Back to Journals » Clinical Ophthalmology » Volume 14
Ocular Complications After Radiation Therapy: An Observational Study
Authors Nuzzi R , Trossarello M, Bartoncini S, Marolo P , Franco P , Mantovani C, Ricardi U
Received 28 May 2020
Accepted for publication 13 August 2020
Published 9 October 2020 Volume 2020:14 Pages 3153—3166
DOI https://doi.org/10.2147/OPTH.S263291
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Raffaele Nuzzi,1 Marta Trossarello,1 Sara Bartoncini,2 Paola Marolo,1 Pierfrancesco Franco,2 Cristina Mantovani,2 Umberto Ricardi2
1Department of Surgical Sciences, Ophthalmology/Eye Clinic, University of Turin, Turin, Italy; 2Department of Oncology, University of Turin, Turin, Italy
Correspondence: Raffaele Nuzzi
Dipartimento di Scienze Chirurgiche, Ophthalmology/Eye Clinic, University of Turin, Oculistica U, Via Cherasco 23, Turin 10126, Italy
Tel +39 368 203 493
Email [email protected]
Aim of the Study: The study aims to quantify the incidence of ocular complications in patients irradiated on the head and neck area in our medical center, stratified by type of neoplasm and radiation dose received.
Materials and Methods: From an existing database of patients radio-treated in our center, we selected 25 patients irradiated in the 2011– 2018 period. The patients had been treated for orbital lymphoma, nasopharyngeal carcinoma and cranial base meningioma. The selected patients received an ophthalmologic evaluation which included a complete ophthalmological and orthoptic assessment.
Results: Our results showed a significantly higher incidence of DES (dry eye syndrome) and corneal complications for eyes receiving a Dmax higher than 40 Gy, as well as for cataract incidence in eyes that had received a Dmax to the lens higher than 5 Gy. We found an overall thinning of the RNFL (retinal nerve fiber layer) in eyes that had received a Dmax higher than 50 Gy, as well as a greater MD (mean deviation) from normal visual field values.
Conclusion: In conclusion, we can say that the study confirms the presence of a correlation between the received radiation dose and the onset of eye complications, despite the small sample.
Keywords: eye, radiotherapy, orbital lymphoma, nasopharyngeal carcinoma, meningioma, secondary/iatrogenic pathology
Introduction and Background
Radiation complications of the eye and orbital structures have been recognized since the late 19th century. Ocular and orbital injuries may occur either as a direct result of irradiation for intraocular tumors, or when the eye is within the entrance or exit beam. Radiation-induced ocular morbidity encompasses a spectrum from transient eyelid erythema to complete loss of vision.1 Radiation protection and modern radiation therapy allow for appropriate treatment programs that minimize complications. Radiation-induced ocular complications are caused by different factors. Acute lesions may occur in the eyelid, conjunctiva, and corneal epithelium. Typical delayed effects include cataracts, glaucoma, and retinopathy. One of the most frequent manifestations of radiation toxicity is a decreased visual acuity.2–4 An overall decrease of at least two Snellen lines have been reported in 26–62% of patients undergoing radiation therapy.1
Several confounding variables make it difficult to evaluate the literature and determine incidence and severity of ocular radiation morbidity. Some papers present only cases of ocular radiation damage without mentioning the total number of patients treated. Other papers include patients treated with irradiation and chemotherapy. Admixture of primary treatment areas with different radiation doses, fractionation schedules and variable follow up make comparison of radiation induced ocular complications in different papers a challenging task.
Dry Eye Syndrome (DES)
The report of the Dry Eye Workshop 2007 indicates radiotherapy as one of the causes of DES with the highest levels of evidence.5 The pathogenetic mechanism of ocular dryness by irradiation is the result of damage to the Meibomian glands, with reduction or absence of the lipid layer of the tear film and evaporation, or damage to the acinar cells of the lacrimal glands.2,6 Woo et al compare the results of the Schirmer test, Break-up time (BUT) test and dry eye OSDI questionnaire obtained from a sample of 40 patients with conjunctival or orbital lymphoma irradiated with an average dose of 37.4 Gy and a healthy sample of 60 people. The study shows lower mean Schirmer values and BUT test values in the irradiated group (9.2 ± 5.1 mm vs. 12.3 ± 5.2 mm and 4.2 ± 2.5 seconds vs. 6.4 ± 2.6 seconds), while OSDI questionnaire scores have significantly higher values in the irradiated group than in the control group (48.1 ± 21.4 vs. 6.2 ± 4.4).9 There are currently no uniform guidelines for the classification of DES. However the literature10 proposes a classification of DES into four grades of severity based on symptoms, tests and objective evidence.
