Back to Journals » Vascular Health and Risk Management » Volume 16
One-Year Outcomes of Peripheral Endovascular Device Intervention in Critical Limb Ischemia Patients: Sub-Analysis of the LIBERTY 360 Study
Authors Mustapha JA , Igyarto Z, O'Connor D , Armstrong EJ, Iorio AR, Driver VR , Saab F, Behrens AN, Martinsen BJ , Adams GL
Received 13 September 2019
Accepted for publication 30 November 2019
Published 10 February 2020 Volume 2020:16 Pages 57—66
DOI https://doi.org/10.2147/VHRM.S230934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Jihad A Mustapha,1,2 Zsuzsanna Igyarto,3 David O’Connor,4 Ehrin J Armstrong,5,6 Anthony R Iorio,7 Vickie R Driver,8 Fadi Saab,2 Ann N Behrens,3 Brad J Martinsen,3 George L Adams9
1College of Osteopathic Medicine, Michigan State University, E. Lansing, MI, USA; 2Advanced Cardiac & Vascular Amputation Prevention Centers, Grand Rapids, MI, USA; 3Cardiovascular Systems, Inc., St. Paul, MN, USA; 4Hackensack University Medical Center, Hackensack, NJ, USA; 5Denver VA Medical Center, Denver, CO, USA; 6Anschutz Medical Campus, University of Colorado, Denver, CO, USA; 7Foot Center of New York, New York College of Podiatric Medicine, New York, NY, USA; 8Department of Orthopedics, Brown University School of Medicine, Providence, RI, USA; 9North Carolina Heart and Vascular, Rex Hospital, UNC School of Medicine, Raleigh, NC, USA
Correspondence: Jihad A Mustapha
Advanced Cardiac & Vascular Amputation Prevention Centers, 1525 East Beltline NE, Suite 101, Grand Rapids, MI 49525, USA
Tel +1 616-447-8220
Email [email protected]
Background: High-risk patients with advanced peripheral artery disease (PAD), including critical limb ischemia (CLI), are often excluded from peripheral endovascular device intervention clinical trials, leading to difficulty in translating trial results into real-world practice. There is a need for prospectively assessed studies to evaluate peripheral endovascular device intervention outcomes in CLI patients.
Methods: LIBERTY 360 is a prospective, observational, multi-center study designed to evaluate the procedural and long-term clinical outcomes of peripheral endovascular device intervention in real-world patients with symptomatic lower-extremity PAD. One thousand two hundred and four patients were enrolled and stratified based on Rutherford Classification (RC): RC2-3 (N=501), RC4-5 (N=603), and RC6 (N=100). For this sub-analysis, RC5 and RC6 patients (RC5-6; N=404) were pooled and 1-year outcomes were assessed.
Results: Procedural complications rarely (1.7%) resulted in post-procedural hospitalization and 89.1% of RC5-6 patients were discharged to home. Considering the advanced disease state in RC5-6 patients, there was a high freedom from 1-year major adverse event rate of 65.5% (defined as target vessel revascularization, death to 30 days, and major target limb amputation). At 1 year, freedom from major amputation was 89.6%. Wounds identified at baseline on the target limb had completely healed in 172/243 (70.8%) of the RC5-6 subjects by 1 year. Additionally, the overall quality of life, as measured by VascuQoL, improved from baseline to 1 year.
Conclusion: LIBERTY investigated real-world PAD patients with independent oversight of outcomes. This analysis of LIBERTY RC5-6 patients demonstrates that peripheral endovascular device intervention can be successful in CLI patients, with low rates of major amputation and improvement in wound healing and quality of life through 1-year follow-up.
LIBERTY 360, https://clinicaltrials.gov/ct2/show/NCT01855412, ClinicalTrials.gov Identifier: NCT01855412.
