Back to Journals » Clinical Ophthalmology » Volume 9
Opacification of hydrophilic intraocular lenses after Descemet stripping automated endothelial keratoplasty
Authors Morgan-Warren P, Andreatta W, Patel A
Received 9 December 2014
Accepted for publication 24 December 2014
Published 10 February 2015 Volume 2015:9 Pages 277—283
DOI https://doi.org/10.2147/OPTH.S78930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Peter J Morgan-Warren, Walter Andreatta, Amit K Patel
Department of Ophthalmology, Solihull Hospital, Heart of England NHS Foundation Trust, Birmingham, UK
Purpose: Opacification of hydrophilic acrylic intraocular lenses (IOLs) is an emerging complication following Descemet stripping automated endothelial keratoplasty (DSAEK). We report six cases and review the current literature.
Methods: In this retrospective, noncomparative, observational case series, patients with IOL opacification after previous DSAEK surgery were identified from corneal clinic records. Case notes were reviewed for demographic details, indication for DSAEK, IOL model, incidence of rebubbling, and postoperative course.
Results: Six patients developed IOL opacification after DSAEK. All patients had Fuchs’ endothelial dystrophy and had previously received hydrophilic acrylic IOL models. Central anterior IOL opacification was noted in all six cases. Five cases (83%) had required rebubbling due to dislocated graft tissue, and one had an early postoperative intraocular pressure (IOP) rise. Five cases (83%) were managed conservatively, and one case with a failed graft underwent redo DSAEK and IOL exchange.
Conclusion: Repeated exposure to intracameral air, raised IOP, and other patient influences may be major etiological factors for IOL opacification after DSAEK. We advise avoiding hydrophilic acrylic IOL models in patients who may require future endothelial keratoplasty.
Keywords: IOL, DSAEK, lamellar keratoplasty, endothelial corneal transplantation
Introduction
The surgical treatment of corneal endothelial pathology has changed significantly with the evolution of posterior lamellar keratoplasty techniques and Descemet stripping automated endothelial keratoplasty (DSAEK) is now widely performed as the procedure of choice for corneal decompensation secondary to endothelial dysfunction.1 DSAEK is associated with excellent visual outcomes, with less corneal astigmatism and a more rapid visual recovery compared to traditional penetrating keratoplasty (PKP).1,2
Complications of DSAEK are well-described, although the most common adverse events do not appear to be detrimental to the ultimate visual outcome.3 Reported complications include graft dislocation, graft rejection, primary graft failure, graft–host interface opacification, pupillary block, cystoid macular edema, and epithelial ingrowth.2,3 It has only recently become apparent that intraocular lens (IOL) complications may occur in pseudophakic patients undergoing DSAEK, with a number of recent reports describing late IOL opacification.4–10 We report a series of six cases of late-onset IOL opacification in pseudophakic patients after DSAEK surgery, and review the current literature on this emerging complication.
Methods
This study was a retrospective, noncomparative, observational case series. The institutional review board of the Research & Development Department, Heart Of England NHS Foundation Trust, Birmingham, UK, ruled that approval was not required for this report. We reviewed clinic records from patients attending the corneal service at Solihull Hospital, Heart of England NHS Foundation Trust, Birmingham, UK. Pseudophakic patients who had undergone DSAEK and later diagnosed with IOL opacification between January 2010 and April 2014, inclusive, were identified from clinic records. Charts were reviewed for demographic data, diagnosis/indication for DSAEK, ocular comorbidity, date of prior cataract surgery, details of IOL implant, date and perioperative details of DSAEK surgery, and postoperative management. For patients who had undergone surgery at another institution before transfer of their case to our clinic, all surgical and perioperative records for both cataract and corneal procedures were obtained for these patients from the referring center.