Complications Affecting the Lacrimal Glands
Lacrimal puncta and canaliculi may also be affected by radiotherapy complications. There are cases of fibrosis and post-radiation obstruction, resulting in epiphora, described in the literature.11,12
Eyelid and Conjunctiva Complications
In patients undergoing radiotherapy, the most frequent eyelid manifestations are blepharitis and ciliary diseases (in particular trichiasis, distichiasis and madarosis). The onset of skin erythema, oedema and flaking is reported by many authors and it occurs within 10–20 days, and then resolves within 2–4 weeks.1,2,6,13 The literature describes the temporary or permanent loss of eyelashes (madarosis).1,6,8 In literature6,8,14 there are also cases of entropion and ectropion in irradiated patients, but this is quite rare.
Corneal Complications
Keratopathies can occur with variable manifestations and in radio-treated patients they generally do not usually occur due to direct damage to the cornea, but as a consequence of the severe dryness of the eye.1,6,14 In radio-treated patients, these lesions occur mainly after treatments with conventional fractioning at a dose of 30–50 Gy. Corneal oedema, on the other hand, tends to occur at higher doses of radiation, from 40 to 50 Gy.8 It has been reported that anterior segment complications affect up to 23% of patients receiving radiation therapy.1,11
Iris Complications
Rare cases of persistent iritis are reported in the literature after a total dose of 70 Gy in conventional fractionation or 30–40 Gy in hypofractioning.14 Neovascularization of the iris can ultimately lead to the onset of neovascular glaucoma, which, according to the literature, can occur in up to 35% of treated eyes, however, there is wide variability in results between studies.15
Cataract
The onset of RT-induced cataract is extremely frequent, with a latency usually of 2 or 3 years, with an interval of 6 to 64 months.15 In elderly patients, an overlap or worsening of a senile cataract may occur. In Stafford et al 48 patients with non-Hodgkin orbital lymphoma received a median dose of 27 Gy, 8 of whom developed cataracts (16.7%).16 Taking into account the wide variability of dose and fractionation it is difficult to estimate the incidence of radiation cataract, Singh et al believe that it can vary from 8% to 83% depending on the characteristics of the treatment.1
Retinal Complications
Radiotherapy on the orbital region can cause radiation retinopathy. When observed with the slit-lamp, cotton wool spots, papillary inflammation, macular oedema and hard exudates can be observed.14,17 The minimum dose capable of generating retinal damage is considered to be 30–35 Gy. However cases of radiation retinopathy with doses as low as 11 Gy have been reported.17 The 2010 Cochrane review, which included 13 randomized clinical trials and a total of 1154 patients irradiated with doses from 7.5 to 24 Gy, had reported that no retinal or optic nerve damage was found in any of the 1154 patients considered.18
Complications Affecting the Optic Nerve
Pathogenesis appears to be linked to both vascular and nervous damage, with the involvement of endothelial cells and the induction of somatic mutations in glial cells, whose metabolism proves to be deficient and ultimately produces demyelination.19,20 The RION (Radiation Induced Optic Neuropathy) typically occurs about 10–20 months after treatment, with a median time of 18 months. However the latency range may vary from 3 months to 8 years after treatment.19,21,22 Mayo et al report a risk of RION near-zero for doses <50 Gy and very low for doses <55 Gy. The risk increases from 3% to 7% for doses of 55 to 60 Gy and from 7% to 20% for doses >60 Gy.19,22 Several authors have shown a variability in the incidence of RION depending on the age of the patients.23,24 There seems to be an increased risk of RION in diabetic patients, however the data are not particularly significant.24
Materials and Methods
This retrospective analysis included 25 patients treated between 2013 and 2018 at our institution. Patients had an average age of 57.42±16.03 years at the time of the visit, ranging between 22 and 80 years old. The patients were selected based on the localization of the tumor and whether or not the irradiation plan included the orbital area. Not all patients contacted accepted to participate in the study.
In 8 cases, patients were suffering from orbital lymphoma (the prescription dose was 24 Gy in 2-Gy daily fractions in 6 patients, while 2 patients received a short-course treatment with a prescribed dose of 4 Gy in 2-Gy daily fractions); 9 patients were irradiated with a prescribed dose of 54 Gy in conventional fractioning for cranial base meningioma and 8 patients received a total dose of 70 Gy in conventional fractioning for nasopharyngeal carcinoma. Most treatments were performed with Volumetric Modulated Arc Therapy (VMAT) technique, 3 patients were treated with Three-dimensional Conformal Radiation Therapy (3D-CRT).
Patients with nasopharyngeal carcinoma received 2 or 3 cycles of neoadjuvant chemotherapy (Cisplatin in combination with another drug among Etoposide, Docetaxel, 5-fluoro-uracil, Epirubicin) and concomitant treatment during radiotherapy with 6 or 7 cycles of Cisplatin. It should be noted that for the patients with orbital lymphoma, the orbit was the main target of irradiation, while for nasopharyngeal carcinomas and cranial base meningiomas the target was represented by other areas, classifying the orbit as a simple organ at risk. This difference of the radiation volumes is reflected in the Dmax values to each ocular structure (crystalline lens, eyeball, optic nerve) of each patient. The four radiation treatment plans implemented are shown in the figures (Figure 1A and B), (Figure 2A and B), (Figure 3A and B), (Figure 4A and B). We invited patients to the ophthalmologic evaluation at the SC Oculistica U of the AOU Città della Salute e della Scienza of Turin, where the evaluation took place after an average period of 45.67 ± 21.17 months from the treatment.
