Back to Journals » Local and Regional Anesthesia » Volume 15
Opioid-Free Segmental Thoracic Spinal Anesthesia with Intrathecal Sedation for Breast and Axillary Surgery: Report of Four Cases
Authors Vincenzi P , Stronati M, Isidori P, Iuorio S, Gaudenzi D , Boccoli G, Starnari R
Received 2 February 2022
Accepted for publication 23 March 2022
Published 9 May 2022 Volume 2022:15 Pages 23—29
DOI https://doi.org/10.2147/LRA.S358157
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Paolo Vincenzi,1 Massimo Stronati,2 Paolo Isidori,1 Salvatore Iuorio,2 Diletta Gaudenzi,3 Gianfranco Boccoli,1 Roberto Starnari2
1Department of General Surgery, IRCSS-INRCA, Ancona, Italy; 2Department of Anaesthesiology, IRCSS-INRCA, Ancona, Italy; 3Department of Perioperative Services, AOU “Ospedali Riuniti di Ancona”, Ancona, Italy
Correspondence: Paolo Vincenzi, Department of General Surgery, IRCSS-INRCA, Via della Montagnola n 81, Ancona, 60127, Italy, Tel + 39 3394578495, Email [email protected]
Purpose: Few studies have described segmental thoracic spinal anesthesia (STSA) as primary anesthesiologic method in breast and axillary surgery, documenting the association of intrathecal local anesthetics and opioids. This case series reports an opioid-free scheme of STSA in four elderly patients undergoing major breast and axillary oncological surgery.
Patients and Methods: STSA was performed in three female patients undergoing unilateral mastectomy ± axillary lymph node dissection (ALND) or sentinel lymph node biopsy for invasive ductal carcinoma and in one male patient undergoing ALND for melanoma metastases. The level of needle insertion was included between T6-8, via a median or paramedian approach. Midazolam (2 mg) and ketamine (20 mg) were used as adjuvants for intrathecal sedation, followed by the administration of hypobaric ropivacaine 0.25% at a dose of 8 mg. The level of sensory blockade achieved was comprised between C2-3 and T11-12. Postoperative analgesia was maintained through continuous intravenous administration of Ketorolac by an elastomeric pump (90 mg over 24 hrs.).
Results: Spinal anesthesia was completed without complications in all patients. Conversion to general anesthesia (GA) and perioperative intravenous sedation were not required. No major postoperative complications and no episodes of postoperative nausea and vomiting (PONV) were reported. No rescue analgesic was administered. All patients were discharged in postoperative day 2 and are alive at 30, 29, 27 and 13 months after surgery, respectively. High grade of satisfaction on the anesthesiologic method was expressed by all cases.
Conclusion: STSA with local anesthetic plus midazolam and ketamine might be considered a safe and effective alternative to GA, even in surgeries involving the breast and axillary region, particularly in elderly and frail patients. Larger prospective studies are required to validate these findings.
Keywords: spinal anesthesia, breast cancer surgery, axillary surgery, intrathecal midazolam, intrathecal ketamine
Introduction
Perioperative care of the elderly comorbid patient has posed a significant challenge to the general surgical community due to increasing age and comorbidities representing the most important risk factors for postoperative morbidity and mortality,1 along with the routine utilization of general anesthesia (GA) in major surgical procedures, potentially responsible for an increased perioperative risk to this growing class of patients.
Accordingly, regional anesthesia (RA), particularly neuraxial blockade, has been increasingly used as a valid and safe technique, which might improve recovery, minimize adverse effects, and provide better outcomes in terms of perioperative morbidity and mortality compared to GA.2–4
Neuraxial blockade in spinal and epidural anesthesia is aimed at preventing the spinal reflex inhibition of diaphragmatic and gastrointestinal function, at suppressing the detrimental responses to surgical stress, and at impending the efferent and afferent nerve signals to and from the spinal cord,5 carrying the potential benefits of decreased respiratory and cardiac complications,2,6 modulation of the neuroendocrine stress response,7 improved perioperative pain management,8 faster return of gastrointestinal function with reduced incidence of postoperative nausea and vomiting (PONV),8,9 adequate prevention of thromboembolic events10 and early patient mobilization.