Keywords: amputation, critical limb ischemia, CLI, endovascular therapy
Introduction
Approximately 18 million Americans have peripheral artery disease (PAD) and 2 million of these patients suffer from critical limb ischemia (CLI),1,2 the end stage of PAD.3 CLI is highly prevalent in older patients with diabetes and/or end-stage renal disease4 and is associated with high risk of amputation and mortality.5 The results following lower extremity amputation can be devastating – 27% of these patients will have one or more re-amputation(s) within 1 year,6 35% will have a higher level of limb loss,7 and 55% will have a contralateral limb amputation within 2–3 years.8 Furthermore, the mortality rates after primary amputation are very high, with rates ranging from 9 to 33% at 1 year6,7,9,10 and 26 to 82% at 5 years.6,9–11 Despite such devastating outcomes, primary amputation remains a common treatment modality for CLI.
The 2016 AHA/ACC Guideline on the management of patients with lower extremity PAD now recommends an evaluation for revascularization options by an interdisciplinary care team before amputation in CLI patients (Class I).12 Interestingly, recent studies demonstrate an increased utilization of endovascular intervention as a first-line approach for CLI patients in the US with a corresponding decrease of in-hospital death and major amputation.13–15
CLI patients, in particular, Rutherford Class 5–6 (RC5-6) patients with ischemic ulcerations or gangrene, are often excluded or under-represented in endovascular intervention clinical trials given their multiple comorbidities and advanced PAD. To the best of our knowledge, no endovascular device clinical studies in the US have been conducted specifically for RC5-6 patients.
In this sub-analysis of the LIBERTY 360 study, we aimed to investigate outcomes through 1 year in CLI RC5-6 patients treated with endovascular intervention.
Materials and Methods
Study Design and Patient Selection
The LIBERTY 360 study design has been previously described.16,17 Briefly, patients were eligible for the study if they were at least 18 years old and had clinical evidence of PAD (Rutherford classification 2 to 6) that required endovascular interventions on one or both limbs that included a target lesion in a native vessel located within or extending into 10 cm above the medial epicondyle to the digital arteries. Patients were excluded from the study if (1) they required a conversion from endovascular intervention to a surgical bypass graft for any lesion in the target area, (2) had an in-stent restenosis in the target area, and this lesion was the only one requiring treatment, or (3) had an anticipated life span of less than 1 year. The study included any FDA-approved technology to treat claudication and CLI. A total of 1204 patients were enrolled at 51 US sites and divided into three groups based on Rutherford Classification (RC): RC2-3 (N=501), RC4-5 (N=603), and RC6 (N=100). The list of participating institutions is provided in Supplementary Appendix 1. Sites were required to comply with the principles of Good Clinical Practices and meet the World Medical Association Declaration of Helsinki requirements. The study protocol was approved by the Institutional Review Board at each participating institution, and all patients gave written informed consent. Patients were followed up in clinic at 30 days, 6 months, and 1 year. For this sub-analysis, RC5 and RC6 patients (N=404) were pooled and 1-year outcomes were assessed.
Endpoints
Procedural success was defined as a final post-procedural result of less than 50% residual stenosis for all treated lesions during index procedure without significant angiographic complications (flow-limiting dissection, perforation, distal embolization, acute vessel closure) as determined by the angiographic core laboratory (SynvaCor, Springfield, IL). Lesion success was defined as a final post-procedural result of less than 50% residual stenosis for a given lesion treated during the index procedure and without significant angiographic complications as determined by the angiographic core laboratory. Major adverse events were defined as a composite of (1) death within 30 days of index procedure, (2) unplanned major (above the ankle) amputation of the target limb, or (3) target vessel revascularization as assessed by the angiographic core laboratory when angiographic images were available. Changes in quality of life (QoL) from baseline were analyzed using the Vascular Quality of Life Questionnaire (VascuQoL, measures PAD related health status). Wounds and wound care visits were assessed on the target limb only; wound status (including wound healing) was collected in-office at 30 days, 6 months, and 12 months.