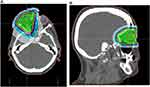 |
Figure 1 (A, B) Treatment plan for 24 Gy orbital lymphoma, transverse and sagittal plane. |
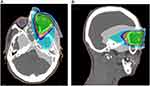 |
Figure 2 (A, B) Treatment plan for 4 Gy orbital lymphoma, transverse and sagittal plane. |
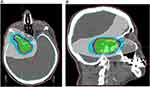 |
Figure 3 (A, B) Example of treatment plan for cranial base meningioma, transverse and sagittal plane. |
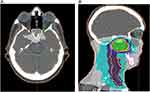 |
Figure 4 (A, B) Example of treatment plan for nasopharyngeal carcinoma, transverse and sagittal plane. |
The ophthalmologic evaluation included the measurement of visual acuity with Snellen boards, slit-lamp observation of the anterior and posterior segment, OCT (Optical Coherence Tomography), color fundus photography, Schirmer I test, Break-up time test (BUT) with fluorescein, Goldmann’s applanation tonometry. The orthoptic evaluation included cover test, ocular motility test, Lang stereo test, red glass test in case of suspected diplopia. The 24–2 computerized visual field and VEP pattern 15ʹ and 60ʹ were also performed. We also used the OSDI (Ocular Surface Dryness Index) questionnaire and a medical history questionnaire produced by us that investigated the complications in the three months immediately following radiotherapy. We calculated the incidence and confidence interval of Dry eye syndrome, and the induced complications in the cornea, the lens, the eyelid, the conjunctiva, as well as in the retina and the optic nerve. We also calculated the mean value, standard deviation and range of BUT, Schirmer value in mm, OSDI score, visual acuity, RNFL, MD and PSD of the computerized visual field. The dose constraints used in the radiation therapy planning were: Dmax 45 Gy for the whole eye, Dmax 54 Gy for the optic nerves, Dmax 5 Gy for the lens, Dmax 54 Gy for the optical chiasm. Based on dose constraints, we compared the data for eyes whose Dmax was higher than dose constraints with those of the eyes whose Dmax was lower than dose constraints, using Wilcoxon-Mann–Whitney tests (for variables not characterized by normal distribution). The parameters compared were visual acuity, Schirmer value, BUT, RNFL, MD and PSD. A comparison was also done in regard to complications incidence, comparing the incidence for eye structure that had received a Dmax higher than dose constraints with those with a lower Dmax, this comparison was performed through Odds ratio. This study was approved by the Ethical Committee “Comitato Etico Interaziendale A.O.U. Città della Salute e della Scienza di Torino – A.O. Ordine Mauriziano di Torino – A.S.L. Città di Torino” (Protocol n° 0033476) and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent to participate in the study.
Results
Dry Eye Syndrome was defined on the basis of the positivity to the Schirmer test (<10 mm) or positivity to the BUT (<5 s) or on the basis of a score >12 on the OSDI questionnaire. Of the total number of patients, the incidence of DES was 76% (CI: 0.64–0.88), affecting 38 eyes out of 50 after an average latency of 45.67±21.63 months. In particular, it had an incidence of 66.67% (CI: 0.40–0.93) in the group of lymphomas irradiated with 24 Gy (average eye Dmax 17.86±9.49 Gy) and 50% (CI: 0.01–0.99) in the group of lymphomas irradiated with 4 Gy (average eye Dmax 2.31±2.20 Gy). In the nasopharyngeal carcinomas group (Average eye Dmax 26.26±17.25 Gy), DES had an incidence of 62.50% (CI: 0.39–0.86), whereas in the meningiomas group (average eye Dmax 15.24±11.06 Gy) it had an incidence of 100% (CI: 1–1). We considered the incidence of DES among the eyes, sorting those eyes that had received a Dmax to the eyeball higher than a 40 Gy cut-off from those whose Dmax was lower than 40 Gy. The resulting Odds Ratio was 2.20. The mean values of Schirmer, BUT and OSDI for each group of tumors and over the total number of patients are shown in Table 1.
 |
Table 1 Mean Schirmer, BUT and OSDI Values Among Our Patients |
Considering 40 Gy as the Dmax to the eyeball cut-off, we compared the Schirmer and BUT value between those patients with Dmax higher than the cut-off and those with lower Dmax. The test used for this comparison was Wilcoxon-Mann–Whitney (the result was p=0.07 for Schirmer values, p=0.31 for BUT values) (Figure 5A and B). The incidence of corneal complications was 46% (IC: 0.32–0.60). In the 24 Gy lymphomas group (average eye Dmax 17.86±9.49 Gy), complications affecting the cornea had an incidence of 33.33% (CI: 0.07–0.60), while in the 4 Gy lymphomas group (average eye Dmax 2.31±2.20 Gy) it was 25% (CI: −0.17–0.67). The incidence in the nasopharyngeal carcinomas group (average eye Dmax 26.26±17.25 Gy) was 37.50% (CI: 0.14–0.61), whereas it was 66.67% (CI: 0.45–0.88) in the meningiomas group (average eye Dmax 15.24±11.06 Gy). We examined the incidence of corneal complications among the eyes, sorting those eyes that had received a Dmax to the eyeball higher than a 40 Gy cut-off from those whose Dmax was lower than 40 Gy. The resulting Odds Ratio was 1.88.
