Back to Archived Journals » Oncolytic Virotherapy » Volume 6
Oncolytic virotherapy including Rigvir and standard therapies in malignant melanoma
Authors Babiker HM, Riaz IB, Husnain M, Borad MJ
Received 8 September 2016
Accepted for publication 20 December 2016
Published 9 February 2017 Volume 2017:6 Pages 11—18
DOI https://doi.org/10.2147/OV.S100072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Faris Farassati
Hani M Babiker,1 Irbaz Bin Riaz,2 Muhammad Husnain,2 Mitesh J Borad3,4
1University of Arizona Cancer Center, 2Department of Internal Medicine, University of Arizona, Tucson, 3Division of Hematology-Oncology, Mayo Clinic Cancer Center, Scottsdale, AZ, 4Department of Molecular Medicine, Center for Individualized Medicine, Mayo Clinic, Rochester, MN, USA
Abstract: The treatment of metastatic melanoma has evolved from an era where interferon and chemotherapy were the mainstay of treatments to an era where immunotherapy has become the frontline. Ipilimumab (IgG1 CTLA-4 inhibitor), nivolumab (IgG4 PD-1 inhibitor), pembrolizumab (IgG4 PD-1 inhibitor) and nivolumab combined with ipilimumab have become first-line therapies in patients with metastatic melanoma. In addition, the high prevalence of BRAF mutations in melanoma has led to the discovery and approval of targeted molecules, such as vemurafenib (BRAF kinase inhibitor) and trametinib (MEK inhibitor), as they yielded improved responses and survival in malignant melanoma patients. This is certainly a burgeoning time in immunotherapy drug development, and the aforementioned efforts along with the recent US Food and Drug Administration approval of talimogene laherparepvec (T-VEC), a recombinant oncolytic herpes virus, have paved the way to exploring the role of additional oncolytic viruses, such as the echovirus Rigvir, as new and innovative treatment modalities in patients with melanoma. Herein, we discuss the current standard of care treatment in melanoma with an emphasis on immunotherapy and oncolytic viruses in development.
Keywords: melanoma, virotherapy, Rigvir
Corrigendum for this paper has been published
Epidemiology
The incidence of melanoma is rising and is currently the fifth most common cancer in the US.1,2 Although melanoma can occur at any age, the median age of diagnosis is 61 years (64 years in males and 57 years in females).1,2 It is the most lethal skin cancer accounting for 80% of skin cancer mortality.2 Although localized melanoma has a 5-year survival of 98.3%, ~50% of these cases progress within 3 years of diagnosis.1 The 5-year survival rates of regional and metastatic melanoma are 62.4% and 16.0%, respectively.2
Background
Oncolytic viruses are therapeutic viruses that are intended to selectively infect and damage cancerous tissues without causing harm to normal surrounding tissues.3 Each virus has specific tropism that determines which tissues are preferentially infected. Some viruses have a natural preference for cancer cells, whereas other viruses can be adapted or engineered to make them cancer specific.3 Oncolytic viruses can kill infected cancer cells in many different ways, ranging from direct virus-mediated cytotoxicity to immune-mediated cell death.4 Although surgical excision remains as the standard of care for early stage disease, metastatic melanoma continues to represent a major therapeutic challenge despite an increasing number of available targeted drugs and immunotherapeutic strategies demonstrating clinical benefits.5,6 Oncolytic virus therapy due to its tolerability and ease of administration has become yet another tool in the armamentarium for treatment of patients with advanced melanoma.5 Rigvir (Rv), a nongenetically engineered oncolytic, nonpathogenic enteric cytopathic human orphan type 7 (ECHO-7) virus adapted and selected for melanoma, was approved and registered in 2004 in Latvia for melanoma therapy.7,8 In this review, we focus on the management strategies for advanced malignant melanoma, including surgical management, immunotherapy, oncolytic virotherapy and the context in which Rv may have a role in therapy for patients with melanoma.
