Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 9 » Issue 1
Once-daily indacaterol 75 µg in moderate- to-severe COPD: results of a Phase IV study assessing time until patients’ perceived onset of effect
Authors Siler TM , LaForce CF, Kianifard F, Williams J, Spangenthal S
Received 7 May 2014
Accepted for publication 4 July 2014
Published 1 September 2014 Volume 2014:9(1) Pages 919—925
DOI https://doi.org/10.2147/COPD.S67356
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Thomas M Siler,1 Craig F LaForce,2 Farid Kianifard,3 James Williams,3 Selwyn Spangenthal4
1Midwest Chest Consultants, St Charles, MO, USA; 2North Carolina Clinical Research, Raleigh, NC, USA; 3Novartis Pharmaceuticals, East Hanover, NJ, USA; 4Charlotte Lung and Health Center, Charlotte, NC, USA
Background: Indacaterol 75 µg once daily is a long-acting β2 agonist approved for maintenance bronchodilator treatment in patients with chronic obstructive pulmonary disease (COPD). The purpose of this study was to evaluate patients' perception of onset of effect with a single dose.
Methods: In this double-blind, crossover, Phase IV study, 40 patients were randomized to receive a single dose of indacaterol 75 µg or placebo via a dry powder inhaler device. The primary variable was time until patient’s perception of onset of effect, using a simple self-administered (nonvalidated) questionnaire that patients answered at nine protocol-specified time points. Exploratory variables included change in forced expiratory volume in 1 second (FEV1) and change in percent predicted FEV1 from predose to postdose (determined 60–75 minutes postdose).
Results: The least-squares mean time to patient’s perception of onset of effect was 25.4 minutes and 23.9 minutes for indacaterol and placebo, respectively. There was no significant effect for treatment, period, or sequence on the time to patient's perception. In addition, no statistically significant differences between treatments were observed for patient's global satisfaction with onset of effect and global expectation of treatment adherence. For the exploratory variable change in FEV1 from predose to postdose, indacaterol showed superiority over placebo with a clinically relevant least-squares mean treatment difference of 0.12 L (P<0.0001). There was little or no association between patient’s perception of time to onset of effect and change in FEV1, or change in percent predicted FEV1. Both treatments were well tolerated.
Conclusion: A single dose of indacaterol 75 µg did not separate from placebo in terms of patient perception of onset, although there was an improvement in FEV1 for indacaterol compared with placebo. Development and use of a validated questionnaire may be needed to address the inconsistency in evaluating this patient-related outcome.
Keywords: bronchodilator, long-acting, perceived onset of action, single dose
Introduction
Indacaterol 75 μg once daily has been demonstrated to be an effective maintenance bronchodilator treatment for patients with moderate-to-severe chronic obstructive pulmonary disease (COPD) in two 12-week Phase III studies. These studies demonstrated a rapid onset of effect (90–100 mL difference from placebo in forced expiratory volume in 1 second [FEV1] at 5 minutes postdose on day 1) and sustained 24-hour bronchodilation over 12 weeks, along with significant improvements versus placebo in dyspnea and health status, and reduced rescue medication use.1,2
Although FEV1 is a standardized and accepted measurement of airflow limitation, it does not assess the impact of lung function improvement in patients.3–5 Patient-reported outcome (PRO) measures that assess changes in a patient’s health status (including the St George’s Respiratory Questionnaire6 [SGRQ] and COPD Assessment Test7) or degree of dyspnea (including the Baseline/Transition Dyspnea Indexes8 and Medical Research Council score9) are considered increasingly pertinent to evaluating the impact of COPD on patients and the long-term management of the disease.3–5
In addition to these validated PRO measures, there is also an interest in PRO measures that assess the impact of bronchodilator treatment with respect to patient perception of onset and satisfaction with onset of effect in patients with COPD. Bronchodilator treatments in COPD have demonstrated an acute improvement in spirometric indices,10 and there is a potential for patient perception of onset of effect to improve adherence to treatment. Although patient-reported assessment of time until the perception of onset of effect based on a questionnaire has been described in asthma and COPD,11,12 overall there is limited information on this topic in the published literature.
This randomized, double-blind, placebo-controlled, crossover, single-dose, Phase IV study assessed the perception of onset of action in patients with moderate-to-severe COPD when treated with a single dose of indacaterol 75 μg or placebo, using a simple patient-reported instrument to detect perception of onset of effect.