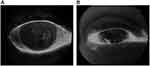 |
Figure 5 (A, B) Eyes with DES. |
The most frequent complication that we found was superficial pointed keratitis, found in 3 eyes belonging to the group of lymphomas, 5 eyes of the nasopharyngeal carcinomas group and 9 eyes of the meningiomas group. Other complications found were bullous keratopathy (2 eyes) corneal epitheliopathy (2 eyes), Leukomas (5 eyes). The patient with bilateral bullous keratopathy is actively treated for it with IVIg at a different center, the patient did not have any known underlying corneal dystrophy, and the keratopathy occurred shortly after the radiation treatment (Figure 6A–D). The incidence of cataract was 54% (CI: 0.40–0.68). In the 24 Gy lymphomas group (average lens Dmax 15.44±11.28 Gy) it was 91.67% (CI: 0.76–1.07), while it was 50% (CI: 0.01–0.99) in the 4 Gy lymphomas group (average lens Dmax 2.13±2.27 Gy). In the nasopharyngeal carcinomas group (average lens Dmax 10.77±11.19 Gy), cataract had an incidence of 50% (CI: 0.26–0.75). The meningiomas group (average lens Dmax 3.72±1.01 Gy) had an incidence of 33.33% (CI: 0.12–0.55). Two of the patients were pseudo-phakic at the time of the visit (3 eyes operated), therefore they were included among the patients with cataracts in the statistical analysis. Considering the incidence of cataract among the eyes, we compared those eyes that had received a Dmax to the eyeball higher than a 5 Gy cut-off with those whose Dmax was lower than 5 Gy. The resulting Odds Ratio was 15.40. In 16% of patients (CI: 0. 06–0.26) there were eyelid complications, with an incidence of 33.33% (CI: 0.07–0.60) in the 24 Gy lymphomas group, 0% in the 4 Gy lymphomas group, 0% in the nasopharyngeal carcinomas group and 22.22% (CI: 0.03–0.41) in the meningiomas group. Chronic blepharitis was the most frequent complication. It was detected in two eyes belonging to the lymphomas group and in four eyes of the meningiomas group. Two eyes belonging to the lymphomas group had ectropion with scleral show. One eye belonging to the meningiomas group had madarosis and distichiasis (Figure 7A–C). The complications of the tear apparatus were not investigated. No patients complained of epiphoras or other symptoms related to potential stenosis of the tear ducts, so no nose-lacrimal exploration or other investigations were performed. No complications were detected in the iris. No patients had iris neovascularization nor ocular hypertension. No patients had radiation retinopathy. No radiation induced optic neuropathy was detected. The intraocular pressure was among normal ranges in all patients and no patient was undergoing any treatment aimed at reducing the intraocular pressure. With the OCT imaging, we calculated the RNFL (Retinal Nerve Fiber Layer) value for each eye. And Mean Deviation (MD) and Pattern Standard Deviation (PSD) were detected with Computerized Visual Field.
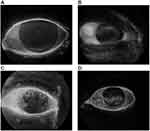 |
Figure 6 (A–D) Eyes with corneal complications. |
 |
Figure 7 (A–C) Eyes with eyelash and eyelid complications. |
The mean values of RNFL, MD and PSD are shown in Table 2. We compared the values of RNFL, MD and PSD among two groups of patients, sorted according to the Dmax received on the optic nerve. We used 54 Gy as the Dmax cut-off (which is the constraint dose for optic nerve). The comparison between those who had received a Dmax higher that 54 and those with lower Dmax was done using Wilcoxon-Mann–Whitney test. The result was p=0.005 for RNFL, p=0.007 for MD and p=0.16 for PSD. We also analyzed how RNFL values changed in relation to the Dmax to the optic nerve, the analysis was done through linear regression and the results are shown in Figure 8A and B. As shown in the tables, the R2 value of the linear regression is 0.21 for the population as a whole, while it raises to 0.72 if we consider the meningiomas group only. The R2 value shows a significant difference in the overall population when the group is evaluated for Dmax to the optic nerve lower or higher than 50 Gy (R2= 0.068 vs. R2=0.35). (Figure 8C). Linear regression analysis was done for MD values as well, showing similar results to the RNFL analysis. The R2 value was higher when we only considered the data concerning those eyes that had received a Dmax to the optic nerve higher than 50 Gy. In particular, the results were R2=0.094 for the whole population and R2=0.24 for the meningiomas population. (Figure 9A and B). As shown in chart 2c and 2d, the R2 value changes dramatically from Dmax lower than 50 Gy to higher than 50 Gy (Figure 9C and D). The mean values of visual acuity for the various groups of neoplasms are shown in Table 3. Among the patients of the meningiomas group, 5 eyes had visual acuity of less than 1 tenth. Of these, one counted the fingers of a hand at a distance of one meter, two eyes distinguished light from shadow, two eyes were unable to distinguish light from shadow. In the statistics, 0 was considered as the visual acuity for these patients. The patients have nevertheless undergone the examination with visual field testing, showing the total loss of field view. We compared the values of visual acuity among two groups of patients, sorted according to the Dmax received on the eyeball, using 40 Gy as the cut-off. The comparison between those who had received a Dmax higher than 40 and those with lower Dmax was done using Wilcoxon-Mann–Whitney test, resulting in a p-value of 0.52. Visual evoked potentials showed some alterations in 4 eyes of the group of lymphomas irradiated with a total dose of 24 Gy, 4 eyes in the group of nasopharyngeal carcinomas and 4 eyes in the group of meningiomas. The complications found were decreased amplitude of evoked potential and increased latency of transmission.
