Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Observation on the Efficacy of a Combined Treatment for Moderate and Severe Androgenetic Alopecia Incorporating Electric Microneedles
Authors Zhang F, Yang YN, Feng JD, Zhao JH, Wan L, Che J, Yan Y, Guo NN, Zhang JY
Received 26 July 2022
Accepted for publication 10 November 2022
Published 29 November 2022 Volume 2022:15 Pages 2573—2581
DOI https://doi.org/10.2147/CCID.S383289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Fan Zhang,1 Yi-Nuo Yang,2 Jin-Di Feng,2 Jing-Hui Zhao,1 Li Wan,1 Jing Che,1 Ying Yan,1 Ning-Ning Guo,1 Jia-Yu Zhang1
1Hair Medical Research Center, Department of Dermatology and Venereology, Beijing Jishuitan Hospital, Beijing, People’s Republic of China; 2School of Clinical Medicine, Peking University School of Medicine, Beijing, People’s Republic of China
Correspondence: Fan Zhang, Hair Medical Research Center, Department of Dermatology and Venereology, Beijing Jishuitan Hospital, No. 31 of Xinjiekou East Street, Xicheng District, Beijing, 100035, People’s Republic of China, Tel +86 10 58516688, Email [email protected]
Objective: To evaluate the effectiveness and safety of a combined treatment for moderate and severe androgenetic alopecia (AGA) involving the use of electric microneedles.
Methods: A total of 83 patients with moderate to severe AGA in the Department of Dermatology at Beijing Jishuitan Hospital were included in this study. The male patients were administered finasteride orally and 5% minoxidil for external use, while the female patients were given spironolactone orally or Diane-35 and 2% minoxidil for external use. All the patients were then treated via electric microneedle therapy alongside the YUFA ®medical care package (Foshan, China) once a week for 1– 28 weeks. The seven-point method and root hair measurement using a hair mirror were adopted to evaluate the efficacy and any adverse reactions of the combined treatment.
Results: Eleven patients were treated for 1– 3 weeks, 60 for 4– 12 weeks, and 12 for more than 12 weeks. The efficacy evaluation using the seven-point method for 12 weeks of treatment indicated a 100% response rate, specifically, a 42.1% mild improvement rate, a 38.6% moderate improvement rate, and a 19.3% marked improvement rate. Besides, the efficacy assessment was also completed with root hair count method and the number of hair roots measured at fixed points were 148.67± 11.15, 158.13± 5.11 and 169.75± 2.06 after treatment time at 16, 20 and 24 weeks, respectively. Of note, a statistical difference in the number of hair roots could be observed during the period of week 20–week 24 (P < 0.01).
Conclusion: The combined treatment of moderate to severe AGA using the electric microneedle technique has a clear effect and can effectively increase the hair density. With a simple operation and mild side effects, the technique has wide application prospects.
Keywords: electric microneedles, androgenic alopecia, combined therapy, efficacy observation
Introduction
Androgenetic alopecia (AGA), an androgen-mediated hair loss characterized by progressive hair follicle miniaturization and shortening of hair growth period, is the most common type of progressive hair loss in men. The clinical symptoms are mainly manifested as a reduction in hair density on both sides of the forehead, hair thinning, gradual extension to the top of the head, and the retreat of the forehead hairline, resulting in an M-shaped hairline,1,2 which led to negative affection of patient’s quality of life and caused them to seek treatments.3,4
Various approaches are currently available for the treatment of AGA, including oral administration of finasteride and spironolactone, topical application of minoxidil, phototherapy, microneedle-based techniques, and hair transplantation. The common strategy drugs are minoxidil and oral finasteride in clinic, which have been approved by the US Food and Drug Administration for the treatment of male androgenetic. Although some patients experience significant hair regrowth intervened by the above common strategies, others simply slow the process of hair loss.5
Electric microneedle, a minimally invasive treatment technique, is a thin needle used to pierce the epidermis and the superficial layers of the dermis on the scalp, which could physically disrupt the stratum corneum of the skin, following by creating temporary and water-laden cavities that facilitate the entry of drug molecules into the epidermis.6 By impairing the integrity of skin barrier, electric microneedles could induce the separation of keratin-forming cells, thus causing the release of various cytokines7,8 such as IL-1a, IL-8, IL-6, TNF-A and granulocyte-macrophage colony stimulating factor (GM-CSF). This will further give rise to dilated blood vessels and keratin-forming cell migration, differentiation as well as proliferation in the dermis to repair the damage of the epidermal layer.9 The healing process after microneedling trauma is divided into three stages: injury, healing and maturation.10 Additionally, multiple studies have reported that as a safe and effective adjunctive therapy for androgenetic alopecia, microneedling treatment performs beneficial effects on promoting hair growth and enhancing the penetration of local therapies.11,12 Therefore, this study aims to evaluate the efficacy and safety of a combined treatment for moderate and severe AGA incorporating electric microneedle techniques.
