Back to Journals » Drug, Healthcare and Patient Safety » Volume 13
Ophthalmic Solution Safety Profile: Active Surveillance of a Sodium Hyaluronate/Chondroitin Sulfate Combination in Peruvian Population
Authors Contreras-Salinas H , Barajas-Hernández M , Baiza-Durán LM, Orozco-Ceja V, Rodríguez-Herrera LY
Received 19 March 2021
Accepted for publication 3 May 2021
Published 27 May 2021 Volume 2021:13 Pages 117—123
DOI https://doi.org/10.2147/DHPS.S311817
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hemalkumar B Mehta
Homero Contreras-Salinas, Mariana Barajas-Hernández, Leopoldo Martín Baiza-Durán, Vanessa Orozco-Ceja, Lourdes Yolotzin Rodríguez-Herrera
Pharmacovigilance Department, Laboratorios Sophia, S.A. de C.V., Guadalajara, Jalisco, Mexico
Correspondence: Lourdes Yolotzin Rodríguez-Herrera Tel +52 33 3001 4200 Ext 1188
Email [email protected]
Background: Sodium hyaluronate/chondroitin sulfate fixed combination plays an essential role in the treatment of keratoconjunctivitis sicca, a multifactorial disease accompanied by ocular symptoms like alteration of the tear film. Despite low or no absorption of such drugs, these can cause secondary effects. An essential tool in the study of medication behavior is active pharmacovigilance. Unlike spontaneous reporting pharmacovigilance, this tool allows an appraisal of adverse drug reactions (ADRs)’ real incidence, a higher capacity to identify safety signals, the relationship with concomitant drugs and pathologies prevalent in the study population. This study aimed to evaluate the safety profile and identify and/or assess adverse reactions in an uncontrolled population.
Methods: Active pharmacovigilance by Drug Event Monitoring was performed. A total of 3 follow-up calls were made for 30 days for the identification of the ADRs, tolerability (ADR severity, seriousness, long term sequelae, and duration) and the possible risks (safety signals, medical interactions) of sodium hyaluronate and chondroitin sulfate (HUM).
Results: Thirty-five ADRs were identified in the 212 patients included in the study (0.17 ADR/patient). The 35 ADRs were classified into 3 System Organ Class (SOC) groups: general disorders and administration site conditions (74.2%), eye disorders (22.9%), and nervous system disorders (2.9%); and 4 Preferred Term (PT) groups: burning sensation (74.2%), followed by blurred vision (20%), ocular pain (2.9%) and headache (2.9%). All the ADRs were categorized as mild and not serious. No statistically significant differences were found in concomitantly medications, posology and age groups.
Conclusion: Good tolerability to the solution was identified, with a low incidence of ADRs. Just the same, all the associated ADRs were consistent with the information found in HUM’s physicochemical profile and the physiopathology of DED. No unknown risks were identified, reinforcing HUM’s safety profile.