Pathogenesis and natural history of melanoma
Malignant melanoma arises from malignant proliferation of neural crest-derived melanocytes, which are pigment-producing cells located in various anatomic sites such as the basal layer (stratum basale) of the epidermis of the skin, the uvea of the eyes, the inner ear, meninges, bones and heart.9 The transformation of melanocytes into melanoma cells may take place in a stepwise manner involving clonal succession and acquisition of genomic alterations in the setting of multifactorial interaction of environmental, genetic and host factors.10,11 Following vertical growth phase, the tumor cells invade deeply into the dermis/hypodermis and eventually penetrate the endothelium of capillaries and enter the blood stream, allowing them to form distant metastases.10 The major cell-autonomous drivers in the pathogenesis of this disease include the classical MAPK, WNT and PI3K signaling pathways.11 Perhaps, the best studied oncogenic mutation in melanoma is that of proto-oncogene B-Raf also known as v-Raf murine sarcoma viral oncogene homolog B (BRAF) that encodes a serine/threonine protein kinase, which acts in the RAS–RAF–MEK–ERK MAPK pathway.11 Activating mutations in BRAF occur in >50% of melanomas with most of the activating mutations being V600E.11,12 Much of the current research has focused on inhibiting the constitutively active BRAF protein kinase in melanoma patients.12 Immune checkpoint inhibitors have also emerged as effective therapies for melanoma.13 Finally, the immunogenicity and easy accessibility for direct injection make melanoma an attractive venue to implement oncolytic virotherapy.
Surgical management of melanoma
The surgical treatment of melanoma has not undergone significant change over the last decade, specifically as it pertains to the treatment of localized primary tumors. The current recommendations for the surgical treatment of localized disease are based on a randomized prospective trial that was conducted several years ago.14 The primary means of diagnosis is excisional biopsy, and wide local resection with negative surgical margins (SMs) still remains the surgical goal. Determining the SM depends on the depth of the tumor Breslow thickness (B). This has been studied in multiple randomized trials, and the current recommendation is to obtain a 1 cm SM for tumor thickness of 1 mm B, 1–2 cm SM for tumor thickness of 1–2 mm B and 2 cm for tumor thickness above 2 mm B.15
A randomized trial studying optimal margins revealed that 1 cm of SM was associated with increased locoregional recurrence rate and risk of melanoma-specific death compared to a 3 cm SM (relative rate of melanoma death was estimated to be 24% higher in the 1 cm group compared to the 3 cm group on univariate analysis [hazard ratio (HR) 1.24; 95% confidence interval (CI) 1.00–1.52; P = 0.05]).16 Subsequent studies comparing 2 cm versus 4 cm SM did not reveal any benefit favoring a wider local resection in melanomas of >2 mm B. There are limited data on melanomas of >4 mm of thickness, and a SM of >3 cm is not beneficial.16
Some patients require a sentinel lymph node biopsy (SLNB). In melanomas of >1 mm B, an SLNB is recommended.16,17 In patients with melanomas of 0.75–1 mm B combined with any other risk factor such as ulceration, age younger than 40 years, Clark level IV or increased mitotic rate, an SLNB is quintessential.15 This stems from the fact that previous studies have indicated sentinel lymph node involvement in 20% of patients with the aforementioned risk factors.18 Complete lymph node dissection consists of anatomically thorough dissection of the involved nodal basin. It must be performed if a sentinel node or any other lymph nodes are positive (stage IIB or IIIC).17
Immunotherapy in melanoma