Statistical Analysis
Data analysis was performed with the SAS Software System (SAS Institute, Inc., Cary, NC). Data are reported as percentage or mean ± standard deviation. For categorical responses, p-values were approximated using a Monte Carlo Approximation of the Fisher’s Exact Test. For ordinal categorical or yes/no responses, p-values were calculated using McNemar’s Test. For continuous variables, p-values were calculated using a paired t-test or ANOVA. Imputation of significant angiographic complications for procedural and lesion success of core lab identified lesions were performed by using site data when the core lab was unable to perform angiographic assessment. Kaplan–Meier method used to obtain estimate of survival rate. Greenwood’s method used to obtain the 95% confidence interval for the estimate. The statistical significance was set at p<0.05.
Results
Patient Characteristics
Patient demographics and baseline characteristics are included in Table 1. Most patients were white males with high prevalence of hypertension, hyperlipidemia, and diabetes. Fifty-seven percent of patients had coronary artery disease and 14.6% had chronic kidney disease requiring hemodialysis. Almost half of the patients (47%) had previous drug therapy for PAD and 43.6% had previous lower-limb endovascular treatment for PAD. Patients with prior lower-limb endovascular treatments had a mean number of 1.3 ± 1.8 lower-limb procedures on their target limb in the prior 3 years. Thirty-one percent of the patients had previous amputations and 9.4% had previous major contralateral limb amputations.
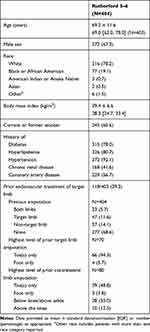 |
Table 1 Demographics and Baseline Characteristics |
Lesion Characteristics and Treatment
More than 76% of target lesions were located below-the-knee (Supplementary Table 1). On average, 1.4 ± 0.7 lesions were treated per subject. Approximately 60% of the lesions were calcified and 73.8% of calcified lesions were moderate to severely calcified per PARC definition.18 As shown in Figure 1, balloon and/or atherectomy were the preferred devices. Percutaneous transluminal angioplasty and the orbital atherectomy system were the most frequently used devices in almost 79% and 48% of the lesions, respectively. Stents were used in 17% of the lesions but with minimal bailout stenting due to angiographic complications or suboptimal result (4.6%).
Procedural and Lesion Success
Procedural success was 76.0%; 83.6% of patients had less than 50% final residual stenosis in all target lesions treated and 88.8% had no severe angiographic complications. Lesion success is presented in Supplementary Table 1.
Discharge Status and Medications
Procedural complications rarely (1.7%) resulted in post-procedural hospitalization and 89.1% of patients were discharged to home. Subjects were taking the following medications at the time of discharge: 91.6% antiplatelet therapy, 10.9% anti-coagulants, 74.8% anti-hyperlipidemic, and 88.4% anti-hypertensive.
Change in Rutherford Category and Quality of Life
RC5-6 patients showed a marked improvement from baseline to 12 months (Supplementary Figure 1). Quality of life improved significantly from baseline to 12 months, as measured by the total score and all subdomains of the VascuQoL (Figure 2) and EQ-5D (p<0.001).
Major Adverse Events and Amputation Free Survival at 12 Months
Freedom from MAEs and amputation free survival is presented in Figure 3 and Table 2. Considering the advanced disease state in RC5-6 patients, there were high rates of 12-month amputation free survival (78.2%) and freedom from major target limb amputation (89.6%).