Results
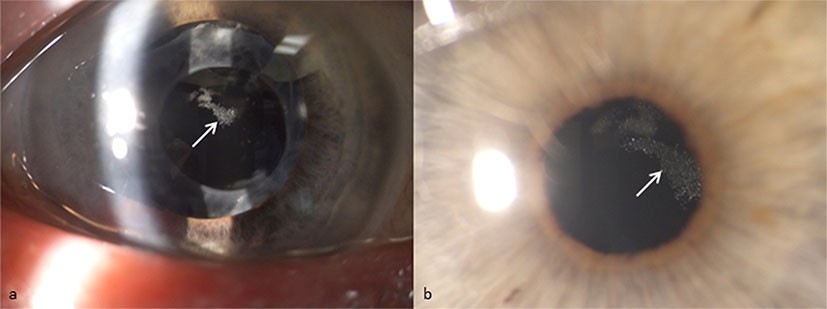
A total of six patients were identified with IOL opacification following DSAEK: three (50%) were male and the mean age was 75.6 years (range 71–81 years). Five of the patients had undergone DSAEK elsewhere, and had subsequently transferred to our unit for ongoing management. We are therefore unable to accurately calculate the incidence from our series. Indication for DSAEK was corneal decompensation secondary to Fuchs’ endothelial dystrophy (FED) in all cases, and all had previously undergone cataract surgery and implantation of hydrophilic acrylic IOL models. Five cases (83%) had required intracameral air injection (‘rebubbling’) for donor graft dislocation in the early postoperative (1–5 days) period. One patient who did not require rebubbling had a postoperative intraocular pressure (IOP) spike on day 2 post-DSAEK, which was managed with oral and topical antihypertensive medical therapy. This patient subsequently developed a corticosteroid-induced rise in IOP 3 months postoperatively, which settled after a switch to a less potent corticosteroid preparation. The standard postoperative medication regime after DSAEK included topical preservative-free 0.1% dexamethasone six times daily and 0.5% chloramphenicol four times daily for 4 weeks, and subsequently 0.1% dexamethasone four times daily for 3 months, tapered over 3 months, and maintained on long-term 0.1% fluorometholone (FML). In all cases, IOL opacification was a central, crystalline opacity within the pupillary margin, diagnosed on average 32 months (range 5–80 months) after DSAEK. Five (83%) patients have been managed conservatively, and one patient with a subsequently failed corneal graft has recently undergone IOL exchange and redo DSAEK. Demographic, clinical, and relevant postoperative details are summarized in Table 1. Clinical images of two patients are illustrated in Figures 1 and 2, respectively.
Discussion
Opacification of IOLs is well-described and associated with many factors, including IOL production processes, surgical techniques, and metabolic conditions.11,12 There are recent reports of hydrophilic acrylic IOL opacification as an emerging late complication of DSAEK (Table 2).4–10 We identified six cases of central IOL opacification in pseudophakic patients after undergoing DSAEK for corneal decompensation secondary to FED. Whilst the pathophysiology is incompletely understood, several features are evident that may enable greater comprehension of this phenomenon and result in changes to practice.
Anterior central IOL opacification after DSAEK was first described in three cases presenting between 7 and 18 months postoperatively.5 A further case presenting approximately 18 months after a combined redo DSAEK and phacoemulsification with hydrophilic IOL underwent IOL explantation,9 and analysis confirmed granular calcification in the central zone of the anterior IOL optic. Calcification has been a common feature of all the explanted IOLs analyzed and confirmed by X-ray spectroscopy and calcium-specific staining methods such as alizarin red.4,7–10 Histological examination of the explanted IOL from our Case 5 confirmed the presence of dystrophic calcification (Figure 2).