Immunotherapy relies on activating the host immune system to attack cancer cells, an effect that is predominantly mediated by T cells. Aldesleukin is a recombinant IL-2 that is approved for the treatment of metastatic melanoma and is associated with a 15%–20% objective response rate.13,19 Ipilimumab, another approved immunotherapeutic drug, is a monoclonal antibody that blocks CTLA-4, leading to activation of T cells that ultimately attack the cancer.20 This is an outpatient, intravenous therapy that has been associated with an objective response rate of 10%–15% and an improvement in the overall survival, and the complete responses achieved may be quite durable. Aldesleukin and ipilimumab are both approved by the US Food and Drug Administration (FDA) for treatment of metastatic melanoma. Tremelimumab is a human therapeutic monoclonal antibody IgG2 that also targets CTLA-4 with a similar mechanism of action to ipilimumab and is still undergoing clinical trials.21 Interferon alfa is FDA approved in adjuvant treatment for patients with high-risk melanoma and has significant immunomodulatory effects.22 Interferon alfa adjuvant monotherapy is the standard of care in lymph node-positive resected melanoma (stage III) and should be considered for patients with negative nodes and a high risk of recurrence (stages IIB and IIC).22–24 Interferon alfa monotherapy has limited utility in the treatment of stage IV melanoma; therefore, it is mainly used in combination with other therapies in the metastatic setting.25
Pembrolizumab is an antibody that blocks the inhibitory ligand of PD-1 located on lymphocytes and also prevents T-cell exhaustion.26 Activation of this receptor leads to the inhibition of the immune response to cancer cells, which express PD-L1 and PD-L2. Normally this effect is necessary to prevent an inappropriate autoimmune disease in healthy patients. These agents have shown superiority with less toxicity compared to ipilimumab in clinical trials.27 Pembrolizumab was recently approved for patients with V600E or V600K mutations and metastatic melanoma who have failed ipilimumab- and BRAF-targeted therapies. A subsequent trial that randomized 834 patients to receive pembrolizumab (at a dose of 10 mg/kg of body weight) every 2 weeks or 3 weeks or four doses of ipilimumab (at 3 mg/kg) every 3 weeks revealed a 6-month progression-free survival of 47.3%, 46.4% or 26.5%, respectively.27 The 12-month survival rates were 74.1%, 68.4% or 58.2%, and the response rates were 33.7%, 32.9% or 11.9%, respectively. This led to its approval as a frontline therapy in patients with advanced melanoma.27
Nivolumab, a humanized IgG4 anti-PD1 monoclonal antibody, and ipilimumab were also studied in combination. Nivolumab showed improved progression-free survival either alone or in combination with ipilimumab and hence was approved either as monotherapy or in combination with ipilimumab as the frontline treatment.28 Vemurafenib, a BRAF kinase inhibitor, has shown improvement in overall and progression-free survival when compared to dacarbazine in patients with metastatic, untreated melanoma harboring the BRAF V600 mutation.29 In addition, trials studying the combination of BRAF and MEK inhibitors such as dabrafenib and trametinib (an MEK1/MEK2 inhibitor) indicated improved survival, and currently ongoing trials are studying the combination with checkpoint inhibitors.30
Oncolytic virotherapy in melanoma
Oncolytic virus immunotherapy is a new form of cancer therapy that uses native or genetically modified viruses to selectively enter, replicate and lyse tumor cells.3,4 This approach has been most widely evaluated in patients with metastatic melanoma.5,31 Oncolytic viruses attack cancer not only by preferentially infecting cancer cells leading to lysis but also by releasing cancer antigens causing an immune attack against the infected malignant cells. Table 1 lists studies in oncolytic viruses highlighting biology, mechanisms of action and trial results.7,32–43 Although surgical excision remains the standard of care for early stage disease, metastatic melanoma continues to represent a major therapeutic challenge, albeit there are an increasing number of emerging targeted drugs and immunotherapeutic treatment strategies demonstrating potential clinical benefits. Oncolytic virus immunotherapy, however, has several features that make it an attractive treatment modality, including the ease of administration, low toxicity profile and potential synergy with other immunotherapeutics such as immune checkpoint inhibitors in advanced melanoma. In 2006, the oncolytic virus H101, a modified adenovirus, was approved in China, for the treatment of squamous cell carcinoma of head and neck.35 The FDA also approved the genetically engineered herpes simplex virus (HSV) called talimogene laherparepvec (T-VEC) to treat advanced melanoma.44–46 A number of other oncolytic viruses are being evaluated in the US for the treatment of melanoma.