Methods
Study design
This was a randomized, double-blind, placebo-controlled, single-dose, crossover, Phase IV study conducted in six centers in the US. Signed approvals for the study were obtained from a central Institutional Review Board (Copernicus Group Independent Review Board).
Patients
This study recruited patients with moderate-to-severe COPD (defined according to the Global Initiative for Chronic Obstructive Lung Disease [GOLD] 2009 criteria),13 aged ≥40 years, and with a smoking history of ≥10 pack-years. Their FEV1 was to be ≤70% and ≥40% of predicted and FEV1/forced vital capacity <70% at screening, both measured postbronchodilator (albuterol 90 μg × four puffs). Patients were also required to have breathing symptoms that interfered with some daily activities, as determined by the investigators. Patients gave their written informed consent. Patients were excluded if they had a history of asthma or repeated COPD exacerbations (two or more in the previous 2 years). Medication-based exclusions included bronchodilators for COPD with a pharmacodynamic activity greater than 48 hours and/or once-daily dosing, and a washout of 48 hours was required for patients on maintenance bronchodilators (eg, formoterol and salmeterol).
Treatments
At visit two (3−10 days postscreening), eligible patients were randomized in a crossover design in a 1:1 ratio to receive a single dose of indacaterol 75 μg followed by placebo at visit three, or placebo at visit two followed by indacaterol 75 μg at visit three (washout of 7−10 days between visits), via a dry powder inhaler device. Patients receiving inhaled corticosteroids at baseline continued this treatment (or the inhaled corticosteroids component alone if taken as a fixed combination with a bronchodilator) at equivalent dose and regimen during the study. Albuterol was available for rescue use. Patients were instructed to withhold albuterol within 6 hours of visit two and visit three and to keep their albuterol with them as rescue medication at all times, including the 6-hour period prior to visits two and three.
Assessments
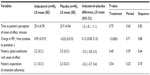
The primary variable was time (in minutes) to patient’s perception of onset of effect, defined as the first time point (5 minutes, 7.5 minutes, 10 minutes, 15 minutes, 20 minutes, 30 minutes, 40 minutes, 50 minutes, and 60 minutes postdose) that the patient responded “yes” to the self-administered statement “I feel that the drug is working in improving my breathing.” This tool was chosen because it was used to assess perceptions of onset of action in a previous study of patients with COPD,11 and a validated instrument was not available for this purpose. Exploratory variables included change in FEV1 and change in percent predicted FEV1 from predose to postdose (60−75 minutes postdose), as well as patient’s global satisfaction with onset of effect and patient’s expectation of treatment adherence (both administered after completion of the 60 minute perception of onset of effect questionnaire; Table 1).
Safety assessments included all adverse events (AEs), serious AEs, vital signs, blood chemistry, urine tests, and electrocardiograms.
Randomization
Patients were randomized to one of the two treatment sequences in this double-blind study (see Treatment section). At visit 2, eligible patients were given the lowest available number on the randomization list. This number assigned them to one of the two treatment sequences. The investigator entered the randomization number on the case report form. The investigator or his/her delegate dispensed the medication kits in sequential order, dispensing the lowest numbered kit first and then the kit with the next highest number to the next randomized study patient.
Statistical methods
The full analysis set (FAS) consisted of all patients to whom the study drug had been assigned through randomization. Patients in the FAS were analyzed according to the treatment sequence to which they were assigned. The safety set consisted of all patients who received at least one dose of either study drug. Patients in the safety set were analyzed according to the treatment they received.
The primary variable was analyzed for the FAS using a repeated-measures analysis of variance model with fixed effects for treatment, period, and sequence and a random effect for patients within sequence. Patients who did not perceive onset of effect by 60 minutes were considered as having a perceived onset of effect at 70 minutes. For analysis of the primary variable, if a patient had missing data for the second period, no imputations were performed.
The change in FEV1 from predose to postdose, the patients’ global satisfaction with the onset of effect, and patients’ expectation of treatment adherence were also analyzed for the FAS with the same repeated-measures analysis of variance model that was used for the analysis of the primary variable.