Our six patients had all been implanted with hydrophilic acrylic IOL models prior to undergoing DSAEK (Table 1), and published cases have also been exclusively associated with hydrophilic acrylic IOLs (Table 2). These IOLs all have a similar water content of 25.5%–26%, although different acrylic polymer compositions, suggesting that the class of IOL is important for the development of opacification, rather than the IOL model per se. Despite macroscopically similar clinical appearances of IOL calcification reported after intraocular gas, there are some differences in the microscopic appearance of the crystalline deposits between different IOL models, possibly reflecting the individual IOL polymer ultrastructure.6–11
The use of intracameral air is an integral part of DSAEK surgery and rebubbling of an incompletely adherent graft in the early postoperative period is reported in the majority of cases that have developed IOL opacification (Table 2), suggesting that repeated exposure of the hydrophilic IOL to intracameral air is responsible. Consistently, five cases in our series (83%) required repeat intracameral air injection within the first postoperative week. The largest series to date of IOL opacification after keratoplasty reported ten cases, including seven after DSAEK, one after deep anterior lamellar keratoplasty (DALK), and two after PKP.7 Six of the DSAEK cases (86%) underwent at least one rebubbling and the DALK case reported was also exposed to repeated intracameral air injections. A recent report described early postoperative rebubbling in all four cases of opacification of Rayner hydrophilic acrylic IOL models after DSAEK, accounting for 40% of cases rebubbled in this series.10 Repeated exposure of hydrophilic IOLs to intracameral air or gas is therefore likely to be a major contributory factor to the development of calcification, and has also been described in other cases of IOL opacification, such as after SF6 and C3F8 gas fill for treatment of Descemet membrane detachment after cataract surgery.13 Anterior central opacification with sparing of the posterior and peripheral IOL optic is a common feature and the pattern of opacification in our cases is comparable (Figures 1 and 2). The distribution of calcification has been attributed to the presence of intracameral gas/air in contact with the anterior IOL surface and relative protection of the peripheral IOL by the iris.6 Interestingly, one case reportedly had a small area of opacification outside the pupillary region, corresponding to an area of preexisting iris atrophy, further supporting the notion of an etiological factor within the anterior chamber.6 The repeated presence of gas or air in the anterior chamber may induce ultrastructural changes on the IOL optic surface within the pupil margin, leading to increased IOL permeability to anterior chamber factors that combine with the IOL material to act as a focus for calcification.6,8 As newer surgical modifications and instruments are being developed, Descemet membrane endothelial keratoplasty (DMEK) has been gaining popularity, although it is associated with high graft detachment rates of up to 63% in the early surgical learning curve.14 Whilst this rate tends to improve with experience,15 IOL opacification may be a potentially greater problem for surgeons performing DMEK or converting from DSAEK, which is associated with a lower incidence of graft dislocations.3,15
Factors other than the repeated presence of intracameral air may also contribute to the development of IOL opacification, as not all reported cases of post-DSAEK IOL calcification have been rebubbled in the postoperative period.5,7,9 IOL opacification is not an inevitable sequel to rebubbling. For example, a series examining 29 DSAEK cases requiring rebubbling did not mention IOL opacity,16 and several smaller series of donor graft dislocations being managed with rebubbling have similarly failed to describe IOL changes.17,18 Use of hydrophobic acrylic or other IOL materials in these cohorts, underreporting of IOL complications, or other factors may account for the lack of IOL opacification described.
Prolonged breakdown of the blood–aqueous barrier (BAB) has been suggested as a contributory factor in IOL opacification, with air or postoperative inflammation inducing a metabolic change in the anterior chamber, leading to an increase in aqueous protein, cells, and calcium content.5,7 Repeated air/gas tamponade may then drive calcium-rich aqueous into hydrophilic IOL substance with subsequent crystallization.6,8 One of the cases in our cohort (Case 5) developed IOL opacification in an eye in which the endothelial graft had failed several years previously, and it is therefore possible that prolonged low-grade intraocular inflammation was unidentified behind a cloudy cornea. Of note, this patient developed significant band keratopathy which required laser phototherapeutic keratectomy (PTK) in the 2 years after DSAEK. He had a past medical history of inflammatory bowel disease, requiring surgical colectomy, and had also previously been prescribed antipsychotic medication, and these factors may have influenced his metabolic status and propensity to calcification, although systemic calcium levels were not raised.