In particular, segmental thoracic spinal anesthesia (STSA), due to its highly selective spinal block, ensures a better control over both induction and surgical anesthesia with increased sensory-motor blockade, improved cardiovascular and respiratory stability, and a reduced requirement for local anesthetics along with a lower risk of toxicity, representing remarkable advantages of this method compared to lumbar spinal anesthesia and other RA procedures, such as thoracic paravertebral block (TPVB).11
Nevertheless, though three recent series from our Institution conducted on a large population of elderly patients already illustrated the feasibility, efficacy and safety of thoracic continuous spinal anesthesia (TCSA) in abdominal and urological surgery,12–14 the secure and reliable use of STSA in patients undergoing major breast surgery and axillary lymph node dissection (ALND) was described only by Elakany et al.15
The authors reported several benefits of this technique over GA in reducing the incidence of PONV and pain with concomitantly minor perioperative administration of intravenous analgesics and reduced length of hospital stay.
For these reasons, we present our experience of STSA with intrathecal sedation in major breast cancer surgery and ALND.
Materials and Methods
Study Design and Population
All cases of axillary and breast surgery performed under the technique of STSA at the Italian National Research Center on Aging (INRCA) conducted on patients aged ≥65 years between January 1, 2019 and January 1, 2021 were reviewed.
Four cases were identified and described below.
Case 1: a 77-year-old woman (BMI 23.3) with a history of systemic arterial hypertension and type-II diabetes mellitus (ASA physical status II), underwent modified left radical mastectomy for invasive ductal carcinoma.
Case 2: a 68-year-old woman (BMI 27.1) with a history of systemic arterial hypertension and hyperthyroidism (ASA physical status II), underwent simple right mastectomy and sentinel lymph node biopsy for invasive ductal carcinoma.
Case 3: a 75-year-old man (BMI 26.3) with a history of systemic arterial hypertension, severe chronic obstructive pulmonary disease, and vertebral compression fracture of T12 (ASA physical status III), underwent ALND for nodal metastasis of a malignant melanoma resected 20 years ago.
Case 4: an 83-year-old woman (BMI 25.7) with a history of hypertensive and ischemic cardiomyopathy with low ejection fraction (ASA physical status III), underwent simple left mastectomy for invasive ductal carcinoma.
The study was in accordance with the INRCA Hospital Institutional Review Board and Helsinki Declaration.
All patients were informed of the possibility of intraoperative conversion to GA if needed.
Written informed consent for publication of medical data and image material was obtained from all subjects or a legal surrogate.
Last follow-up date for this study was December 31, 2021.
Segmental Thoracic Spinal Anesthesia Technique
500 mL of lactated Ringer’s solution were administered before the procedure.
Under fully aseptic technique with the patient in sitting position, the correct intervertebral space, located between T6 and T8, was identified and the skin of the puncture site infiltrated with 3 mL of 2% lidocaine. The puncture was performed via a median or a paramedian approach using a 25-gauge Whitacre needle (B. Braun Medical Inc., Melsungen, Germany), as shown in Figure 1.
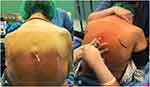 |
Figure 1 Insertion of the needle at T6-T7: (A) paramedian approach (Case 1), (B) median approach (Case 2). |
Once clear flow of cerebrospinal fluid was established, preservative free midazolam (Accord Healthcare, Milan, Italy) at a dose of 2 mg and preservative free ketamine (Molteni Pharmaceuticals, Florence, Italy) at a dose of 20 mg were injected as adjuvants for intrathecal sedation, followed by the administration of hypobaric ropivacaine 0.25% (Bioindustria Pharmaceuticals, Novi Ligure, Italy) at a dose of 8 mg (1 mL of ropivacaine 1% diluted with 3 mL of distilled water for a total volume given equal to 3.2 mL).
Procedure-related paresthesias, pain, or any difficulty during spinal puncture were documented in each case.