 |
Table 2 Mean RNFL, MD, PSD Values Among Our Patients |
 |
Table 3 Mean Visual Acuity Among Our Patients |
The orthoptic evaluation showed a slight deficit of one or more extrinsic ocular muscles in 4 eyes of the group of lymphomas irradiated with 24 Gy, while two patients had diplopia. The deficient muscles were the lateral rectus muscle for the first eye, the medial rectus muscle and inferior oblique muscle for the second eye, the medial rectus muscle, superior rectus muscle and inferior oblique muscle for the third eye and the medial and lateral rectus muscles for the fourth eye. These muscles corresponded to those infiltrated by neoplasm. There were no muscle deficits in the group of lymphomas irradiated with 4 Gy and no diplopia was present in any of the patients. In the group of nasopharyngeal carcinomas, one eye had a deficiency of an extrinsic ocular muscle (lateral rectum) and the same patient had diplopia. In the group of meningiomas, 5 eyes had a deficiency of one or more extrinsic muscles. One patient had diplopia. The deficient muscles were the small oblique for the first eye, the lower rectum for the second eye, the lateral rectum for the third eye and the upper rectum for the fourth and fifth eye. Four patients in the meningioma group could not be evaluated for diplopia as monocles.
Discussion
Technical and nontechnical factors may influence the incidence of radiation-induced ocular complications. Technical factors are fraction size, length of treatment, fractionation, type of radiation, radiosensitivity of the irradiated tissues, and the age of the patient. Non-technical factors included the presence of comorbidities in the compromised tissue. Concurrent chemotherapy or targeted therapy may also alter radiation tolerance.
Variations in the radiation dose, fractionation schedules, and the use or not of protective measures make it difficult to define and compare the incidence and severity of ocular morbidity. In addiction, a wide spectrum of effects can be observed depending on the histologic type of tumor, latent period, tumor volume, and location of tumor with respect to adjacent structures. The age of the patients, the associated medical condition, and co-treatment with chemotherapy also influence these effects considerably.
With these limits, our study showed an incidence of Dry Eye Syndrome higher than 60% for groups that had received a Dmax to the eye higher than 15 Gy. This results are consistent with other results in literature.2,15 Hsu et al, in fact, reports the onset of DES in 63% of the 20 patients irradiated for palpebral tumors with prescribed doses ranging from 26 to 63 Gy (median 60 Gy),2 while Kennerdell et al reports the results of a study in 54 orbital lymphoma patients irradiated with a total dose of 24 Gy in conventional fractioning. Acute DES was found in 50% of patients during the study, with chronic persistence of DES in 33%.15
In particular, we analyzed how the DES incidence changed when the eye was subjected to a Dmax higher than 40 Gy, showing an Odds Ratio of 2.20. The OR confirms the significant role of radiation higher than 40 Gy in causing DES onset.
The overall incidence of DES in our sample was 76%. The data are difficult to compare with those in the literature, as other studies in the literature take into account very heterogeneous populations in terms of dose received.
One study worth reporting is that of Bhandare et al, who considers 78 patients irradiated for various head and neck neoplasms with total prescribed doses ranging from 20 to 70 Gy. Of the 78 irradiated patients, 40 (51.2%) developed DES and all patients with DES experienced keratitis. The incidence of DES increased progressively from 6% for doses of 35–39.99 Gy to 50% for doses of 45–49.99 Gy, up to 90% for doses of 60–64.99 Gy. None of the patients who had DES had received a dose of <35 Gy. The latency between irradiation and onset of DES was also variable depending on the total dose received and the type of fractioning they had undergone. In particular, they observed a median latency of 1.2 years for patients undergone a once-daily fraction with less than 1.8 Gy/fraction, while median latency was 0.4 years for doses higher than 1.8 Gy/fraction.11
While comparing Schirmer values between the eyes irradiated with Dmax lower and higher than 40 Gy, a non-significant difference emerged, with a p=0.07 in the Wilcoxon-Mann–Whitney test, this could imply a trend, but it does not reach the statistical significance level. The p-value was not significant for the BUT value comparison (p=0.31).