The postoperative course of published cases has been variable. Of the three cases reported by Patryn et al5 only one had been rebubbled, and the other two had very minimal and short-lived intraocular inflammation. The seven DSAEK case series all had complicated postoperative courses with rebubbling and intraocular inflammation, and even the one case that was not rebubbled had a prolonged anterior chamber inflammatory reaction and posterior synechiae.7 Schmidinger et al reported the presence of posterior synechiae in their case, which did not undergo rebubbling.9 Our single case (Case 4) who did not require rebubbling had a spike in IOP in the immediate postoperative period and a later corticosteroid-induced IOP rise. Whilst the influence of raised IOP has not been widely considered, it is possible that IOP raised above a certain threshold may be an initiating event for anterior chamber factors to induce IOL calcification. We cannot rule out the possibility that IOP or antiglaucoma medications may be contributing factors. Thus, prolonged intraocular inflammation, raised IOP, or other patient metabolic factors may increase the risk of developing IOL opacification after DSAEK.
Whilst there are many indications for endothelial transplantation, all the patients we identified had a diagnosis of FED. Furthermore, over 90% of reported cases have occurred in FED patients (Table 2),4–10 although IOL opacification after DSAEK has also been described in patients with pseudophakic bullous keratopathy and Axenfeld–Rieger’s anterior segment dysgenesis.8,19 It is possible that the underlying ocular condition itself may play a role in the pathogenesis of IOL opacification after DSAEK. FED is not considered to be an inflammatory disease, but it is conceivable that differences in aqueous humor proteome in FED patients could predispose to IOL calcification.20 Furthermore, differential expression of genes related to ion transport, pump function, and stress response has been reported in the corneal endothelium of FED patients compared to normal controls.21 Altered expression of aquaporins and solute transporters has also been reported in FED and PBK patients and further understanding of the genetic basis of corneal endothelial conditions may reveal other potential patient-related factors.22,23
Management of IOL opacification after DSAEK depends on the nature of the patient’s symptoms. Where intervention is indicated, neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has failed to disperse the calcification opacity (Table 2).4–9 However, it should be considered that Nd:YAG laser treatment of the posterior capsule may compromise a safe IOL exchange if subsequently required.7 Attempts to surgically remove the opacity have also failed (Table 2),5–9 reflecting the fact that the opacity is not merely a surface membrane but calcification within the IOL substance. IOL exchange is the only intervention that has enabled visual improvement (Table 2),4–10 and may require iris-fixated or sutured/glued IOL in cases where capsular integrity is insufficient for in-the-bag or sulcus implantation.8,24
In summary, we report six cases of IOL opacification after DSAEK, of which the majority required rebubbling of the dislocated donor endothelial graft in the early postoperative period. Repeated exposure to intracameral air is likely to be a significant etiological factor, although excessive postoperative inflammation, raised IOP, or other local or systemic factors may also contribute. Steps should be taken to minimize IOP spikes and inflammation. We suggest that hydrophilic IOL models should be avoided in patients undergoing cataract surgery who are at risk of postoperative corneal decompensation. Furthermore, as lamellar keratoplasty is now the treatment of choice for endothelial dysfunction, it is important that clinicians are alert to the emergence of this newly reported late complication in pseudophakic patients with hydrophilic IOLs.
Acknowledgment
This manuscript is derived from a poster abstract submitted to the American Academy of Ophthalmology, Chicago, IL, USA, October 18–21, 2014.
Disclosure
The authors report no conflicts of interest in this work.