 |
Figure 3 Kaplan–Meier curves for freedom from major adverse events (A) and amputation free survival (B) with number of subjects at risk. Abbreviation: MAE, major adverse events. |
 |
Table 2 Freedom from Major Adverse Events and Amputation Free Survival at 30 Days, 6 Months, and 12 Months |
Wound Healing on Target Limb
Significant improvement was noted in the number of wounds from baseline to 12 months (Table 3). At 12 months, wounds identified at baseline on the target limb had completely healed in 172/243 (70.8%) of the RC5-6 subjects (RC5: 144/198 (72.7%); RC6: 28/45 (62.2%)). When assessing paired data, the percentage of subjects not seeing a wound care specialist improved significantly from 31.8% (N=69) at baseline to 66.4% (N=144) at 12 months; however, at 12 months 5.1% (N=11) began seeing a wound care specialist as compared to baseline. Of subjects seeing a wound care specialist at 12 months, 27.4% visited monthly, 20.5% once every 2 weeks, 39.7% weekly, and only 12.3% more than 2 times per week.
 |
Table 3 Target Limb Wound Healing |
Discussion
CLI patients are under-represented in endovascular therapy clinical trials for multiple reasons: they are challenging to treat due to their comorbidities and long-term follow-up of this challenging population is near impossible because of their high mortality rate. In addition, treatment of CLI patients has not yet been standardized, and primary amputation was the first-line therapy for 22 to 67% of these patients until the mid-2010s,5,19 although less than 5% of the CLI patients should have primary amputation due to the severity of their disease.20
A recent meta-analysis of six trials that compared bypass surgery with angioplasty found no evidence to favor bypass surgery over angioplasty in terms of the effect on death, improvement of symptoms, amputation rate, need for further procedure, or long-term mortality.21 However, that study showed that angioplasty was associated with decreased procedural complications and shorter hospital stay compared with bypass surgery and concluded that endovascular treatment should be used in patients with CLI as they are high-risk surgical candidates.21 Mustapha et al22 found the same in their population-based cohort study using Medicare data of CLI patients treated with endovascular- or surgical revascularization or primary major amputation. Long-term survival and healthcare cost were comparable between the revascularization techniques but with lower major amputation rates following endovascular therapy. In addition, primary major amputation was associated with shorter survival time, higher risk of subsequent amputation, and higher costs compared with revascularization techniques.
Despite the evidence-based preventive effect of revascularization on limb amputation in CLI patients, recent studies reported that 13.5%23 and 23%15 of CLI patients were still managed with major amputation and minor amputation or conservative therapy, respectively, instead of endovascular revascularization.
Not all CLI patients are the same. CLI is a broad definition and includes patients with Rutherford classification 4 to 6, where RC4 is characterized by ischemic rest pain, RC5 with minor tissue loss, and RC6 with major tissue loss – ulcer, gangrene.24 Previous studies found that the comorbidities and lesion characteristics of patients with rest pain (RC4) differed significantly from those with tissue loss (RC5-6).25,26 RC4 patients were significantly less likely than RC5-6 to have diabetes, end-stage renal disease, or chronic heart failure.25–27 Also, RC5-6 patients had longer lesions, more TASC C and D lesions, and fewer patent below-the-ankle arteries compared to RC4 patients.26 In addition, CLI patients without wounds had better prognosis after intervention than those with wounds.25 RC4 patients are also treated differently compared to RC5-6, as they more often undergo revascularization and less often undergo primary amputation. Retrospectively reviewing diagnosis and procedural data from the largest public health insurance in Germany, the authors found that only ~50% of RC5-6 patients had any revascularization compared to 71% of RC4 patients and 27.5% of them had in-hospital amputation compared to only 1.6% of RC4.27 Reinecke and co-authors27 found a high mortality rate in RC5-6 patients which was only comparable with that of some aggressive types of cancer, as they noted it appropriately.