OSDI questionnaires reached the highest scores in the meningioma group, followed by 24 Gy lymphomas and nasopharyngeal carcinomas. The severe dryness, determined by a score >33, was found in four out of nine patients of the meningioma group, while only one out of six patients for the group of lymphomas irradiated with 24 Gy and one out of eight for the group of nasopharyngeal carcinomas, confirming the tendency of the group of meningiomas to show a more severe dryness (as shown by the incidence values too).
With regard to corneal lesions, the highest incidence was in the group of meningiomas, followed by nasopharyngeal carcinomas and lymphomas. Patients with meningioma and nasopharyngeal carcinoma were also those with the most severe complications (epitheliopathy, bullous keratopathy, leucomas), while the group of lymphomas had mainly SPK. Incidence in the group of lymphomas irradiated with 4 Gy was not considered statistically significant due to the confidence interval (−0.17–0.67), presumably due to the small sample size. The overall incidence in our sample was 46%.
Our results showed much higher incidences of corneal complications than in Muller et al25 Muller et al, in fact, reported an incidence of 9.8% of keratitis in 102 patients irradiated with 50 Gy dose, in our sample we found an incidence of 37.50% in patients irradiated with 70 Gy and 66.67% in patients irradiated with 54 Gy. Not only were the incidences much higher in comparable groups in terms of received dose, but also in the group of lymphomas irradiated with 24 Gy there were higher incidences.
The incidence comparison between those eyes irradiated with a Dmax lower than 40 Gy and those with Dmax higher than 40 Gy showed an OR=1.88, confirming a significantly higher incidence of corneal complications when Dmax is higher than 40 Gy.
Cataract incidence showed a strong correlation with the dose received. The group most affected was in fact the group of lymphomas irradiated with 24 Gy (Dmax to the lens 15.44±11.28 Gy) and had a 54% incidence, the incidence for the nasopharyngeal carcinoma was 50% (Dmax to the lens 10.77±11.19 Gy), while the group of meningiomas was the one with the lowest incidence (Dmax to the lens 3.72±1.01 Gy), with 33.33% incidence. The incidence in the groups of lymphomas irradiated with 4 Gy was not considered significant due to the extremely large CI (0.01–0.99).
The incidence in the eyes with lens Dmax lower than 5 Gy (lens constraint dose) and the incidence in the eyes with lens Dmax higher than 5 Gy was significantly different, with an extremely high OR value (OR=15.40).
Of the three pseudo-phakic eyes at the time of examination, one eye belonged to a 43-year-old patient with orbital lymphoma irradiated with 24 Gy who had been operated on at the age of 42. The eye concerned was the eye that had undergone radiotherapy (Dmax to the lens 25.7 Gy). The age of the patient makes the detection of cataracts particularly significant. The contralateral eye was also affected by initial sclerosis of the lens.
Stafford et al, who evaluated 48 patients irradiated with a dose of 27 Gy, identified 8 patients with cataract (16.7%).16 Comparing these results with those obtained by us for lymphomas irradiated with 24 Gy, our only group with a comparable dose, shows that in our sample the incidence is significantly higher (96.67%). It is not clear what was the average age in the study by Stafford et al, so it is not possible to clarify whether the difference was due to the different average age of the samples.
Eyelid complications were only present in the groups of meningiomas and lymphomas irradiated with 24 Gy, but the incidences were considered not statistically significant due to large confidence intervals, the overall incidence was 16%. The most important eyelid complication was found in a patient with meningioma, who on the irradiated side had distichiasis and madarosis, complications described in the literature,14 while the contralateral eye had no complication. Jeganathan et al showed the onset of madarosis and distichiasis only for doses greater than 50 Gy,14 our data are in line with what has been reported. The study did not detect any complications in the lacrimal drainage system, iris and retina. Our results were not in line with those of the literature, radiation retinopathy was in fact described in 50% of patients irradiated with doses of 60 Gy, while in our study was not found any. No RION was detected, although, three eyes belonging to the meningioma group showed anomalies in the thickness of the papilla at OCT. The review by Mayo et al reported 0 cases of RION in irradiated patients with less than 50 Gy, 3–7% in those irradiated with 55–60 Gy and 7–20% in those irradiated with more than 60 Gy.22 Our results cannot be considered concordant, because in no group, even in those with a dose of 54 and 70 Gy, there were cases of RION. However, in the meningioma group, we found alterations in the thickness of the optic nerve.
