPGS-ALA films.22
Effect on the cumulative drug permeated after 24 hours (Y3)
The permeation of VNP from the prepared transdermal films showed a marked variation in the cumulative drug permeated after 24 hours ranging from 57.72% to 97.29% according to the level of factors in the formulations as shown in Figure 2. All the studied factors (X1, X2, and X3) showed a significant effect on the cumulative amount of VNP permeated after 24 hours (Y3) as illustrated in Figure 3C. The percentage of ALA in TPGS (X1) showed an antagonistic significant effect on the cumulative drug permeation after 24 hours (Y3) with a P-value of 0.0001. On the other hand, the percentage of citral (X2) and the percentage of PG (X3) showed a synergistic significant effect on the same response with P-values of 0.0061 and 0.0405, respectively (Table 3). The magnitude of the effect of the three factors on Y3 is illustrated in Figure 4D–F.
Incorporation of X1 at high level (30%) in VNP-TPGS-ALA transdermal films led to a controlled-release pattern. At the same levels of X2 and X3, when the X1 increased from 0% to 30%, Y3 decreased from 86.65% (F8) to 57.72% (F11), from 88.15% (F15) to 64.41% (F6), from 97.29% (F10) to 66.21% (F14), and from 92.15% (F2) to 64.69% (F12). This could be due to low aqueous solubility of ALA.
On the other hand, as the percentage of citral (X2, penetration enhancer) increased, the percent of VNP permeated via the rat skin increased. At the same levels of X1 and X3, when X2 increased from 1% to 3%, Y3 increased from 86.65% (F8) to 97.29% (F10), from 66.43% (F3) to 73.1% (F4), and from 57.72% (F11) to 66.21% (F14). This finding could be attributed to the presence of citral in the film which may interact with some components of the skin causing increased fluidity in the intercellular lipid lamellae, and/or leach out some of the structural components, which increases the level of drug penetrated through the barrier membrane.46,47 The penetration enhancer may also reduce the capacity of drug binding to the skin, thereby improving drug transport.43,48 A similar permeation-enhancing effect of citral was reported by Ali et al, who developed glibenclamide transdermal matrix system.49
The same finding was found with X3; as the percentage of PG (X3, plasticizer) increased, the percent of VNP permeated via the rat skin increased. At the same levels of X1 and X2, when the X3 increased from 1% to 5%, Y3 increased from 88.15% (F15) to 92.15% (F2), from 66.43% (F3) to 71.85% (F1), and from 64.41% (F6) to 64.69% (F12). It has been reported that the selection of a suitable plasticizer and its proper percentage have an impact not only on the mechanical properties of the film but also on its permeability of drugs.50 So, the incorporation of PG (X3) as a plasticizer increases the mobility of the chain of the film-forming polymer leading to increased amount of drug release. The plasticizer will interpose itself between the polymer chains and interact with the forces held together by extending and softening the polymer matrix.47,51 Another explanation that may enforce the obtained finding is the presence of hydroxyl groups in PG that would strongly interact with water and/or with hydroxypropyl methylcellulose via hydrogen bonding. Hence, the use of PG allowed for more water absorption by the film, thus facilitating VNP diffusion.32,52 This result was in accordance with Vora et al who observed a permeation-enhancing effect of PG when used as a plasticizer in carvedilol transdermal systems.53
Optimization of VNP-TPGS-ALA transdermal films
The optimum combination of the factors was obtained by numerical optimization. The results deduced optimum VNP-TPGS-ALA transdermal film formulation of 16.62% for ALA percentage in TPGS, 3.0% for citral concentration, and 2.18% for PG concentration. The deduced optimized VNP-TPGS-ALA transdermal film formulation was prepared and evaluated. The results of the observed and the predicted values are presented in Table 4. According to these results, the optimized combination of independent factors of VNP-TPGS-ALA transdermal film formulation produced the desired elongation percent and controlled drug permeation.