Keywords: post-marketing surveillance, adverse drug reaction, artificial tears, dry eye disease
Introduction
Dry Eye Disease (DED) or keratoconjunctivitis sicca, is a multifactorial disease accompanied by ocular symptoms like alteration of the tear film resulting in damage to the eye’s surface. It is associated with symptoms of eye discomfort.1,2 Although there are no formal prevalence studies of this disease in Latin American countries, many reports suggest a higher prevalence of severe symptoms and clinical diagnosis of DED in the Hispanic population than in the Caucasian population.3,4 Eye lubricants are the first line of management for DED and a constant at all levels of treatment. They are characterized by being hypo or isotonic solutions containing electrolytes, surfactants, and various types of viscous agents. The main variables in ocular lubricants’ formulations are related to the selection or concentration of electrolytes, osmolarity, type of visco-polymer system, and the presence or absence of preservatives.5 The combination of sodium hyaluronate/chondroitin sulfate plays an important role in the treatment of keratoconjunctivitis sicca since the application of both components increases the stability of the tear film, improvement of the ocular microenvironment, as well as the decrease of inflammation due to the decrease of the presence of CD4+ lymphocytes.6,7 Despite the low or no absorption of such medications, these can cause secondary effects.8
Knowledge regarding a product’s safety profile may expand over time due to the greater number of patients with different traits exposed to the product, varying from those patients included under strict selection criteria as is required in clinical trials.9 Hence, drugs’ safety monitoring is essential, especially after their prolonged use.10,11
Post-marketing surveillance studies are highly relevant since once a product is marketed new information about its safety will be generated, potentially impacting the risk/benefit balance associated with it. Therefore, the evaluation of such information is a continuous process that pharmacovigilance undertakes besides detecting, evaluating, and understanding ADRs or related problems. This is done mainly through spontaneous reporting systems (SRS), which are intended to periodically assess whether the risk/benefit ratio of the drug remains favorable; or otherwise, to determine if there is a need to implement actions and reduce the risks.12,13 An essential tool in the study of medication post-marketing behavior is active pharmacovigilance. Unlike spontaneous reporting pharmacovigilance, this tool allows an appraisal of ADRs’ real incidence, a higher capacity to identify safety signals, the relationship with concomitant medicines, pathologies, and lifestyle characteristics prevalent in the study population.13,14 Drug Event Monitoring is a structured method of active pharmacological monitoring, which allows patients to be followed up at pre-specified intervals using questionnaires designed to obtain the data of interest. This system allows detailed information to be obtained on the events and evolution of a large number of patients.15
The aim of this study was to evaluate the safety profile and to identify and/or assess adverse reactions associated with the use of Humylub Ofteno®, a fixed combination of Sodium Hyaluronate 0.1% and Chondroitin Sulfate 0.18% (HUM) (Laboratorios Sophia, S.A. de C.V., México), in an uncontrolled population.
Methods
Study Design
Active pharmacovigilance by Drug Event Monitoring was applied to Peruvian population from February 28, 2018 (first enrolled patient) to April 5, 2020 (last completed patient). The study was conducted according to the Declaration of Helsinki; likewise, the study’s protocol and its corresponding informed consent form were reviewed and approved by an ethics committee (see Ethics approval section). Patients who received a HUM prescription by an ophthalmologist (on his/her own initiative) were derived to a member of the pharmacovigilance unit of Laboratorios Sophia, S.A. de C.V. stationed in Perú and were informed about the enrollment process and invited to participate. If the patient agreed to participate, the informed consent was signed. All the admitted patients signed the informed consent before enrolling in the study. In the case of patients under 18 years old (yo), the parent or legal guardian signed the informed consent. A total of 3 follow-up calls were performed.
First call: It was performed on day three after the patient signed the informed consent during which a questionnaire divided into the following sections was applied. I. Personal data (age, gender, nationality, pregnancy or breastfeeding), II. Characteristics of the drug and its prescription (dose, route of administration, start and end date of treatment, expiration date, batch), III. Patient’s medical history (diagnosis, concomitant drugs used and their dose, route of administration, start of application), in addition to conducting the first interrogation aimed at identifying ADRs (start date, description of intensity, ADR duration, need of treatment, re-challenge, existence of a similar preceding ADR with the same drug, de-challenge, response to dose modification, existence of other cause different to drug application that may have explained the ADR). Second and third calls: days 7 and 30 after the enrollment process, respectively. These interrogations were aimed at identifying ADRs as mentioned above.