For the reasons mentioned above and also per the reporting standards of the Society of Vascular Surgery for endovascular treatment of PAD,28 as ‘rest pain and tissue loss patients should not be grouped together in reporting outcomes’, we present the outcomes for the LIBERTY RC5-6 patients only, and not for all CLI patients (RC4-6). This is the first study to show the endovascular treatment outcomes in RC5-6 patients in the US. Notably, in the LIBERTY 360 RC5-6 patients, 65.1% of lesions were below-the-knee only, 46.4% were longer than 10 cm and approximately 60% were calcified. The preferred devices were balloon and/or atherectomy and orbital atherectomy was the most frequently used atherectomy device. Procedural complications rarely (1.7%) resulted in post-procedural hospitalization and 89.1% of RC5-6 patients were discharged to home. Agarwal et al,23 using State Inpatient Databases from three US states from 2009 to 2013, studied trends and factors affecting readmission in CLI patients, 16.6% of whom had endovascular revascularization alone, and found that only 33.6% of primary CLI admissions were discharged home from the hospital. On the other hand, in a retrospective study of 219 CLI patients treated with atherectomy and balloon angioplasty in an outpatient center, all patients were discharged home the same day, including 51.6% RC5-6 patients.29
Considering the advanced disease state in RC5-6 patients, there was a high rate of 12-month amputation free survival (AFS, 78.1%). The 1-year AFS was 83.1% in the above-mentioned outpatient study,29 while in another retrospective study of 809 RC5-6 patients with diabetes, the 1-year AFS was 75% for the endovascular group compared with 69% in the bypass group.30 Only 10.4% of the LIBERTY RC5-6 patients had a major amputation at 1 year – much lower than the 22% to 67%5,31–34 primary major amputation rates seen in the literature for CLI patients that did not receive endovascular intervention (Figure 4).35
The mortality rate in LIBERTY RC5-6 patients was 13.3% at 1 year, very comparable to the outpatient study where the death rate was 11.4% for the entire cohort29 and much lower than the 30% 1-year mortality rate in a retrospective US study36 of patients who underwent lower extremity amputation. This rate is very similar though to the mortality rate in a large German retrospective study,27 where 34.2% of the RC6 patients died within the first 12 months after index hospitalization. Only 29.5% of these patients were treated with endovascular therapy and less than half of them had any revascularization procedure during index hospitalization.
It has been shown that endovascular revascularization improves wound healing in CLI patients37 and direct blood flow to the wounds is a positive predictor of wound healing.38 There was a significant improvement in the number of wounds on the target limb from baseline to 12 months in LIBERTY RC5-6 patients.
Quality of life (QoL), as measured by the total score and all subdomains of the VascuQoL, also improved significantly from baseline to 12 months. A recently published Japanese prospective study found the same; the health-related QoL was significantly improved compared to baseline in the CLI patients alive at 1 year after revascularization.39 A handful of observational studies that compared the effects of primary amputation and revascularization on QoL concluded that revascularization should be the preferred approach in patients with CLI. However, critics say that CLI patients have poor health prospects and life expectancy, irrespective of the treatment mode,40 and a focus on QoL research is needed to prescribe the ideal treatment for patients suffering from CLI.41 This study adds more evidence to the scarce literature and LIBERTY data supporting a revascularization approach for CLI will likely be corroborated by the BEST-CLI trial42 that compares surgical bypass and endovascular therapy in CLI patients and also provide data about the impact of these treatments on the QoL of the study population.
Limitations
This was a post hoc analysis of LIBERTY 360 – an observational, non-randomized study of endovascular therapies and was not powered to assess clinical outcomes in RC5-6 CLI patients. As this study was sponsored by a company whose principal endovascular strategy is atherectomy, bias may be attributed to physician selection of orbital atherectomy in a high number of cases. Additionally, wound data collection was limited in the LIBERTY 360 study; exact wound area, wound volume, and the date of wound healing were not collected. Possible over or underreporting of outcomes is possible due to subject withdrawal prior to 12 months.
Conclusions
LIBERTY investigated real-world PAD patients with independent oversight of outcomes. The findings in this novel “all-comers“ PAD study reveal hope for RC5-6 CLI patients and suggest that “primary amputation” in RC5-6 may not be necessary – peripheral endovascular device intervention can be successful in this patient population.