The mean value of RNFL was in fact in the ranges (>95 µm) for all groups of neoplasms, except for meningiomas, which had a lower mean RNFL value than the other groups. The meningioma group also showed a difference in mean RNFL between those eyes that had received a Dmax to the optic nerve lower than 54 Gy and those with an optic nerve Dmax higher than 54 Gy. The Wilcoxon-Mann–Whitney test provided statistically significant results for RNFL (p=0.005) and for MD values (p=0.007), while it was not significant for PSD (p=0.16). The linear regression analysis was particularly significant, as it shows an important difference in R2 value once the cut off of 50 Gy to the optic nerve is exceeded (R2= 0.068 for optic nerve Dmax lower than 50 Gy vs. R2=0.35 for optic nerve Dmax higher than 50 Gy). A similar result was found in the regression analysis of the MD values (R2= 0.00025 for optic nerve Dmax lower than 50 Gy vs. R2=0.56 for optic nerve Dmax higher than 50 Gy). The visual fields showed the values of MD and PSD mostly deviated within the group of meningiomas (mean MD −4.46±13.83 dB, mean PSD 3.63±3.40 dB). The reduction of the mean RNFL that we noticed was mostly due to an overall thinning of all the quadrants. A few patients presented a slight focal thinning limited to one or two quadrants, but that did not lead to any significant reduction of the mean RNFL nor to a matching focal field deficit, so these results were not analyzed more thoroughly. The p-value of the Wilcoxon-Mann–Whitney test for visual acuity was not statistically significant (p=0.52). However, in the meningioma group we detected a lower mean visual acuity (5.89±4.34 tenths) than in the other groups, result that is consistent with the higher Dmax received on the eyeball (38.11±17.83 Gy). The VEPs showed alteration in terms of latency or amplitude of signal transmission in 12 to 50 eyes, but we do not consider the tests to be particularly significant due to the absence of a baseline. In addition, the altered tests were characterized by a high number of fixation losses. None of the studies that we researched in literature took into account the results of visual fields and evoked potentials. A comparison with the literature data is therefore not possible. Our study has some inherent limitations. A limitation is the retrospective way and the lack of a baseline reference. Patients, in fact, had not undergone an ophthalmological and orthoptic evaluation before receiving radiation therapy. This has prevented a systematic evaluation and comparison of the pre- and post-radiotherapy status of patients. The study also lacks a control group, due to its retrospective nature. For this reason, we chose to set a Dmax cut off and compare the complications between those eyes that had received a Dmax higher than the cut off or lower through statistical analysis. Another limitation of our study is the small sample, although this is in line with that of many other studies already published in the literature. The 4 Gy lymphoma group was the smallest, with only 2 patients, often leading to non-significant results with regard to that group. Another important limitation is the heterogeneity of pathologies considered in the study. The three neoplasms considered had very different treatment volumes, with the orbit being the main target for the lymphoma treatment, while in the nasopharyngeal carcinomas and in the meningiomas the orbit was an organ at risk, having the main target located in different areas (nasopharynx and cranial base). Consequently, the ocular structures had very different Dmax depending on the pathology and the volume of irradiation for each patient.
Most severe radiation-induced ocular complications such as cataracts, glaucoma, and retinopathy are late effects. The latency period ranges from a few months to several years, depending on the total radiation dose, fraction size, radiation field, and fractionation. A limitation of our study is the short follow-up.
In conclusion, our study confirms a correlation between the radiation dose received and the onset of ocular complications. The linear regression analysis showed how the RNFL and MD values drastically changed after a 50 Gy Dmax cut off. The OR were particularly significant as well, showing how the incidence of complications changed after exceeding the radiation doses that are usually unanimously considered safe.
Finally, it is important to emphasize how the problem of potential radiation-induced complications on the eye should be contextualized in terms of risk-benefit ratio. Sorour et al reported the case of a woman with choroidal melanoma previously treated with plaque brachytherapy, which has relapsed. The therapeutic choice for the relapse was between an enucleation and GKR (Gamma Knife Radiosurgery), the second one was chosen, allowing a better quality of life for the patient, despite the potential hazard risk. The GKR dose planning was done taking into account the previous treatment, in order to reduce the risk of potential complications.26
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
No potential conflict of interest was reported by any of the authors.
References
1. Singh AD, Damato B. Ocular complications of radiotherapy. In.: Clinical Ophthalmic Oncology: Basic Principles and Diagnostic Techniques.
2. Hsu A, Frank SJ, Ballo MT, et al. Postoperative adjuvant external-beam radiation therapy for cancers of the eyelid and conjunctiva. Ophthal Plast Reconstr Surg. 2008;24(6):444–449. doi:10.1097/IOP.0b013e31818be098
3. Astradsson A, Wiencke AK, Munck Af Rosenschold P, et al. Visual outcome after fractionated stereotactic radiation therapy of benign anterior skull base tumors. J Neurooncol. 2014;118(1):101–108. doi:10.1007/s11060-014-1399-0
4. Astradsson A, Munck Af Rosenschöld P, Feldt-Rasmussen U, et al. Visual outcome, endocrine function and tumor control after fractionated stereotactic radiation therapy of craniopharyngiomas in adults: findings in a prospective cohort. Acta Oncol (Madr). 2017;56(3):415–421. doi:10.1080/0284186X.2016.1270466