Categorization
The patients were classified as: children (0–12 yo), adolescents (>12–18 yo), adults (>18–60 yo), or geriatric (>60 yo). All ADRs were listed according to the Medical Dictionary for Regulatory Activities (MedDRA) v22 in System Organ Class (SOC) and Preferred Term (PT). The drugs were cataloged according to “ATC/DDD Index 2020, World Health Organization” and the comorbidities with High Level Term (HLT) according to MedDRA v22. The incidences of ADRs were registered according to “The Council for International Organizations of Medical Sciences (CIOMS)”.16
Once the information of the ADRs was obtained, the severity was assessed using the ADR Severity Assessment Scale (Modified Hartwig and Siegel)17 and subsequently, the causal relationships were assessed, in accordance with the Naranjo algorithm (Definite, Probable, Possible, Doubtful and Not assessable).18
Data Management
The data obtained in each of the calls were recorded in the follow-up contact form and sent to the pharmacovigilance unit of Laboratorios Sophia, S.A. de C.V. (UFTLS), afterward, the data was compiled in an excel document (Microsoft Office® 365 ProPlus., Washington, Redmond, USA.), and ADRs underwent evaluation of causality and severity.
Outcome Analysis
Identification of the ADRs, tolerability (ADR severity, seriousness, long term sequelae, and duration) and the possible risks of HUM (safety signals, medical interactions) was performed.
Statistical Analysis
Quantitative variables were described as mean (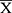 ) ± Standard Deviation (SD), qualitative variables were described as frequencies and percentages. A chi-square test was performed to compare proportions and Fisher’s exact for small-sized samples. In this study, the statistical significance was 2-sided set at a p-value ≤0.05. SPSS (version 21; SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis.
) ± Standard Deviation (SD), qualitative variables were described as frequencies and percentages. A chi-square test was performed to compare proportions and Fisher’s exact for small-sized samples. In this study, the statistical significance was 2-sided set at a p-value ≤0.05. SPSS (version 21; SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis.
Results
In this study 252 non-controlled patients were recruited, none of them withdrew their informed consent. Nevertheless, the percentage of patients not contacted was 15.9% (n=40) due to: call not answered (57.5%, n=23), the phone number did not correspond to the person who signed the informed consent (27.5%, n=11), the phone number did not exist (15%, n=6). A total of 212 outpatients were enrolled; 123 females 58.0% and 89 males 42% (Children n=1, Adolescents n=2, Adults n=110, Geriatrics n=99) (Table 1). The most frequent therapeutic indication for the use of HUM was dry eye (n=178; 84%), post-surgical treatment (n=30; 14%) and eye irritation (n=4; 2%). Fifty-five percent of the patients enrolled in the study used other medications simultaneously with HUM, finding that the therapeutic group most commonly used was antiglaucoma preparations and miotics (8%) followed by antiinflammatory agents, non-steroids (6.6%). Statistical analysis was carried out to corroborate whether the concomitant administration of those drugs increased the incidence of ADRs, finding no statistically significant difference in any of the therapeutic groups of studied drugs (Table 2). Similarly, a square chi test was performed to compare the posology ADRs’ incidence: 1 drop c/3h (n=4; 1 ADR), 1 drop c/4h (n=23; 3 ADRs), 1 drop c/6h (n=20; 5 ADRs), 1 drop c/8 (n=82; 16ADRs), 1 drop c/12h (n=70; 7 ADRs), 1 drop c/24 (n=13; 3 ADRs); nevertheless, no statistically significant differences were found (X2(5)=4551, p=0.4731). On the other hand, the 57.6% of patients enrolled in the study who suffered one or more comorbidities were cataloged by HLT as follows: vascular hypertensive disorders (11.3%; n=24), glucose metabolism disorders (8.4%; n=18), glaucoma and ocular hypertension (7.6%; n=16), conjunctival infections, irritations and inflammations (7.6%; n=16), immunology and allergic investigations (4.7%; n=10), gastritis (2.4%; n=5) and others (5.7%; n=11).