The patients were then placed in lateral decubitus on the side opposite to the surgical site, in a slight anti-Trendelenburg position for 5 to 10 min.
The level of the sensory blockade was tested using pinprick tests. When an adequate sensory block from the lower border of the clavicle (C2-C3) to the inferior costal margin (T11-T12) was achieved, the surgery was allowed to start.
In the case of patient anxiety and discomfort, an intravenous midazolam bolus (0.02–0.05 mg/kg) or a continuous infusion of propofol (1–3 mg/kg/h) were administered.
All patients were spontaneously breathing in Venturi masks on 28–40% FiO2.
Standard hemodynamic and clinical monitoring, particularly the level of sensory blockade, were applied to all patients.
Hypotension, defined as a decrease in systolic blood pressure of more than 15% of the basal pre-anesthetic value, was managed with boluses of ephedrine at a dose of 6 mg.
The level of sedation was assessed at the same time points through the Modified Ramsay Sedation Scale.16
Postoperative analgesia started at the end of surgery through continuous intravenous administration of Ketorolac by an elastomeric pump (90 mg over 24 hrs).
At the end of surgical procedure, patients were admitted to the Post-anesthesia Care Unit (PACU), where they all were monitored for at least 6 h and then transferred to the Surgical Unit.
Results
During all anesthetic and surgical procedures no major complications and no need for conversion from STSA to GA and for perioperative administration of intravenous sedative agents were reported.
Intraoperative hypotension was documented only in cases 1 and 3 and resolved with a single bolus of ephedrine, whereas level of sedation recorded during surgery was: 4 for the first 15 min and then 2–3 for the remaining portion of surgery in Case 1; 2–3 for the whole duration of surgery in Case 2 and 3 for the entire operation in the other 2 cases.
Length of surgery was 60 min, 50 min, 75 min and 40 min, for Case 1, 2, 3 and 4, respectively, while duration of spinal anesthesia was included between 120 min and 165 min for each case.
Postoperative course was uneventful in all cases; no patient needed mechanical ventilation or presented any neurologic consequences related to spinal anesthesia and no episodes of PONV were reported or rescue analgesic was administered after surgery.
All patients were discharged in postoperative day 2 and expressed high grade of satisfaction on the anesthesiologic method.
As of the date of last follow-up, all patients are alive at 30, 29, 27 and 13 months after surgery, respectively.
Discussion
To the best of our knowledge, this is the first report on the use of “opioid-free” STSA for breast cancer surgery and ALND in older patients with comorbidities, expanding its potential role as a primary anesthesiologic method alternative to GA.
Indeed, we already described our extensive experience in the utilization of STSA in high-risk patients undergoing major abdominal and urological surgeries, both in an elective and urgent context, reporting its safety, feasibility and efficacy.12–14
Strengths of this method documented in previous works11,17 and confirmed in our series12–14 rely on improved intraoperative hemodynamic control and perioperative analgesia in addition to avoiding invasive airway management and postoperative mechanical ventilation, leading to fewer negative repercussions on the cardiorespiratory system and decreased perioperative morbidity compared to GA,2–4,6 as well as other potential benefits including a minor risk of local recurrence and distant metastasis by attenuating the immunosuppression associated with the surgical neuroendocrine stress response when applied in oncological surgery.18,19
Indeed, a highly selective and exclusive sensory block of the cervical roots without any impact on the motor function of the diaphragm allows to safely conduct this method in patients with chronic pulmonary disease and reduced reserve, representing the group that might benefit the most from a neuraxial approach, such as our case 3.20
Nevertheless, we were able to find in literature only one randomized study mentioning the use of STSA in surgical procedures involving the breast and the axillary region.15
According to the authors, in a cohort of 40 patients with breast cancer scheduled for unilateral mastectomy and ALND, this technique was significantly associated with improved post-operative analgesia, reduced incidence of PONV, faster discharge from PACU and shorter overall length of in-hospital stay compared to GA.15
This trial presented a regimen of spinal anesthesia based on a combination of intrathecal local anesthetics (bupivacaine) and opioids (fentanyl), the last ones potentially associated with adverse effects such as respiratory depression, urinary retention, PONV and pruritus, as extensively documented in literature.21–23
Therefore, as reported in our previous work on TCSA, we developed a technique that allows sparing of the neuraxial opioid, replacing it with other adjuvants, specifically midazolam and ketamine,13 whose benefits consist in improved duration and quality of spinal anesthesia, with reduced onset time of the sensory-motor blockade and delayed recovery time of the sensory block leading to enhanced perioperative analgesia, together with a mild intraoperative sedation and a decreased incidence of PONV without negative effects on perioperative hemodynamics, significant side effects and neurotoxicity, though only limited evidences exist in literature.24–26
Accordingly, we decided to extend this approach to surgeries involving the breast and axillary region, with apparently promising initial results as detailed in our four cases.