Visualization of skin penetration of the optimized VNP-TPGS-ALA transdermal film using fluorescence laser microscope
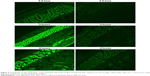
Visualization of the permeation extent of the prepared optimized FITC-dextran-loaded TPGS-ALA transdermal film was carried out using a fluorescence laser microscope in comparison with raw FITC-dextran-loaded transdermal film. The images revealed marked widespread fluorescence intensity in skin tissue from FITC-dextran-loaded TPGS-ALA transdermal film formulation after 0.5, 2, and 4 hours compared to raw FITC-dextran-loaded transdermal formulation as shown in Figure 5. Laser microscope results clearly revealed penetration enhancement from FITC-dextran-loaded TPGS-ALA transdermal film formulation through different skin layers. This result could be attributed to the entrapment of drug in the micellar structure of TPGS-ALA that enhances its penetration through skin layers compared with raw film. The results revealed the ability of penetration enhancement through skin tissue attained by the optimized TPGS-ALA transdermal film formulation when compared with raw transdermal formulation.
Conclusion
TPGS and ALA were utilized as a nanostructured micellar formulation for transdermal penetration enhancement of VNP. The Box–Behnken experimental design proved to be a useful tool to optimize and validate the results of the desired formulation. The optimized TPGS-ALA micelles-loaded transdermal film showed ability to improve permeation of VNP through skin layers compared with raw VNP-loaded transdermal film. The results deduced optimum VNP-TPGS-ALA transdermal film formulation of 16.62%, 3.0%, and 2.18% for X1, X2, and X3, respectively. The marked widespread fluorescence intensity clearly revealed penetration enhancement through skin tissue attained by the optimized TPGS-ALA transdermal film formulation after 0.5, 2, and 4 hours compared with raw transdermal formulation. These findings highlighted the potential of VNP-TPGS-ALA micelles-loaded film for enhancing the transdermal delivery of the loaded drug.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no G-99-166-38. The authors, therefore, acknowledge with thanks, the DSR for technical and financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
Kidd PM. A review of nutrients and botanicals in the integrative management of cognitive dysfunction. Altern Med Rev. 1999;4(3):144–161. | ||
Kong L, Song C, Ye L, Guo D, Yu M, Xing R. The Effect of vinpocetine on human cytochrome P450 Isoenzymes by using a cocktail method. Evid Based Complement Alternat Med. 2016;2016:5017135. | ||
Zhang YS, Li JD, Yan C. An update on vinpocetine: new discoveries and clinical implications. Eur J Pharmacol. 2018;819:30–34. | ||
Patyar S, Prakash A, Modi M, Medhi B. Role of vinpocetine in cerebrovascular diseases. Pharmacol Rep. 2011;63(3):618–628. | ||
El-Laithy HM, Shoukry O, Mahran LG. Novel sugar esters proniosomes for transdermal delivery of vinpocetine: preclinical and clinical studies. Eur J Pharm Biopharm. 2011;77(1):43–55. | ||
Ahmed OA, Badr-Eldin SM, Tawfik MK, Ahmed TA, El-Say KM, Badr JM. Design and optimization of self-nanoemulsifying delivery system to enhance quercetin hepatoprotective activity in paracetamol-induced hepatotoxicity. J Pharm Sci. 2014;103(2):602–612. | ||
Ahmed TA, El-Say KM. Development of alginate-reinforced chitosan nanoparticles utilizing W/O nanoemulsification/internal crosslinking technique for transdermal delivery of rabeprazole. Life Sci. 2014;110(1):35–43. | ||
Badr-Eldin SM, Ahmed OA. Optimized nano-transfersomal films for enhanced sildenafil citrate transdermal delivery: ex vivo and in vivo evaluation. Drug Des Devel Ther. 2016;10:1323–1333. | ||
Alexander A, Dwivedi S, Ajazuddin A, et al. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 2012;164(1):26–40. | ||
Zhang Z, Tan S, Feng SS. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–4906. | ||