 |
Table 1 Characteristics of Included Patients |
 |
Table 2 Risk of Using HUM with Concomitant Drugs |
Thirty-five ADRs were identified in the 212 patients included in this study (0.17 ADR/patient). 22 ADRs in adults (0.2 ADR/patient) and 13 ADRs in geriatrics (0.13 ADR/patient); no ADRs were reported in children or adolescents (Table 3). No significant differences for ADR incidence were found between adults and geriatrics groups through the Chi Square test (X2(1)=1.763, p=0.1842). The ADRs were classified into 3 SOC: general disorders and administration site conditions, eye disorders and nervous system disorders; and 4 PT: burning sensation 74.2% [Recovery Time (RT): 2 seconds to 5 minutes], blurred vision 20% [RT: 5 minutes up to 1 hour], ocular pain 2.9% [RT: 10 minutes] and headache 2.9% [RT: 5 hours]. The most frequent causality was probable (n=25) in 71% of the cases, followed by possible (n=5) 14%, doubtful (n=4) 12% and finally definite (n=1) 3%. All the ADRs were categorized as mild and not serious (n=35).
 |
Table 3 Adverse Drug Reactions |
Discussion
One of the aims of the study is the identification and classification of the ADRs.16 In this post-marketing study the ADRs were classified in 4 PT, all of them mild and not severe, with the following incidence: “frequent” in the case of burning sensation and blurred vision, and “infrequent” in the case of ocular pain and headache (Table 3). Due to the limited bibliographic safety information about sodium hyaluronate and chondroitin sulfate, a search was conducted regarding the most frequent ADRs in artificial tears, and different sources showed 3 ADR repeatedly reported in regards to artificial tears: burning sensation, ocular pain, and blurred vision.19–21 These results are consistent with what was observed in this study, where these 3 ADRs were described; however, one of the ADRs identified as “headache” was not detected in the revised literature.
During this study, the main ADR was burning sensation, which is one of the principal ADRs in patients who use artificial tears like HUM.21–23 Besides, the most frequent indication of these drugs was dry eye disease (DED), which exhibits pre-existing damage due to insufficient ocular lubrication, and consequently an inflammatory process, neurosensorial abnormalities, and damage to the ocular surface secondary to the frictional forces of blinking.1,24 This could largely explain the appearance of burning sensation or pain after using ophthalmic drugs in several patients since being HUM in contact with the damaged tissue could cause a small transitory burning or pain. However, an improvement of this ADR has been demonstrated with subsequent administrations.19,20,25
Another ADR described after HUM application was blurred vision. Furthermore, multiple studies indicate that this is one of the most frequent ADRs associated with the use of artificial tears,21–23 and it is related to the viscosity of this type of pharmaceutical preparations since artificial tears adhere to the surface of the eye.20 However, it is essential to point out that blurred vision is transitory, and blinking or manipulating the lower eyelid usually solves this problem.19 The bibliography even describes that the increase of the viscosity in this type of solution is beneficial due to a greater permanence on the ocular surface.21,23,26,27
The last ADR, headache, was observed in one patient and it was analyzed using Bradford-Hill’s criteria;28 however, due to the low causal relationship (doubtful), low biological plausibility, as well as the fact that no information was found in different sources relating headache to the use of sodium hyaluronate, chondroitin sulfate or artificial tears, it was not possible to relate the use of HUM with this ADR.
On the other hand it is essential to establish a safety profile for posology and age groups due the over-the-counter medications do not require a medical prescription; consequentially, in this study, we did not find a statistically significant difference in the incidence of ADRs in different posology and age groups; this coincides with reported in the literature, since there is no limitation of use in dosage or increased risk in different strata of the population.29
Another aspect relevant of the study is the drugs’ interactions.16 It has been described in the literature that the use of artificial tears increases the permanence of different compounds on the ocular surface, and can increase the exposure time of ophthalmic drugs and thus increase the incidence of ADRs.8,30 Even some non-ophthalmic drugs may also have interactions with ophthalmic medications;31 however, this type of interactions are not well documented. For this reason, we conducted a statistical analysis of the use of HUM and therapeutic groups of drugs used by patients where no significant difference was found between the risk of using HUM and patients’ medications.