The concern for potential spinal cord injury related to the procedure has been one of the main reasons limiting the widespread use of STSA, though its safety was demonstrated by magnetic resonance imaging investigations showing how the subarachnoid space is wider at the thoracic level in comparison to the lumbar level with the spinal cord lying more anteriorly from mid to lower thoracic level,27 thus allowing the safe insertion of a needle at this level.
In relation to this evidence, a large clinical series conducted on a cohort of patients undergoing elective surgery with the technique of STSA revealed similar incidences of paresthesia to that reported in lumbar spinal anesthesia, without any neurologic injuries or sequelae.28
Similarly, severe direct complications of the technique (eg, spinal hematomas or infections) were not recorded in our cases as well as in our previous works on STSA,12–14 validating its security.
Lastly, the delivery of the anesthetic directly at the required level of the body allows to perform a highly selective neuraxial blockade, with a rapid onset of action, a reduced dose of local anesthetic required and a limited caudal spread with concomitant minor vasodilation and risk of hypotension, representing all described benefits of this method compared to lumbar anesthesia.12,13,29
For the recent past years, TPVB has been described as one of the preferred anesthesiologic technique in breast surgery together with pectoral nerve blocks and serratus anterior plane blocks,30 tough this method might present several drawbacks, including the need for multiple injections with large volume of local anesthetics and the onset of delayed or patchy sensory block, in association with potential risks and complications such as pneumothorax, Horner’s Syndrome, inadvertent injection or spread to the epidural space responsible for perioperative hypotension and failure of technique, the last one occurring in 10–15% of cases, independently from adopting the ultrasound-guided or landmark procedure.31
In addition, according to a recent review, TPVB has minimal effect in decreasing the incidence of PONV and the perioperative requirement of intravenous opioids,31 which might have negative immunomodulatory effects and promote tumor angiogenesis, leading to an increased risk of cancer recurrence and metastasis.32
Therefore, in our view, all approaches to decrease their utilization, such as STSA, should be advocated, particularly in oncological surgery.
Moreover, all the above-mentioned anesthetic procedures are generally combined with GA, limiting their use in patients with important comorbidities. Indeed, as far as we are aware, the use of TPBV and intravenous sedation without delivering GA in breast surgery was mentioned exclusively in one retrospective work in literature.33
Nevertheless, of the 28 surgeries described, approximately half consisted of mastectomies, whereas the remaining were represented by minor procedures such as local excisions, wire-localized biopsies, and cavity re-excisions,33 allowing to conduct these surgical procedures under the exclusive utilization of paravertebral block and intravenous sedation.
Conclusions
Our small series suggest STSA as a safe, reliable, and adequate anesthesiologic method in surgeries involving the breast and axillary region, particularly in frail and elderly patients, representing a reasonable alternative to GA in this peculiar class of patients, though very limited experiences have been described so far in literature.