Oncolytic HSVs in melanoma
The herpes simplex virus type 1 (HSV-1) is an alphaherpesvirus that is a well-known human pathogen.34 T-VEC and HF-10 are both recombinant herpes viruses that have undergone clinical evaluation in advanced melanoma.44,47 T-VEC was recently approved by the FDA for the treatment of advanced melanoma based on achieving a durable response benefit. T-VEC is a first-in-class oncolytic virus based on a modified HSV-1 designed to selectively replicate in and lyse tumor cells while promoting regional and systemic antitumor immunities. A phase III trial comparing T-VEC that has been genetically engineered to express GM-CSF, to GM-CSF alone, in patients with surgically non-resectable but injectable melanoma demonstrated therapeutic benefit favoring T-VEC.44 The durable response rate, the primary end point, was significantly higher with T-VEC (16.3%; 95% CI, 12.1%–20.5%) than with GM-CSF (2.1%; 95% CI, 0%–4.5%; odds ratio, 8.9; P < 0.001). The overall response rate (ORR) also favored the T-VEC arm (26.4%; 95% CI, 21.4%–31.5% versus 5.7%; 95% CI, 1.9%–9.5%). In addition, the median overall survival was 23.3 months (95% CI, 19.5–29.6 months) with T-VEC and 18.9 months (95% CI, 16.0–23.7 months) with GM-CSF (HR, 0.79; 95% CI, 0.62–1.00; P = 0.051).44 A phase Ib trial investigated the safety of the combination of T-VEC and ipilimumab, and the results indicated tolerability and an ORR of 41% (24% complete response, 18% partial response) and stable disease in 35%.48 HF10 is a spontaneously occurring, oncolytic, mutant HSV-1 that is also being studied for the treatment of melanoma.49 The role of intratumoral injection of HF-10 in patients with refractory superficial cancers including melanoma was also studied in a phase I trial, and the results indicated tolerability and preliminary signs of efficacy.47
Oncolytic coxsackieviruses in melanoma
Coxsackieviruses A13 (CVA13), A15 (CVA15), A18 (CVA18), and A21 (CVA21) have been studied as potential oncolytic therapies in melanoma.50 CVA21 is a human enterovirus C that causes the common cold and targets cells that express specific virus receptors such as ICAM-1 causing cell lysis. A phase I/phase II trial involving 57 patients with stage IIIC–IV melanoma studied multi-dose intralesional therapy with CVA21.51 The primary end point of the study was immune-related progression-free survival (irPFS), and this was achieved in 21 of 57 (38.6%) evaluable patients displaying irPFS at 6 months with a median irPFS of 4.2 months. The ORR utilizing the immune-related response evaluation criteria in solid tumors (ir-RECIST) was 28.1% (16 of 57 evaluable patients) with a ≥6 months durable response rate of 19.3% (eleven of 57 patients). The 1-year survival rate was 75.4% (43 of 57 patients), and the median time to response was 2.8 months.51 Currently, CVA21 is being studied in combination with other immunotherapeutic agents such as anti-CTLA-4 and anti-PD-1 agents.52
Oncolytic reovirus in melanoma
Reovirus serotype 3-dearing strain (Reolysin) is a naturally occurring, non-enveloped, double-stranded RNA virus. Reovirus has been shown to replicate in cells harboring a mutated or activated RAS pathway.53–55 A phase II trial of 21 patients with metastatic melanoma investigating the intravenous administration of reovirus serotype 3-dearing strain showed tolerability to the regimen without any dose reductions implemented.56 Posttreatment biopsy samples were obtained in 71% of the patients, and only 62% of the samples contained adequate tumor for correlative analysis. In two patients, productive reoviral replication (viral antigen coexpression with tubulin) was demonstrated, despite increase in neutralizing antibody titers. There were no objective response rates, yet one patient had 75%–90% tumor necrosis in resected metastatic lesions.56 The median times to progression and survival were 45 days (range 13–96 days) and 165 days (range 15 days–15.8 months), respectively. Studies are underway to investigate the role of Reolysin combined with other immunotherapies.57
Rv in melanoma
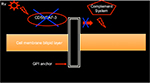