Abbreviations
AFS, amputation free survival; ATK, above-the-knee; BMS, bare-metal stent; BTK, below-the-knee; CLI, critical limb ischemia; DCB, drug-coated balloon; DES, drug-eluting stent; MAE, major adverse events; OAS, orbital atherectomy system; PAD, peripheral artery disease; PARC, Peripheral Academic Research Consortium; PTA, percutaneous transluminal angioplasty; QoL, quality of life; RC, Rutherford Classification; TASC, Trans-Atlantic Inter-Society Consensus; TVR, target vessel revascularization.
Data Sharing Statement
Individual-deidentified participant data and study-related documents will not be made available.
Acknowledgements
The authors would like to thank Ryan Bolduan, BA, for his critical review of this manuscript. This paper/the abstract of this paper was presented at the Society for Cardiovascular Angiography and Interventions’ (SCAI) 41st Annual Scientific Sessions as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Catheterization and Cardiovascular Interventions: https://doi.org/10.1002/ccd.27553.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. Each author participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Cardiovascular Systems, Inc. sponsored the LIBERTY 360 Study (ClinicalTrials.gov Identifier: NCT01855412).
Disclosure
JAM reports consulting agreements with Cardiovascular Systems, Inc., Medtronic, Bard Peripheral Vascular, Terumo, and Philips. Equity ownership CardioFlow. ZI is employed by and owns stock in CSI, during the conduct of the study. DO reports consulting agreement with CSI, a consulting agreement and grants from Boston Scientific, outside the submitted work. EJA reports consulting agreements with Cardiovascular Systems Inc., Abbott vascular, Boston scientific, Intact vascular, Gore, Medtronic, and Philips, outside the submitted work. FS reports speaker and consulting fees from CSI. ANB is employed by and owns stock in CSI. BJM is employed by and owns stock in CSI. GLA reports personal fees from Cardiovascular Systems, Philips, Abbott Vascular, Cook Medical and Gore, outside of the submitted work; and received consulting fees from Bard Peripheral Vascular, Terumo Interventional Systems, Medtronic, Boston Scientific, Spectranetics, and CSI. The authors report no other conflicts of interest in this work.
References
1. Yost M. Critical Limb Ischemia. Vol I United States Epidemiology Atlanta (GA). The Sage Group; 2010.
2. Schiavetta A, Maione C, Botti C, et al. A Phase II trial of autologous transplantation of bone marrow stem cells for critical limb ischemia: results of the Naples and Pietra ligure evaluation of stem cells study. Stem Cells Transl Med. 2012;1(7):572–578. doi:10.5966/sctm.2012-0021
3. Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51(1):230–241. doi:10.1016/j.jvs.2009.08.073
4. Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999;56(4):1524–1533. doi:10.1046/j.1523-1755.1999.00668.x
5. Abu Dabrh AM, Steffen MW, Undavalli C, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62(6):1642–1651.e3. doi:10.1016/j.jvs.2015.07.065
6. Jindeel A, Narahara KA. Nontraumatic amputation: incidence and cost analysis. Int J Low Extrem Wounds. 2012;11(3):177–179. doi:10.1177/1534734612457031
7. Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480–486. doi:10.1016/j.apmr.2004.06.072
8. Pasquina PF, Miller M, Carvalho AJ, et al. Special considerations for multiple limb amputation. Curr Phys Med Rehabil Rep. 2014;2(4):273–289. doi:10.1007/s40141-014-0067-9
9. Schofield CJ, Libby G, Brennan GM, et al. Mortality and hospitalization in patients after amputation: a comparison between patients with and without diabetes. Diabetes Care. 2006;29(10):2252–2256. doi:10.2337/dc06-0926
10. Tentolouris N, Al-Sabbagh S, Walker MG, Boulton AJM, Jude EB. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: a 5-year follow-up study. Diabetes Care. 2004;27(7):1598–1604. doi:10.2337/diacare.27.7.1598