Abbreviations
ALND, axillary lymph node dissection; ASA, American society of anesthesiologists; BMI, body mass index; FiO2, fraction of inspired oxygen; GA, general anesthesia; INRCA, Italian national research center on aging; PACU, post-anesthesia care unit; PONV, postoperative nausea and vomiting; RA, regional anesthesia; STSA, segmental thoracic spinal anesthesia; TCSA, thoracic continuous spinal anesthesia; TPVB, thoracic paravertebral block.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. McLean RC, McCallum IJD, Dixon S, O’Loughlin P. A 15-year retrospective analysis of the epidemiology and outcomes for elderly emergency general surgical admissions in the North East of England: a case for multidisciplinary geriatric input. Int J Surg. 2016;28:13–21. doi:10.1016/j.ijsu.2016.02.044
2. Guay J, Choi P, Suresh S, Albert N, Kopp S, Pace NL. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of cochrane systematic reviews. Cochrane Database Syst Rev. 2014;(1):CD010108. doi:10.1002/14651858.CD010108.pub2
3. Antoniou SA, Adasonla K, Hajibandeh S, et al. Loco-regional versus general anaesthesia for elective endovascular aneurysm repair – results of a cohort study and a meta-analysis. Vasa. 2018;47(3):209–217. doi:10.1024/0301-1526/a000688
4. Hill K, Macfarlane AJ. Does regional anaesthesia improve outcome? Anaesth Intensive Care Med. 2018;19(11):619–623. doi:10.1016/j.mpaic.2018.08.018
5. Halaszynski TM. Pain management in the elderly and cognitively impaired patient: the role of regional anesthesia and analgesia. Curr Opin Anaesthesiol. 2009;22(5):594–599. doi:10.1097/ACO.0b013e32833020dc
6. Savas JF, Litwack R, Davis K, Miller TA. Regional anesthesia as an alternative to general anesthesia for abdominal surgery in patients with severe pulmonary impairment. Am J Surg. 2004;188(5):603–605. doi:10.1016/j.amjsurg.2004.07.016
7. Milosavljevic SB, Pavlovic AP, Trpkovic S, AN Ilić, Sekulic AD. Influence of spinal and general anesthesia on the metabolic, hormonal, and hemodynamic response in elective surgical patients. Med Sci Monit. 2014;20:1833–1840. doi:10.12659/MSM.890981
8. McIsaac DI, Cole ET, McCartney CJL. Impact of including regional anaesthesia in enhanced recovery protocols: a scoping review. Br J Anaesth. 2015;115:ii46–ii56. doi:10.1093/bja/aev376
9. Clemente A, Carli F. The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol. 2008;74(10):549–563.
10. Turan A, Bajracharya GR, Leung S, et al. Association of neuraxial anesthesia with postoperative venous thromboembolism after noncardiac surgery. Anesth Analg. 2019;128(3):494–501. doi:10.1213/ANE.0000000000003394
11. Kumar CM, Corbett WA, Wilson RG. Spinal anaesthesia with a micro-catheter in high-risk patients undergoing colorectal cancer and other major abdominal surgery. Surg Oncol. 2008;17(2):73–79. doi:10.1016/j.suronc.2007.10.025
12. Spannella F, Giulietti F, Damiani E, et al. Thoracic continuous spinal anesthesia for high-risk comorbid older patients undergoing major abdominal surgery: one-year experience of an Italian geriatric hospital. Minerva Anestesiol. 2020;86(3):261–269. doi:10.23736/S0375-9393.19.13896-5
13. Vincenzi P, Starnari R, Faloia L, et al. Continuous thoracic spinal anesthesia with local anesthetic plus midazolam and ketamine is superior to local anesthetic plus fentanyl in major abdominal surgery. Surg Open Sci. 2020;2(4):5–11. doi:10.1016/j.sopen.2020.07.002
14. Castellani D, Faloia L, Dellabella M, et al. Radical cystectomy in frail octogenarians in thoracic continuous spinal anesthesia and analgesia: a pilot study. Ther Adv Urol. 2018;10(11):343–349. doi:10.1177/1756287218795427