Echoviruses (ECHO) are positive single-stranded RNA viruses of the Enterovirus genus and Picornaviridae family.34 They are generally transmitted by fecal contamination and cause a range of human illnesses, including diarrhea and aseptic meningitis. Currently, 32 serotypes of echovirus have been identified. Rv is a wild-type ECHO-7 virus.7,8 Limited data are available in the English scientific literature describing the pre-clinical and clinical efficacies of Rv. In pre-clinical evaluations, Rv has been shown to have both humoral and T-cell mediated anti-neoplastic effects.8,58,59 Oncolytic viruses in general kill cancer cells by directly invading and lysing the cells and by releasing cancer antigens leading to an immune system attack targeting cancer cells carrying the same antigen. CD55/DAF-3 is a glycosylphosphatidylinositol (GPI)-anchored protein, encoded by the CD55 gene, that regulates the complement system on the cell surface and is a targeted receptor for both coxsackieviruses and echoviruses. Echoviruses (Rv) bind to and inhibit CD55/DAF-3, thus leading to complement activation and cancer cell hemolysis (Figure 1).60
Rv has been studied by scientific teams in the Soviet Union (now Latvia) led by Aina Muceniece with initial reports from her group in the 1960s and 1970s.7 Rv was approved in Latvia in 2004, making it the first therapeutic virus to achieve regulatory approval.7 It was subsequently approved in the country of Georgia in 2015. Both of these regulatory approvals do not appear to be supported by clinical data of the rigor that is typically warranted in the US, European Union and Japan, considered to be the most rigorous in the regulatory review of novel cancer therapeutics. Despite the limitations on the available data, the story of Rv remains an intriguing one, given that it is a wild-type virus, among other viruses, and hence could rapidly undergo translation if safety and efficacy can be confirmed by other investigators. A potential benefit for Rv in comparison to T-VEC includes potential for higher specificity of cancer cells as the latter is taken up by normal cells. However, there is currently insufficient information to compare both oncolytic viruses due to the lack of prospective and comparative trials.
A retrospective evaluation of melanoma patients with a range of disease stages (IB [n = 17], IIA [n = 16], IIB [n = 12], IIC [n = 7]) was conducted (n = 79, 52 patients treated with Rv and 27 controls).7 Patients were treated at a number of centers in Latvia. Rv was administered after surgical resection at a dose of 2 × 106 tissue culture infective dose50 (2 mL solution with 106 tissue culture infective dose50/mL) intramuscularly for 3 consecutive days every 4 weeks for an initial 3-month period. Thereafter, the treatment was monthly during the first year, every 6 weeks during the first half of the second year, every 2 months during the second half of the second year and every 3 months during the third year. No statistically significant difference (despite a numerically superior difference in the Rv group) was observed in terms of disease progression between Rv and observation groups. A lower mortality (higher survival) was noted in patients receiving Rv. However, the data should be interpreted with caution, given the retrospective nature of the study, lack of control arm or randomization and other biases inherent with studies that are not prospective in nature. From a safety perspective, it was reported that there were no severe treatment-related toxicities. However, detailed data supporting this were not provided in the study publication, making it difficult to interpret objectively. Moreover, limited data are available to objectively evaluate efficacy of Rv relative to ECHO-7 neutralizing antibodies. The authors in the aforementioned manuscript allude to the fact that the prevalence of neutralizing antibodies against ECHO-7 in the general population has not been reported and that levels do not correlate with efficacy of therapy. However, it appears that more corroborating information is needed in this regard.