11. Faglia E, Clerici G, Clerissi J, et al. Early and five-year amputation and survival rate of diabetic patients with critical limb ischemia: data of a cohort study of 564 patients. Eur J Vasc Endovasc Surg. 2006;32(5):484–490. doi:10.1016/j.ejvs.2006.03.006
12. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):1465–1508. doi:10.1016/j.jacc.2016.11.008
13. Goodney PP, Tarulli M, Faerber AE, Schanzer A, Zwolak RM. Fifteen-year trends in lower limb amputation, revascularization, and preventive measures among medicare patients. JAMA Surg. 2015;150(1):84–86. doi:10.1001/jamasurg.2014.1007
14. Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: from 2003–2011. J Am Coll Cardiol. 2016;67(16):1901–1913. doi:10.1016/j.jacc.2016.02.040
15. Armstrong EJ, Ryan MP, Baker ER, Martinsen BJ, Kotlarz H, Gunnarsson C. Risk of major amputation or death among patients with critical limb ischemia initially treated with endovascular intervention, surgical bypass, minor amputation, or conservative management. J Med Econ. 2017;20(11):1148–1154. doi:10.1080/13696998.2017.1361961
16. Adams GL, Mustapha J, Gray W, et al. The LIBERTY study: design of a prospective, observational, multicenter trial to evaluate the acute and long-term clinical and economic outcomes of real-world endovascular device interventions in treating peripheral artery disease. Am Heart J. 2016;174:14–21. doi:10.1016/j.ahj.2015.12.013
17. Mustapha J, Gray W, Martinsen BJ, et al. One-year results of the LIBERTY 360 study: evaluation of acute and midterm clinical outcomes of peripheral endovascular device interventions. J Endovasc Ther. 2019;26(2):143–154. doi:10.1177/1526602819827295
18. Patel MR, Conte MS, Cutlip DE, et al. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC). J Am Coll Cardiol. 2015;65(9):931–941. doi:10.1016/j.jacc.2014.12.036
19. Allie DE, Hebert CJ, Lirtzman MD, et al. Critical limb ischemia: a global epidemic.A critical analysis of current treatment unmasks the clinical and economic costs of CLI. EuroIntervention. 2005;1(1):75–84.
20. Rudofker EW, Hogan SE, Armstrong EJ. Preventing major amputations in patients with critical limb ischemia. Curr Cardiol Rep. 2018;20(9):74. doi:10.1007/s11886-018-1019-2
21. Antoniou GA, Georgiadis GS, Antoniou SA, Makar RR, Smout JD, Torella F. Bypass surgery for chronic lower limb ischaemia. Cochrane Database Syst Rev. 2017;4:CD002000. doi:10.1002/14651858.CD002000.pub3
22. Mustapha JA, Katzen BT, Neville RF, et al. Determinants of long-term outcomes and costs in the management of critical limb ischemia: a population-based cohort study. J Am Heart Assoc. 2018;7(16):e009724. doi:10.1161/JAHA.118.009724
23. Agarwal S, Pitcavage JM, Sud K, Thakkar B. Burden of readmissions among patients with critical limb ischemia. J Am Coll Cardiol. 2017;69(15):1897–1908. doi:10.1016/j.jacc.2017.02.040
24. Hardman RL, Jazaeri O, Yi J, Smith M, Gupta R. Overview of classification systems in peripheral artery disease. Semin Interv Radiol. 2014;31(4):378–388. doi:10.1055/s-0034-1393976
25. O’Brien-Irr MS, Dosluoglu HH, Harris LM, Dryjski ML. Outcomes after endovascular intervention for chronic critical limb ischemia. J Vasc Surg. 2011;53(6):1575–1581. doi:10.1016/j.jvs.2011.01.068
26. Tsuchiya T, Iida O, Shiraki T, et al. Clinical characteristics of patients with Rutherford category IV, compared with V and VI. SAGE Open Med. 2015;3:2050312115597087. doi:10.1177/2050312115597087
27. Reinecke H, Unrath M, Freisinger E, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36(15):932–938. doi:10.1093/eurheartj/ehv006
28. Stoner MC, Calligaro KD, Chaer RA, et al. Reporting standards of the society for vascular surgery for endovascular treatment of chronic lower extremity peripheral artery disease: executive summary. J Vasc Surg. 2016;64(1):227–228. doi:10.1016/j.jvs.2016.03.432
29. Dattilo R, Dattilo A, Colby S. Outcomes of patients treated for critical limb ischemia in an outpatient endovascular center. Vasc Manag. 2018;15(6):E49–E52.