Guo Y, Luo J, Tan S, Otieno BO, Zhang Z. The applications of Vitamin E TPGS in drug delivery. Eur J Pharm Sci. 2013;49(2):175–186. | ||
Raju A, Muthu MS, Feng SS. Trastuzumab-conjugated vitamin E TPGS liposomes for sustained and targeted delivery of docetaxel. Expert Opin Drug Deliv. 2013;10(6):747–760. | ||
Mi Y, Zhao J, Feng SS. Vitamin E TPGS prodrug micelles for hydrophilic drug delivery with neuroprotective effects. Int J Pharm. 2012;438(1–2):98–106. | ||
Zhao J, Mi Y, Feng SS. Targeted co-delivery of docetaxel and siPlk1 by herceptin-conjugated vitamin E TPGS based immunomicelles. Biomaterials. 2013;34(13):3411–3421. | ||
Wang G, Yu B, Wu Y, Huang B, Yuan Y, Liu CS. Controlled preparation and antitumor efficacy of vitamin E TPGS-functionalized PLGA nanoparticles for delivery of paclitaxel. Int J Pharm. 2013;446(1–2):24–33. | ||
Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19(2):227–250. | ||
Alan C, Kocoglu H, Resit Ersay A, Anil Kurt H, Ertung Y, Alan H. Biochemical changes in cavernosal tissue caused by single sided cavernosal nerve resection and the effects of alpha lipoic acid on these changes. Actas Urol Esp. 2010;34(10):874–881. Spanish. | ||
Keegan A, Cotter MA, Cameron NE. Effects of diabetes and treatment with the antioxidant alpha-lipoic acid on endothelial and neurogenic responses of corpus cavernosum in rats. Diabetologia. 1999;42(3):343–350. | ||
Udupa A, Nahar P, Shah S, Kshirsagar M, Ghongane B. A comparative study of effects of omega-3 Fatty acids, alpha lipoic Acid and vitamin e in type 2 diabetes mellitus. Ann Med Health Sci Res. 2013;3(3):442–446. | ||
Mitkov MD, Aleksandrova IY, Orbetzova MM. Effect of transdermal testosterone or alpha-lipoic acid on erectile dysfunction and quality of life in patients with type 2 diabetes mellitus. Folia Med (Plovdiv). 2013;55(1):55–63. | ||
Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, Rihn BH. Alpha-lipoic acid in liver metabolism and disease. Free Radic Biol Med. 1998;24(6):1023–1039. | ||
Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004;21(2):114–121. | ||
Shinto L, Quinn J, Montine T, et al. A randomized placebo-controlled pilot trial of Omega-3 fatty acids and alpha lipoic acid in alzheimer’s disease. J Alzheimers Dis. 2013;38(1):111–120. | ||
Fava A, Pirritano D, Plastino M, et al. The effect of lipoic acid therapy on cognitive functioning in patients with alzheimer’s disease. J Neurodegener Dis. 2013;2013:454253. | ||
Kofuji K, Nakamura M, Isobe T, Murata Y, Kawashima S. Stabilization of α-lipoic acid by complex formation with chitosan. Food Chem. 2008;109(1):167–171. | ||
Nishiura H, Sugimoto K, Akiyama K. A novel nano-capsule of α-lipoic acid as a template of core-shell structure constructed by self-assembly. J Nanomed Nanotechnol. 2013;04(01):1–7. | ||
Ahmed OAA. Development and single dose clinical pharmacokinetics investigation of novel zein assisted-alpha lipoic acid nanoencapsulation of vardenafil. Sci Rep. 2018;8(1):15802. | ||
Abourehab MA, Ahmed OA, Balata GF, Almalki WH. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood-brain barrier. Int J Nanomedicine. 2018;13:3679–3687. | ||
Ahmed OA, Badr-Eldin SM. In situ misemgel as a multifunctional dual-absorption platform for nasal delivery of raloxifene hydrochloride: formulation, characterization, and in vivo performance. Int J Nanomedicine. 2018;13:6325–6335. | ||
El-Say KM, El-Helw AR, Ahmed OA, et al. Statistical optimization of controlled release microspheres containing cetirizine hydrochloride as a model for water soluble drugs. Pharm Dev Technol. 2015;20(6):738–746. | ||
Ahmed OA, Afouna MI, El-Say KM, Abdel-Naim AB, Khedr A, Banjar ZM. Optimization of self-nanoemulsifying systems for the enhancement of in vivo hypoglycemic efficacy of glimepiride transdermal patches. Expert Opin Drug Deliv. 2014;11(7):1005–1013. | ||
El-Say KM, Ahmed OA, Aljaeid BM, Zidan AS. Matrix-type transdermal films to enhance simvastatin ex vivo skin permeability. Pharm Dev Technol. 2017;22(4):492–499. | ||