The associations between time until patient’s perception of onset of effect, and change in FEV1, and change in percent predicted FEV1 from predose to postdose were examined by computing Pearson’s correlation coefficients and Spearman’s rank correlation coefficients. The association between patient’s global satisfaction with onset of effect and patient’s expectation of treatment adherence was examined by computing Spearman’s rank correlation coefficient.
All statistical tests were conducted against a two-sided alternative hypothesis and evaluated at a significance level of 0.05. All statistical analyses were performed using SAS®, Version 9.1.3 (SAS Institute, Inc., Cary, NC, USA).
Sample size calculation
The sample size was calculated based on the primary variable, time (in minutes) until patient’s perception of onset of effect. By assuming a within-patient correlation of 0.50, a between-patient standard deviation of 14, a significance level of 0.05, and a power of 87% to detect a difference of 7.5 minutes between the true means using a paired t-test, it was calculated that 36 patients were needed for randomization.14 Allowing for 10% of patients who may not complete both treatment periods, it was estimated that approximately 40 patients would need to be randomized to the two treatment sequences.
Results
Patients
Forty patients were randomized, with 20 patients in the indacaterol/placebo (IP) sequence and 20 patients in the placebo/indacaterol (PI) sequence. The FAS and safety set also included 20 patients each in the IP and PI sequences. The demographic data of patients are presented in Table 2.
  | Table 2 Demographics of the patient population (full analysis set) (means [standard deviations] are reported, unless otherwise stated) |
Most patients were Caucasian (90.0%), male (67.5%), and current smokers (60.0%). The mean age was 61.5 years and the mean time since diagnosis of COPD was 8.5 years.
Primary and exploratory variables
For patients receiving indacaterol and placebo, the least-squares (LS) mean time until patient’s perception of onset of effect was 25.4 minutes and 23.9 minutes, respectively (Table 3). The LS mean of the IP difference was 1.5 minutes (P=0.75). The period effect (P=0.63) and sequence effect (P=0.82) were not statistically significant. The cumulative frequencies for time to patient’s perception of onset of effect are summarized in Table 4 and Figure 1. The mean values for onset of effect in the combined sequences for indacaterol and placebo were 25.58 minutes and 23.88 minutes, respectively.
  | Figure 1 Cumulative distribution functions for time to patient’s perception of onset of effect by treatment. |
For the exploratory variable change in FEV1 from predose to postdose, indacaterol showed superiority over placebo with an LS mean treatment difference of 0.12 L (P<0.0001; Table 3). The mean (SD) predose FEV1 (L) of first dose for indacaterol and placebo was 1.53 (0.606) and 1.59 (0.530), respectively. The improvement in FEV1 with indacaterol 75 μg from predose to postdose was considered to be clinically relevant.3 The magnitudes of the Pearson’s and Spearman’s correlation coefficients (for combined treatments and combined sequences) indicated little or no association between patient’s perception of time until onset of effect and change in FEV1, and between patient’s perception of time until onset of effect and change in percent predicted FEV1 (correlation coefficients ranging from −0.06 to 0).
The Spearman’s correlation coefficients (for combined treatments and combined sequences) for examining the association between patient’s global satisfaction with onset of effect and patient’s expectation of treatment adherence (60 minutes postdose) ranged from 0.65 to 0.96, indicating a moderate to strong positive association between these two variables. For patient’s global satisfaction with onset of effect, there was a small improvement with indacaterol compared with placebo (LS mean 2.2 vs 2.5; difference −0.2), but this was not statistically different (P=0.40; Table 3). Similarly, for patient’s expectation of treatment adherence score, the LS mean treatment difference (−0.2) between indacaterol (2.1) and placebo (2.3) indicated a small improvement but was not significant (P=0.54; Table 3).
Safety
Overall, five patients (12.5%) receiving indacaterol and one patient (2.5%) receiving placebo experienced at least one AE after study treatment. Cough was the most frequently occurring AE, reported by two patients (5.0%) receiving indacaterol and no patients receiving placebo. Other AEs reported by patients in the indacaterol group included rhinorrhea, throat irritation, dyspepsia, pyrexia, muscle spasm, and headache (one case per AE [2.5%]). COPD worsening was reported by one patient in the placebo group. One patient in the placebo group discontinued due to AEs. No serious AEs or deaths were reported in the study.