30. Lo ZJ, Lin Z, Pua U, et al. Diabetic foot limb salvage-a series of 809 attempts and predictors for endovascular limb salvage failure. Ann Vasc Surg. 2018;49:9–16. doi:10.1016/j.avsg.2018.01.061
31. Allie DE, Hebert CJ, Ingraldi A, Patlola RR, Walker CM. 24-carat gold, 14-carat gold, or platinum standards in the treatment of critical limb ischemia: bypass surgery or endovascular intervention? J Endovasc Ther. 2009;16(Suppl 1):I134–I146. doi:10.1583/08-2599.1
32. Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. J Vasc Surg. 2011;53(2):330–339.e1. doi:10.1016/j.jvs.2010.08.077
33. Abou-Zamzam AM, Gomez NR, Molkara A, et al. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg. 2007;21(4):458–463. doi:10.1016/j.avsg.2006.12.006
34. Baser O, Verpillat P, Gabriel S, Li W. Prevalence, incidence, and outcomes of critical limb ischemia in the US medicare population | vascular disease management. Vasc Dis Manag. 2013;10(2):E26–E36.
35. Mustapha J, Martinsen BJ, Igyarto Z. LIBERTY 360° study presentation at amp 2016 reveals hope for Rutherford-6 CLI patients. Cath Lab Dig. 2016;24(10).
36. Shah SK, Bena JF, Allemang MT, et al. Lower extremity amputations: factors associated with mortality or contralateral amputation. Vasc Endovascular Surg. 2013;47(8):608–613. doi:10.1177/1538574413503715
37. Bae J-I, Won JH, Han SH, et al. Endovascular revascularization for patients with critical limb ischemia: impact on wound healing and long term clinical results in 189 limbs. Korean J Radiol. 2013;14(3):430–438. doi:10.3348/kjr.2013.14.3.430
38. Kobayashi N, Hirano K, Nakano M, et al. Wound healing and wound location in critical limb ischemia following endovascular treatment. Circ J. 2014;78(7):1746–1753. doi:10.1253/circj.CJ-14-0171
39. Iida O, Takahara M, Soga Y, et al. Prognostic impact of revascularization in poor-risk patients with critical limb ischemia: the priority registry (Poor-risk patients with and without revascularization therapy for critical limb ischemia). JACC Cardiovasc Interv. 2017;10(11):1147–1157. doi:10.1016/j.jcin.2017.03.012
40. Bosma J, Vahl A, Wisselink W. Systematic review on health-related quality of life after revascularization and primary amputation in patients with critical limb ischemia. Ann Vasc Surg. 2013;27(8):1105–1114. doi:10.1016/j.avsg.2013.01.010
41. Steunenberg SL, Raats JW, Te Slaa A, de Vries J, van der Laan L. Quality of life in patients suffering from critical limb ischemia. Ann Vasc Surg. 2016;36:310–319. doi:10.1016/j.avsg.2016.05.087
42. Menard MT, Farber A, Assmann SF, et al. Design and rationale of the best endovascular versus best surgical therapy for patients with critical limb ischemia (BEST-CLI) trial. J Am Heart Assoc. 2016;5:7. doi:10.1161/JAHA.116.003219
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