A separate retrospective case series highlighted three advanced cancer patients (those with melanoma, small cell lung cancer and histiocytic sarcoma).61 Rv was administered on the same schedule as described in the previous study with the only notable exception being that treatment was continued beyond year 3 if indicated. A patient with stage IV melanoma with liver and lymph node involvement received Rv for ~3 years (and ongoing) and had stable disease with some shrinkage of lymph node lesions and stability of liver lesions. These data are provocative but given that some patients with melanoma can have indolent disease or even spontaneous tumor regressions, it is not entirely clear what the impact of Rv was in this particular patient. A patient with stage III small cell lung cancer and histiocytic sarcoma exhibited some evidence of tumor shrinkage as well, and these data are indicative of potential preliminary evidence of antitumor activity. Larger prospective trials would help confirm these initial findings.
Conclusion
The current standard of care treatment for localized melanoma includes surgical resection and adjuvant interferon in certain cases. However, the treatment of metastatic melanoma has witnessed a burgeoning development of immunotherapeutic and targeted drugs, and now trials evaluating the additional benefit of oncolytic viruses are ongoing. Therapeutic oncolytic viruses (virotherapeutics) represent a novel class of anticancer treatments with a unique mechanism of action, a good safety profile and an effective and promising treatment in malignant melanoma. There are currently ongoing trials in oncolytic viruses, including HSVs (such as T-VEC), coxsackieviruses (such as CVA13), reoviruses (such as Reolysin) and echoviruses (such as Rv). Rv, a wild-type ECHO virus, represents an intriguing possibility among other oncolytic viruses in this context and has been studied mostly in Latvia. Currently, limited retrospective data from Latvia are available. Larger prospective randomized studies and evaluations by investigators outside of Latvia will allow for more rigorous assessments and future development of Rv as a cancer virotherapeutic.
Disclosure
The authors report no conflicts of interest in this work.
References
Little EG, Eide MJ. Update on the current state of melanoma incidence. Dermatol Clin. 2012;30(3):355–361. | ||
DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. | ||
Russell SJ, Peng K-W. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28(7):326–333. | ||
Russell SJ, Peng K-W, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–670. | ||
Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107(10):1373–1379. | ||
Redman JM, Gibney GT, Atkins MB. Advances in immunotherapy for melanoma. BMC Med. 2016;14:20. | ||
Doniņa S, Strēle I, Proboka G, et al. Adapted ECHO-7 virus Rigvir immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res. 2015;25(5):421–426. | ||
Glinkina LS, Bruvere RZ, Venskus DR, Garklava RR, Muceniece AJ. [The cellular immunity indices of patients with malignant melanoma using the viral immunomodulator Rigvir]. Vopr Onkol. 1992;38(5):540–547. | ||
Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–1678. | ||
Liu J, Fukunaga-Kalabis M, Li L, Herlyn M. Developmental pathways activated in melanocytes and melanoma. Arch Biochem Biophys. 2014;563:13–21. | ||
Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. | ||
Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–819. | ||
Faghfuri E, Faramarzi MA, Nikfar S, Abdollahi M. Nivolumab and pembrolizumab as immune-modulating monoclonal antibodies targeting the PD-1 receptor to treat melanoma. Expert Rev Anticancer Ther. 2015;15(9):981–993. | ||
Cohn-Cedermark G, Rutqvist LE, Andersson R, et al. Long term results of a randomized study by the Swedish Melanoma Study Group on 2-cm versus 5-cm resection margins for patients with cutaneous melanoma with a tumor thickness of 0.8-2.0 mm. Cancer. 2000;89(7):1495–1501. | ||