Additionally, a low incidence of ADR/patient was found. All ADRs presented were classified as mild, and 83% of the patients recovered completely within 5 minutes. None of the patients discontinued treatment due to ADR incidence. This correlates to what is mentioned in the literature where low incidences of ADRs and high tolerability are described for artificial tears.19,20
Limitations of Study
This study’s limitations were that the patient’s medical knowledge may limit the data collection through a direct patient interview (for example, characteristics of the prescription, concomitant medication and data from the patient’s medical history) as well as some ADRs require a physician’s expertise. However, it has some advantages, such as individualized pharmacovigilance, continuous monitoring, and more detailed information on adverse events in a large number of patients.
Conclusion
In this study, good tolerability for the drug was proven, with a low incidence of ADRs cataloged in 3 PTs associated with the drug; 2 of them were cataloged with the incidence frequent and one as infrequent. All the associated ADRs were consistent with the information found in HUM’s physicochemical profile and DED physiopathology. All the ADRs showed a short recovery time; no unknown risks were identified, reinforcing HUM’s safety profile.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Ethics Approval
This study was approved by the “Comité Institucional de Bioética (CIB), Vía libre”, located in Lima, Perú (Approval Number 4205, Dic-18-2018). This study was conducted in compliance with international guidelines and in accordance with strictest international ethical regulations for research.
Acknowledgments
This study was sponsored by Laboratorios Sophia, S.A. de C.V. (Zapopan, Jalisco, México). The sponsor provided support in the form of salaries for authors (HCS, MBH, LMBD, VOC and LYRH), but did not have any additional role in the data collection. The authors thank Alejandra Sánchez Rios, MD for the medical writing support.
Disclosure
Homero Contreras-Salinas, Mariana Barajas-Hernández, Leopoldo Martín Baiza-Durán, Vanessa Orozco-Ceja, Lourdes Yolotzin Rodríguez-Herrera are employees of Laboratorios Sophia S.A. de C.V. The authors report no other conflicts of interest.
References
1. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510.
2. Phadatare S, Momin M, Nighojkar P, Askarkar S, Singh K. A comprehensive review on dry eye disease: diagnosis, medical management, recent developments, and future challenges. Adv Pharm. 2015;2015:1–12.
3. Hom M, Land P. Prevalence and severity of symptomatic dry eyes in hispanics. Optom Vis Sci. 2005;82(3):206–208. doi:10.1097/01.OPX.0000156310.45736.FA
4. Graue-Hernández E, Serna-Ojeda J, Estrada-Reyes C, Navas A, Arrieta-Camacho J, Jimenez-Corona A. Dry eye symptoms and associated risk factors among adults aged 50 or more years in Central Mexico. Salud Publica Mex. 2018;60(5, sep–oct):520. doi:10.21149/9024
5. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628.
6. Duran L, Delgado JÁ, Rubio YC, et al. Estudio de la seguridad y eficacia del hialuronato de sodio y sulfato de condroitín versus carboximetilcelulosa en el síndrome de ojo seco. Rev Mex Oftalmol. 2008;82:86–90.
7. Belalcázar-Rey S, Sánchez Huerta V, Ochoa-Tabares JC. et al. Efficacy and safety of sodium hyaluronate/chondroitin sulfate preservative-free ophthalmic solution in the treatment of dry eye: a clinical trial. Curr Eye Res. 2020:1–11. doi:10.1080/02713683.2020.1849733
8. Durairaj C. Ocular Pharmacokinetics BT - Pharmacologic Therapy of Ocular Disease. Whitcup SM, Azar DT, eds. Springer International Publishing; 2017:31–55.
9. Beninger P. Pharmacovigilance: an overview. Clin Ther. 2018;40(12):1991–2004. doi:10.1016/j.clinthera.2018.07.012
10. Garotta F, Messa A. Safety profile of ceftizoxime: an Italian experience on 14,007 patients. J Chemother. 1991;3(Suppl 2):36–38.