Elkady EF, Tammam MH, Mohamed AA. Development and Validation of an RP-HPLC Method for the Determination of Vinpocetine and Folic Acid in the Presence of a Vinpocetine Alkaline Degradation Product in Bulk and in Capsule Form. J AOAC Int. 2017;100(3):671–676. | ||
Ahmed OA, Rizq WY. Finasteride nano-transferosomal gel formula for management of androgenetic alopecia: ex vivo investigational approach. Drug Des Devel Ther. 2018;12:2259–2265. | ||
Hulström D, Svensjö E. Intravital and electron microscopic study of bradykinin-induced vascular permeability changes using FITC-dextran as a tracer. J Pathol. 1979;129(3):125–133. | ||
Yeon JH, Na D, Choi K, Ryu SW, Choi C, Park JK. Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed Microdevices. 2012;14(6):1141–1148. | ||
Thavarajah R, Mudimbaimannar VK, Elizabeth J, Rao UK, Ranganathan K. Chemical and physical basics of routine formaldehyde fixation. J Oral Maxillofac Pathol. 2012;16(3):400–405. | ||
Prentø P, Lyon H. Commercial formalin substitutes for histopathology. Biotech Histochem. 1997;72(5):273–282. | ||
Meng X, Liu J, Yu X, Li J, Lu X, Shen T. Pluronic F127 and D-α-Tocopheryl Polyethylene Glycol Succinate (TPGS) mixed micelles for targeting drug delivery across The Blood Brain Barrier. Sci Rep. 2017;7(1):2964. | ||
Yang C, Wu T, Qi Y, Zhang Z. Recent advances in the application of Vitamin E TPGS for Drug delivery. Theranostics. 2018;8(2):464–485. | ||
Li W, Peng J, Yang Q, et al. α-Lipoic acid stabilized DTX/IR780 micelles for photoacoustic/fluorescence imaging guided photothermal therapy/chemotherapy of breast cancer. Biomater Sci. 2018;6(5):1201–1216. | ||
Lin S-Y, Lee C-J, Lin Y-Y. Drug-polymer interaction affecting the mechanical properties, adhesion strength and release kinetics of piroxicam-loaded Eudragit E films plasticized with different plasticizers. J Control Release. 1995;33(3):375–381. | ||
Cho CW, Choi JS, Kim SJ, Shin SC. Enhanced transdermal delivery of loratadine from the EVA matrix. Drug Deliv. 2009;16(4):230–235. | ||
Ahmed TA, El-Say KM. Transdermal film-loaded finasteride microplates to enhance drug skin permeation: Two-step optimization study. Eur J Pharm Sci. 2016;88:246–256. | ||
Nesseem DI, Eid SF, El-Houseny SS. Development of novel transdermal self-adhesive films for tenoxicam, an anti-inflammatory drug. Life Sci. 2011;89(13–14):430–438. | ||
Magnusson BM, Runn P. Effect of penetration enhancers on the permeation of the thyrotropin releasing hormone analogue pGlu-3-methyl-His-Pro amide through human epidermis. Int J Pharm. 1999;178(2):149–159. | ||
Carelli V, di Colo G, Nannipieri E, Serafini MF. Bile acids as enhancers of steroid penetration through excised hairless mouse skin. Int J Pharm. 1993;89(2):81–89. | ||
El-Say KM, Ahmed TA, Badr-Eldin SM, Fahmy U, Aldawsari H, Ahmed OAA. Enhanced permeation parameters of optimized nanostructured simvastatin transdermal films: ex vivo and in vivo evaluation. Pharm Dev Technol. 2015;20(8):919–926. | ||
Ali A, Trehan A, Ullah Z, Aqil M, Sultana Y. Matrix type transdermal therapeutic systems of glibenclamide: Formulation, ex vivo and in vivo characterization. Drug Discov Ther. 2011;5(1):53–59. | ||
Crawford RR, Esmerian OK. Effect of plasticizers on some physical properties of cellulose acetate phthalate films. J Pharm Sci. 1971;60(2):312–314. | ||
Qussi B, Suess WG. The influence of different plasticizers and polymers on the mechanical and thermal properties, porosity and drug permeability of free shellac films. Drug Dev Ind Pharm. 2006;32(4):403–412. | ||
Mali S, Sakanaka LS, Yamashita F, Grossmann MVE. Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr Polym. 2005;60(3):283–289. | ||
Vora N, Lin S, Madan PL. Development and in-vitro evaluation of an optimized carvedilol transdermal therapeutic system using experimental design approach. Asian J Pharm Sci. 2013;8(1):28–38. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.