Grotz TE, Markovic SN, Erickson LA, et al. Mayo clinic consensus recommendations for the depth of excision in primary cutaneous melanoma. Mayo Clin Proc. 2011;86(6):522–528. | ||
Hayes AJ, Maynard L, Coombes G, et al. Wide versus narrow excision margins for high-risk, primary cutaneous melanomas: long-term follow-up of survival in a randomised trial. Lancet Oncol. 2016;17(2):184–192. | ||
Berrocal A, Espinosa E, Marín S, et al. Spanish Multidisciplinary Melanoma Group (GEM) guidelines for the management of patients with advanced melanoma. Eur J Dermatol. 2015;25(5):392–403. | ||
Phan GQ, Messina JL, Sondak VK, Zager JS. Sentinel lymph node biopsy for melanoma: indications and rationale. Cancer Control. 2009;16(3):234–239. | ||
Keilholz U, Conradt C, Legha SS, et al. Results of interleukin-2-based treatment in advanced melanoma: a case record-based analysis of 631 patients. J Clin Oncol. 1998;16(9):2921–2929. | ||
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. | ||
Ribas A, Hanson DC, Noe DA, et al. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte–associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist. 2007;12(7):873–883. | ||
Hauschild A. Adjuvant interferon alfa for melanoma: new evidence-based treatment recommendations? Curr Oncol. 2009;16(3):3–6. | ||
Grob JJ, Dreno B, de la Salmonière P, et al. Randomised trial of interferon alpha-2a as adjuvant therapy in resected primary melanoma thicker than 1.5 mm without clinically detectable node metastases. French Cooperative Group on Melanoma. Lancet. 1998;351(9120):1905–1910. | ||
Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14(1):7–17. | ||
Richards JM, Gale D, Mehta N, Lestingi T. Combination of chemotherapy with interleukin-2 and interferon alfa for the treatment of metastatic melanoma. J Clin Oncol. 1999;17(2):651–657. | ||
McDermott J, Jimeno A. Pembrolizumab: PD-1 inhibition as a therapeutic strategy in cancer. Drugs Today (Barc). 2015;51(1):7–20. | ||
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. | ||
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. | ||
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. | ||
Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. | ||
Dharmadhikari N, Mehnert JM, Kaufman HL. Oncolytic virus immunotherapy for melanoma. Curr Treat Options Oncol. 2015;16(3):326. | ||
Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6(7):821–825. | ||
Myers R, Greiner S, Harvey M, et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005;12(7):593–599. | ||
Parato KA, Senger D, Forsyth PAJ, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5(12):965–976. | ||
Xia ZJ, Chang JH, Zhang L, et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng. 2004;23(12):1666–1670. | ||
Geevarghese SK, Geller DA, de Haan HA, et al. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum Gene Ther. 2010;21(9):1119–1128. | ||
Muster T, Rajtarova J, Sachet M, et al. Interferon resistance promotes oncolysis by influenza virus NS1-deletion mutants. Int J Cancer. 2004;110(1):15–21. | ||
Pecora AL, Rizvi N, Cohen GI, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol. 2002;20(9):2251–2266. | ||
Park SH, Breitbach CJ, Lee J, et al. Phase 1b trial of biweekly intravenous pexa-vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus in colorectal cancer. Mol Ther. 2015;23(9):1532–1540. | ||
McAneny D, Ryan CA, Beazley RM, Kaufman HL. Results of a phase I trial of a recombinant vaccinia virus that expresses carcinoembryonic antigen in patients with advanced colorectal cancer. Ann Surg Oncol. 1996;3(5):495–500. | ||
Zajac P, Oertli D, Marti W, et al. Phase I/II clinical trial of a nonreplicative vaccinia virus expressing multiple HLA-A0201-restricted tumor-associated epitopes and costimulatory molecules in metastatic melanoma patients. Hum Gene Ther. 2003;14(16):1497–1510. | ||