5. Dry Eye Workshop (DEWS) Committee. 2007 report of the Dry Eye WorkShop (DEWS). Ocul Surf. 2007;5(2):63–204.
6. Gordon KB, Char DH, Sagerman RH. Late effects of radiation on the eye and ocular adnexa. Int J Radiat Oncol Biol Phys. 1995;31(5):1123–1139. doi:10.1016/S1278-3218(97)82945-9
7. Stephens LC, Schultheiss TE, Peters LJ, Ang KK, Gray KN. Acute radiation injury of ocular adnexa. Arch Ophthalmol. 1988;106(3):389–391. doi:10.1001/archopht.1988.01060130415032
8. Barabino S, Raghavan A, Loeffler J, Dana R. Radiotherapy-induced ocular surface disease. Cornea. 2005;24(8):909–914. doi:10.1097/01.ico.0000154235.64359.d3
9. Woo YJ, Ko J, Ji YW, Kim T, Yoon JS. Meibomian gland dysfunction associated with periocular radiotherapy. Cornea. 2017;36(12):1486–1491. doi:10.1097/ICO.0000000000001377
10. Behrens A, Doyle J, Stern L. Dysfunctional tear syndrome. A delphi approach to treatment recommendation. Cornea. 2006;25:900–907. doi:10.1097/01.icu.0000512373.81749.b7
11. Bhandare N, Moiseenko V, Song WY, Morris CG, Bhatti T, Mendenhall WM. Severe dry eye syndrome after radiotherapy for head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2012;82(4):1501–1508. doi:10.1016/j.ijrobp.2011.05.026
12. Petsuksiri J, Frank SJ, Garden AS, et al. Outcomes after radiotherapy for squamous cell carcinoma of the eyelid. Cancer. 2008;112(1):111–118. doi:10.1002/cncr.23143
13. Durkin SR, Roos D, Higgs B, Casson RJ, Selva D. Ophthalmic and adnexal complications of radiotherapy. Acta Ophthalmol Scand. 2007;85(3):240–250. doi:10.1111/j.1600-0420.2006.00822.x
14. Jeganathan VSE, Wirth A, MacManus MP. Ocular risks from orbital and periorbital radiation therapy: a critical review. Int J Radiat Oncol Biol Phys. 2011;79(3):650–659. doi:10.1016/j.ijrobp.2010.09.056
15. Kennerdell JS, Flores NE, Hartsock RJ. Low-dose radiotherapy for lymphoid lesions of the orbit and ocular adnexa. Ophthalmic Plast Reconstr Surg. 1999;15(2):129–133. doi:10.1097/00002341-199903000-00012
16. Stafford SL, Kozelsky TF, Garrity JA, et al. Orbital lymphoma: radiotherapy outcome and complications. Radiother Oncol. 2001;59(2):139–144. doi:10.1016/S0167-8140(00)00328-5
17. Gupta A, Dhawahir-Scala F, Smith A, Young L, Charles S. Radiation retinopathy: case report and review. BMC Ophthalmol. 2007;7(6). doi:10.1186/1471-2415-7-6
18. Evans J, Sivagnanavel V, Chong V. Radiotherapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2010;1–90. doi:10.2217/FMEB2013.13.272
19. Danesh-Meyer HV. Radiation-induced optic neuropathy. J Clin Neurosci. 2008;15(2):95–100. doi:10.1016/j.jocn.2007.09.004
20. Miller NR. Radiation-induced optic neuropathy: still no treatment. Clin Exp Ophthalmol. 2004;32(3):233–235. doi:10.1111/j.1442-9071.2004.00809.x
21. McClellan RL, El Gammal T, Kline LB. Early bilateral radiation-induced optic neuropathy with follow-up MRI. Neuroradiology. 1995;37(2):131–133. doi:10.1007/BF00588629
22. Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76(3):S28–S35. doi:10.1016/j.ijrobp.2009.07.1753
23. Parsons JT, Bova FJ, Ph D, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30(4):765–773. doi:10.1016/0360-3016(94)90347-6
24. Bhandare N, Monroe AT, Morris CG, Bhatti MT, Mendenhall WM. Does altered fractionation influence the risk of radiation-induced optic neuropathy? Int J Radiat Oncol Biol Phys. 2005;62(4):1070–1077. doi:10.1016/j.ijrobp.2004.12.009
25. Muller K, Naus N, Nowak PJCM, et al. Fractionated stereotactic radiotherapy for uveal melanoma, late clinical results. Radiother Oncol. 2012;102(2):219–224. doi:10.1016/j.radonc.2011.06.038
26. Sorour OA, Mignano JE, Duker JS. Gamma knife radiosurgery for locally recurrent choroidal melanoma following plaque radiotherapy. Int J Retina Vitreous. 2018;4(23):1–5. doi:10.1186/s40942-018-0123-1
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