Discussion
In this Phase IV, single-dose study, the effect of active treatment (indacaterol 75 μg once daily) and placebo on patient’s perception of onset of effect was evaluated with a simple patient-reported instrument in patients with moderate-to-severe COPD. The study did not show a significant treatment, period, or sequence effect for the primary variable, time until patient’s perception of onset of effect. However, a statistically significant improvement was observed in FEV1 with indacaterol compared with placebo (Table 3). The treatment difference of 0.12 L (P<0.0001) in FEV1 at 60–75 minutes postdose for indacaterol versus placebo was clinically meaningful3 and was comparable with the improvement reported in trough FEV1 with indacaterol 75 μg versus placebo by Kerwin et al.2 Therefore, the absence of patient perception of onset of effect occurred despite a strong bronchodilator response.
Evaluation of patient perceptions of treatment effects involves significant challenges. In particular, it is recognized that subjective responses to placebo may be similar to those reported for active treatments, despite marked differences in objective measures. For example, Wechsler et al15 compared the effects of the bronchodilator albuterol with two placebo interventions and no intervention in patients with asthma. Although albuterol significantly improved FEV1, whereas the placebo interventions did not, self-reported outcomes were similar between the active and placebo groups. Wechsler et al15 concluded that placebo treatments can result in clinically meaningful effects that may rival those of active treatments in patients with asthma. Other studies have also indicated that placebo exerts positive effects on subjectively assessed outcomes (such as pain and depression) but not on objectively evaluated outcomes.16,17 In 610 patients with asthma, Wise et al17 demonstrated that placebo given with enhanced messages to increase the expectation of benefit significantly increased subjective outcomes but had no effect on objective outcomes. The results of our study are consistent with these findings.
Disparities between subjective and objective assessments have been noted in other trials of COPD treatments. For example, significant improvements (P<0.05) in FEV1 and health status (SGRQ) were noted at week 12 in patients receiving indacaterol 75 μg, compared with placebo, in two pivotal Phase III studies.2 In both the studies, the improvements in FEV1 met the prespecified criterion for clinical relevance (120 mL difference versus placebo), and the differences in the SGRQ scores approached clinical significance (defined as a four-point difference versus placebo).2,18 However, dyspnea (measured using the Transition Dyspnea Index) was significantly improved with indacaterol 75 μg versus placebo in one study but not in the other.1 Disparities between different assessments were also observed in a study by Lindberg et al11 that used the same instrument employed in our study to evaluate budesonide/formoterol, salmeterol/fluticasone, salbutamol, and placebo in patients with COPD. In this trial, significant objective differences were recorded in lung function between active treatments, but patient perceptions of the onset of action were similar. Of note, 61% of placebo recipients answered “yes” when asked whether their medication was working (versus 81%–84% for the active treatments). As well as raising concerns regarding the sensitivity of the questionnaire, Lindberg et al11 noted that patients may have been in a “steady state” with regard to symptoms, and many may have been relatively asymptomatic, thus compromising the ability of the instrument to assess treatment effects.
Studies investigating the effect of single-dose bronchodilators on lung function, exercise capacity, and walking performance in patients with COPD have reported improvements in objective measures (lung function, walking performance, and arterial oxygen saturation), as well as subjective measures (perceived breathlessness, exertion, chest pain, and fatigue).19–21 In contrast to the current study, where there was no physical challenge prior to the assessments, these earlier trials involved an exercise test. It can be hypothesized that an improvement in subjective assessments could have been seen with indacaterol if the study involved a physical challenge, such as a 6-minute walk test.
We assessed the single-dose effect of indacaterol 75 μg (US approved dose) versus placebo on patient perception of onset of effect. The sensitivity of the instrument used in this study and the symptom severity of the patients enrolled may have influenced the results. An effect on patient perception of onset of effect with indacaterol may have been observed with a different instrument with a higher sensitivity, or had the study been conducted among patients with more severe impairment (eg, with severe to very severe air flow obstruction or with acute symptoms such as dyspnea or fatigue as a result of exercise). Patients with COPD may need the use of rescue medication for immediate relief of bronchospasm. Our study allowed the use of albuterol as a rescue medication for rapid relief of acute dyspnea.