Galanis E, Hartmann LC, Cliby WA, et al. Phase I Trial of Intraperitoneal Administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70(3):875–882. | ||
Regules JA, Beigel JH, Paolino KM, et al. A recombinant vesicular stomatitis virus Ebola vaccine – preliminary report. N Engl J Med. Epub 2015 Apr 1. | ||
Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. | ||
Kaufman HL, Bines SD. OPTIM trial: a phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 2010;6(6):941–949. | ||
Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17(3):718–730. | ||
Gildener-Leapman N, Ferris RL, Ohr J, et al [webpage on the Internet]. A phase I trial of intratumoral administration of HF10 in patients with refractory superficial cancer: immune correlates of virus injection. J Clin Oncol. [cited Aug 26, 2016]. Available from: http://meetinglibrary.asco.org/content/115064-132. Accessed December 21, 2016. | ||
Puzanov I, Milhem MM, Ingemar Andtbacka RH, et al [webpage on the Internet]. Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. J Clin Oncol. [cited Sep 2, 2016]. Available from: http://meetinglibrary.asco.org/content/128142-144. Accessed December 21, 2016. | ||
Watanabe D, Goshima F, Mori I, Tamada Y, Matsumoto Y, Nishiyama Y. Oncolytic virotherapy for malignant melanoma with herpes simplex virus type 1 mutant HF10. J Dermatol Sci. 2008;50(3):185–196. | ||
Au GG, Lindberg AM, Barry RD, Shafren DR. Oncolysis of vascular malignant human melanoma tumors by Coxsackievirus A21. Int J Oncol. 2005;26(6):1471–1476. | ||
Ingemar Andtbacka RH, Curti BD, Kaufman H, et al [webpage on the Internet]. CALM study: a phase II study of an intratumorally delivered oncolytic immunotherapeutic agent, coxsackievirus A21, in patients with stage IIIc and stage IV malignant melanoma. J Clin Oncol. [cited Aug 26, 2016]. Available from: http://meetinglibrary.asco.org/content/134927-144. Accessed December 21, 2016. | ||
Shafren D, Quah M, Wong Y, Andtbacka RH, Kaufman HL, Au GG. Combination of a novel oncolytic immunotherapeutic agent, CAVATAK (coxsackievirus A21) and immune-checkpoint blockade significantly reduces tumor growth and improves survival in an immune competent mouse melanoma model. J Immunother Cancer. 2014;2(suppl 3):125. | ||
Stoeckel J, Hay JG. Drug evaluation: Reolysin – wild-type reovirus as a cancer therapeutic. Curr Opin Mol Ther. 2006;8(3):249–260. | ||
Prestwich RJ, Errington F, Ilett EJ, et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. 2008;14(22):7358–7366. | ||
Gujar SA, Marcato P, Pan D, Lee PWK. Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity. Mol Cancer Ther. 2010;9(11):2924–2933. | ||
Galanis E, Markovic SN, Suman VJ, et al. Phase II Trial of Intravenous Administration of Reolysin® (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther. 2012;20(10):1998–2003. | ||
Roulstone V, Pedersen M, Kyula J, et al. BRAF- and MEK-targeted small molecule inhibitors exert enhanced antimelanoma effects in combination with oncolytic reovirus through ER stress. Mol Ther. 2015;23(5):931–942. | ||
Glinkina LS, Bruvere RZ. [The reaction of the T-immunity system in patients with malignant skin melanoma and stomach cancer to active nonspecific immunotherapy]. Vopr Onkol. 1992;38(6):659–666. Article in Russian. | ||
Glinkina LS, Heisele OG, Garklava RR, Muceniece AJ. [The humoral immunity indices of patients with malignant skin melanoma using the viral immunomodulator Rigvir]. Vopr Onkol. 1992;38(5):534–540. Article in Russian. | ||
Blue CE, Spiller OB, Blackbourn DJ. The relevance of complement to virus biology. Virology. 2004;319(2):176–184. | ||
Alberts P, Olmane E, Brokāne L, et al. Long-term treatment with the oncolytic ECHO-7 virus Rigvir of a melanoma stage IV M1c patient, a small cell lung cancer stage IIIA patient, and a histiocytic sarcoma stage IV patient-three case reports. APMIS. 2016;124(10):896–904. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.