References
Price MO, Price FW. Descemet’s stripping endothelial keratoplasty. Curr Opin Ophthalmol. 2007;18(4):290–294. | ||
National Institute for Health and Care Excellence. Corneal endothelial transplantation. [IPG304] 2009;1–10. Available from: https://www.nice.org.uk/guidance/ipg304. Accessed January 6, 2015. | ||
Lee BL, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(9):1818–1830. | ||
Werner L, Wilbanks G, Ollerton A, Michelson J. Localized calcification of hydrophilic acrylic intraocular lenses in association with intracameral injection of gas. J Cataract Refract Surg. 2012;38(4):720–721. | ||
Patryn E, van der Meulen IJ, Lapid-Gortzak R, Mourits M, Nieuwendaal CP. Intraocular lens opacifications in Descemet stripping endothelial keratoplasty patients. Cornea. 2012;31(10):1189–1192. | ||
Dhittal A, Spalton DJ, Goyal S, Werner L. Calcification in hydrophilic intraocular lenses associated with injection of intraocular gas. Am J Ophthalmol. 2012;153(6):1154–1160.e1. | ||
Neuhann IM, Neuhann TF, Rohrbach JM. Intraocular lens calcification after keratoplasty. Cornea. 2013;32(4):e6–e10. | ||
Fellman MA, Werner L, Liu ET, et al. Calcification of a hydrophilic acrylic intraocular lens after Descemet-stripping endothelial keratoplasty: case report and laboratory analyses. J Cataract Refract Surg. 2013;39(5):799–803. | ||
Schmidinger G, Pemp B, Werner L. [Opacification of an intraocular lens: calcification of hydrophilic intraocular lenses after gas tamponade of the anterior chamber]. Ophthalmologe. 2013;110(11):1066–1068. German. | ||
De Cock R, Fajgenbaum MA. Calcification of Rayner hydrophilic acrylic intra-ocular lenses after Descemet’s stripping automated endothelial keratoplasty. Eye (Lond). 2014;28(11):1383–1384. | ||
Werner L. Causes of intraocular lens opacification or discoloration. J Cataract Refract Surg. 2007;33(4):713–726. | ||
Werner L. Calcification of hydrophilic acrylic intraocular lenses. Am J Ophthalmol. 2008;146(3):341–343. | ||
Saeed MU, Singh AJ, Morrell AJ. Sequential Descemet’s membrane detachments and intraocular lens haze secondary to SF6 or C3F8. Eur J Ophthalmol. 2006;16(5):758–760. | ||
Price MO, Giebel AW, Fairchild KM, Price FW Jr. Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116(12):2361–2368. | ||
Dirisamer M, Ham L, Dapena I, et al. Efficacy of descemet membrance endothelial keratoplasty; clinical outcome of 200 consecutive cases after a learning curve of 25 cases. Arch Ophthalmol. 2011;129(11):1435–1443. | ||
Clements JL, Bouchard CS, Lee WB, et al. Retrospective review of graft dislocation rate associated with descemet stripping automated endothelial keratoplasty after primary failed penetrating keratoplasty. Cornea. 2011;30(4):414–418. | ||
Terry MA, Hoar KL, Wall J, Ousley P. Histology of dislocations in endothelial keratoplasty (DSEK and DLEK): a laboratory-based, surgical solution to dislocation in 100 consecutive DSEK cases. Cornea. 2006;25(8):926–932. | ||
Bahar I, Kaiserman I, McAllum P, Slomovic A, Rootman D. Comparison of posterior lamellar keratoplasty techniques to penetrating keratoplasty. Ophthalmology. 2008;115(9):1525–1533. | ||
Park J, Habib N. Opacification of IOL implants following endothelial keratoplasty. Paper presented at: Congress of the ESCRS, October 2013; Amsterdam, Netherlands. | ||
Richardson MR, Segu ZM, Price MO, et al. Alterations in the aqueous humor proteome in patients with Fuchs endothelial dystrophy. Mol Vis. 2010;16:2376–2383. | ||
Gottsch JD, Bowers AL, Margulies EH, et al. Serial analysis of gene expression in the corneal endothelium of Fuchs’ dystrophy. Invest Ophthalmol Vis Sci. 2003;44(2):594–599. | ||
Kenney MC, Atilano SR, Zorapapel N, Holguin B, Gaster RN, Ljubimov AV. Altered expression of aquaporins in bullous keratopathy and Fuchs’ dystrophy corneas. J Histochem Cytochem. 2004;52(10):1341–1350. | ||
Vithana EN, Morgan PE, Ramprasad V, et al. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet. 2008;17(5):656–666. | ||
Kumar DA, Agarwal A. Glued intraocular lens: a major review on surgical technique and results. Curr Opin Ophthalmol. 2013;24(1):21–29. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.