11. Varenna M, Bertoldo F, Di Monaco M, Giusti A, Martini G, Rossini M. Safety profile of drugs used in the treatment of osteoporosis: a systematical review of the literature. Reumatismo. 2013;65(4):143–166. doi:10.4081/reumatismo.2013.143
12. Li X, Li H, Deng J, et al. Active pharmacovigilance in China: recent development and future perspectives. Eur J Clin Pharmacol. 2018;74(7):863–871. doi:10.1007/s00228-018-2455-z
13. Mann M, Mengistu A, Gaeseb J, et al. Active surveillance versus spontaneous reporting for first-line antiretroviral medicines in namibia: a cost–utility analysis. Drug Saf. 2016;39(9):859–872. doi:10.1007/s40264-016-0432-y
14. Moses C, Celi LA, Marshall J. Pharmacovigilance: an active surveillance system to proactively identify risks for adverse events. Popul Health Manag. 2013;16(3):147–149. doi:10.1089/pop.2012.0100
15. ICH harmonized tripartite guideline. Pharmacovigilance planning; November 18, 2004. Available from: https://database.ich.org/sites/default/files/E2E_Guideline.pdf.
16. Council for International Organization of Medical Sciences (CIOMS). Working Group IV Benefit-Risk Balance for Marketed Drugs: Evaluating Safety Signals.
17. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–2232.
18. Naranjo A, Busto U, Sellers E, et al. A method of estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi:10.1038/clpt.1981.154
19. Marner K, Møller PM, Dillon M, Rask-Pedersen E. Viscous carbomer eye drops in patients with dry eyes. Acta Ophthalmol Scand. 1996;74(3):249–252. doi:10.1111/j.1600-0420.1996.tb00086.x
20. Wang I-J, Lin I-C, Hou Y-C, Hu F-R. A comparison of the effect of carbomer-, cellulose- and mineral oil-based artificial tear formulations. Eur J Ophthalmol. 2007;17(2):151–159. doi:10.1177/112067210701700202
21. Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2:CD009729. doi:10.1002/14651858.CD009729.pub2
22. Zopf Y, Rabe C, Neubert A, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64(10):999–1004. doi:10.1007/s00228-008-0494-6
23. Lievens C, Berdy G, Douglass D, et al. Evaluation of an enhanced viscosity artificial tear for moderate to severe dry eye disease: a Multicenter, Double-Masked, Randomized 30-Day Study. Cont Lens Anterior Eye. 2019;42(4):443–449. doi:10.1016/j.clae.2018.12.003
24. Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15(3):404–437.
25. Brodwall J, Alme G, Gedde-Dahl S, et al. A comparative study of polyacrylic acid (Viscotears) liquid gel versus polyvinylalcohol in the treatment of dry eyes. Acta Ophthalmol Scand. 1997;75(4):457–461. doi:10.1111/j.1600-0420.1997.tb00413.x
26. Simmons PA, Liu H, Carlisle-Wilcox C, Vehige JG. Efficacy and safety of two new formulations of artificial tears in subjects with dry eye disease: a 3-month, multicenter, active-controlled, randomized trial. Clin Ophthalmol. 2015;9:665–675. doi:10.2147/OPTH.S78184
27. Nepp J, Schauersberger J, Schild G, et al. The clinical use of viscoelastic artificial tears and sodium chloride in dry-eye syndrome. Biomaterials. 2001;22(24):3305–3310. doi:10.1016/S0142-9612(01)00167-3
28. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300.
29. Sodium hyaluronate. Micromedex; 2020. Available from: https://www.micromedexsolutions.com/.
30. Mochizuki H, Yamada M, Hato S, Nishida T. Fluorophotometric measurement of the precorneal residence time of topically applied hyaluronic acid. Br J Ophthalmol. 2008;92(1):108–111. doi:10.1136/bjo.2007.121533
31. Hughes WF. Drug-induced ocular side effects and drug interactions. Arch Ophthalmol. 1983;101(3):497.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