Conclusion
In summary, a single dose of indacaterol 75 μg was not associated with a significant difference in patient perception of benefit compared with placebo, despite significant improvement in FEV1. There were no differences in time to perception of onset of effect between indacaterol and placebo. It may be that a more sensitive instrument or an exercise challenge study would be required to address the discrepancy between a patient’s perception of effect and the significant bronchodilation achieved. As has been shown in other studies, significant improvements in physiologic function do not always correlate directly with patients’ perception of relief.
Acknowledgments
The authors thank the patients and staff at the participating study centers and David Young (Novartis, Horsham, UK) for critically reviewing the manuscript. The study was funded by Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA); Anneliese LaRose was the clinical trial lead. Melanie Stephens, a professional medical writer (CircleScience, London, UK) funded by Novartis Pharmaceuticals Corporation, assisted in the preparation of the manuscript.
Disclosure
Thomas M Siler has received research support from Arena, Boehringer Ingelheim, Daiichi Sankyo, Dey Laboratories, Forest Research Institute, Genentech, GlaxoSmithKline, Johnson and Johnson, Merck, Novartis, Pearl Therapeutics, Schering, and Sunovion, and has received honoraria from AstraZeneca, Boehringer Ingelheim, Forest, Novartis, and UCB. Craig F LaForce is a consultant to Novartis and has received honoraria from Merck and Co. He is the principal investigator on trials with the following pharmaceutical companies: Array, Boehringer Ingelheim, GlaxoSmithKline, Merck Research Laboratories, Novartis, Pfizer, Roxane Laboratories, Teva, Sunovion, and Watson Pharmaceuticals. Selwyn Spangenthal has received honoraria from AstraZeneca, Forest, Mylan Labs, and Novartis. Farid Kianifard and James Williams are employees of Novartis Pharmaceuticals.
References
Gotfried MH, Kerwin EM, Lawrence D, Lassen C, Kramer B. Efficacy of indacaterol 75 μg once-daily on dyspnea and health status: results of two double-blind, placebo-controlled 12-week studies. COPD. 2012;9:629–636. | |
Kerwin EM, Gotfried MH, Lawrence D, Lassen C, Kramer B. Efficacy and tolerability of indacaterol 75 μg once daily in patients aged ≥40 years with chronic obstructive pulmonary disease: results from 2 double-blind, placebo-controlled 12-week studies. Clin Ther. 2011;33:1974–1984. | |
Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31:416–469. | |
Jones P, Miravitlles M, van der MT, Kulich K. Beyond FEV(1) in COPD: a review of patient-reported outcomes and their measurement. Int J Chron Obstruct Pulmon Dis. 2012;7:697–709. | |
Jones PW, Price D, van der Molen T. Role of clinical questionnaires in optimizing everyday care of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:289–296. | |
Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. | |
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline LN. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–654. | |
Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. | |
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. | |
Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31:742–750. | |
Lindberg A, Szalai Z, Pullerits T, Radeczky E. Fast onset of effect of budesonide/formoterol versus salmeterol/fluticasone and salbutamol in patients with chronic obstructive pulmonary disease and reversible airway obstruction. Respirology. 2007;12:732–739. | |
O’Connor RD, Patrick DL, Parasuraman B, Martin P, Goldman M. Comparison of patient-reported outcomes during treatment with adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler versus fixed-dose fluticasone propionate/salmeterol dry powder inhaler in patients with asthma. J Asthma. 2010;47:217–223. | |
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Diagnosis, Management, and Prevention of COPD; 2009. Available at: http://www.goldcopd.org/. Accessed August 6, 2014. | |
Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23:1921–1986. | |
Wechsler ME, Kelley JM, Boyd IO, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365:119–126. | |
Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–1602. | |
Wise RA, Bartlett SJ, Brown ED, et al. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol. 2009;124:436–438. | |
Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19:398–404. | |
Brouillard C, Pepin V, Milot J, Lacasse Y, Maltais F. Endurance shuttle walking test: responsiveness to salmeterol in COPD. Eur Respir J. 2008;31:579–584. | |
Grove A, Lipworth BJ, Reid P, et al. Effects of regular salmeterol on lung function and exercise capacity in patients with chronic obstructive airways disease. Thorax. 1996;51:689–693. | |
Okudan N, Gok M, Gokbel H, Suerdem M. Single dose of tiotropium improves the 6-minute walk distance in chronic obstructive pulmonary disease. Lung. 2006;184:201–204. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.