15. Abdelhamid S, Elakany M. Segmental thoracic spinal has advantages over general anesthesia for breast cancer surgery. Anesth Essays Res. 2013;7(3):390–395. doi:10.4103/0259-1162.123263
16. Sheahan CG, Mathews DM. Monitoring and delivery of sedation. Br J Anaesth. 2014;113:ii37–ii47. doi:10.1093/bja/aeu378
17. Ellakany M. Thoracic spinal anesthesia is safe for patients undergoing abdominal cancer surgery. Anesth Essays Res. 2014;8(2):223–228. doi:10.4103/0259-1162.134516
18. Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain, Behav Immun. 2013;30:S32–S40. doi:10.1016/j.bbi.2012.03.006
19. Li T, Chen L, Zhao H, et al. Both Bupivacaine and Levobupivacaine inhibit colon cancer cell growth but not melanoma cells in vitro. J Anesth. 2019;33(1):17–25. doi:10.1007/s00540-018-2577-6
20. Bayrak M, Altintas Y. Comparing laparoscopic cholecystectomy in patients with chronic obstructive pulmonary disease under spinal anesthesia and general anesthesia. BMC Surg. 2018;18(1):65. doi:10.1186/s12893-018-0396-1
21. Sultan P, Gutierrez MC, Carvalho B. Neuraxial morphine and respiratory depression: finding the right balance. Drugs. 2011;71(14):1807–1819. doi:10.2165/11596250-000000000-00000
22. Sultan P, Halpern SH, Pushpanathan E, Patel S, Carvalho B. The effect of intrathecal morphine dose on outcomes after elective cesarean delivery: a meta-analysis. Anesth Anal. 2016;123(1):154–164. doi:10.1213/ANE.0000000000001255
23. Raffaeli W, Marconi G, Fanelli G, Taddei S, Borghi GB, Casati A. Opioid-related side-effects after intrathecal morphine: a prospective, randomized, double-blind dose-response study. Eur J Anaesthesiol. 2006;23(7):605–610. doi:10.1017/S026502150600038X
24. Yegin A, Sanli S, Dosemeci L, Kayacan N, Akbas M, Karsli B. The analgesic and sedative effects of intrathecal midazolam in perianal surgery. Eur J Anaesthesiol. 2004;21(8):658–662. doi:10.1017/S0265021504008129
25. Abd El-Rahman AM, Mohamed AA, Mohamed SA, Mostafa MAM. Effect of intrathecally administered ketamine, morphine, and their combination added to bupivacaine in patients undergoing major abdominal cancer surgery a randomized, double-blind study. Pain Med. 2018;19(3):561–568. doi:10.1093/pm/pnx105
26. Chattopadhyay A, Maitra S, Sen S. Midazolam in Subarachnoid Block: current evidence. ISRN Anesthesiol. 2013;2013:1–7. doi:10.1155/2013/202835
27. Imbelloni LE, Quirici MB, Ferraz Filho JR, Cordeiro JA, Ganem EM. The anatomy of the thoracic spinal canal investigated with magnetic resonance imaging. Anesth Analg. 2010;1(2):1–4. doi:10.1213/ANE.0b013e3181d5aca6
28. Imbelloni LE, Pitombo PF, Ganem EM. The incidence of paresthesia and neurologic complications after lower spinal thoracic puncture with cut needle compared to pencil point needle. Study in 300 patients. J Anesth Clin Res. 2010;1(2):1–4. doi:10.4172/2155-6148.1000106
29. Imbelloni L. Spinal anesthesia for laparoscopic cholecystectomy: thoracic vs. Lumbar technique. Saudi J Anaesth. 2014;8(4):477–483. doi:10.4103/1658-354x.140853
30. Sherwin A, Buggy DJ. Anaesthesia for breast surgery. BJA Educ. 2018;18(11):342–348. doi:10.1016/j.bjae.2018.08.002
31. Terkawi AS, Tsang S, Sessler DI, et al. Systematic review improving analgesic efficacy and safety of thoracic paravertebral block for breast surgery: a mixed-effects meta-analysis. Pain Physician. 2015;18:757–780. doi:10.36076/ppj.2015/18/E757
32. Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis. Can J Anesth. 2016;63(2):184–192. doi:10.1007/s12630-015-0523-8
33. Simpson J, Ariyarathenam A, Dunn J, Ford P. Breast surgery using thoracic paravertebral blockade and sedation alone. Anesthesiol Res Pract. 2014;2014:127467. doi:10.1155/2014/127467
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