Materials and Methods
Clinical Information
General Data
A total of 83 patients treated in the outpatient Department of Dermatology at Beijing Jishuitan Hospital were included in the study. Among them, 56 were male and 27 were female, with an age range of 19–62 years (average age = 30.96 ± 7.12 years). The hair of AGA female patients was mainly characterized by diffusedly thinning and soft in the top of the head and the hairline margin and showed no change in the position of the forehead hairline, which usually also accompanied by the symptoms of increased scalp oil secretion.13
The group included 69 cases with a moderate basic and specific (BASP, a system to classify the types of pattern hair loss. In detail, the basic types and specific types represent the shape of the anterior hairline and the density of hair on frontal and vertex areas,14 respectively.) rating and 14 cases with a severe BASP rating. The Hamilton rating was also applied to the patients who had been treated for more than 12 weeks, with the rating found to be above Grade 3a and all the patients found to present typical clinical manifestations.
Inclusion and Exclusion Criteria
The following inclusion criteria applied:
- Patients without local use of germinal drugs (eg, minoxidil) or other local treatments four weeks prior to the initial diagnosis.
- Patients not administered with medication involving systemic germinal or antiandrogenic effects for eight weeks prior to the initial visit.
Meanwhile, the following exclusion criteria applied:
- Pregnant patients or patients in lactation.
- Patients with a history of heart or liver disease, renal insufficiency, hypertension, tumors, or nervous/mental illness.
- Patients with other types of alopecia, such as alopecia areata, hair loss induced by autoimmune thyroid disease and systemic lupus erythematosus, or alopecia caused by other factors (eg, drugs).
- Patients with hair follicle atrophy resulting in a loss of hair growth function.
- Patients having received minoxidil or other drugs that may affect hair growth within 24 weeks.
- Patients who demonstrate poor drug compliance and cannot tolerate drug treatment.
Methods
Treatments
The male patients were treated with finasteride (Axelfin, 1 mg/d) orally, 5% minoxidil (Dafeixin, standard content = 5%, dosage = 1 mL each time, BID) topically and electric microneedle therapy using a F6 electric microneedle instrument (Bohui Meizui Bioengineering Technology Co. Ltd., Guangdong) alongside a medical care package, which was administered once a week with continuous 20 min each treatment. The female patients were given spironolactone (Spironolactone tablets, specification content = 20 mg/tablet, dosage = 1 tablet/time, TID) or ethinyl estradiol cyproterone tablets (trade name: Diane-35, specification content = cyproterone acetate [2 mg] and ethinyl estradiol [0.035 mg], dosage = 1 tablet/day, continuous administration for 21 days), and topical 2% minoxidil liniment (Dafeixin, specification content concentration). The above treatments were terminated when the patient’s hair volume was deemed to be satisfactory.
Efficacy Evaluation
The patients were treated once a week for 15–20 min each session, and the efficacy and any adverse reactions were evaluated every four weeks during the treatment period.
After 12 weeks of treatment, a comparison and evaluation were performed using the seven-point method with comprehensive consideration of head-top photos and microscope tests before and after the treatment. Here, a marked improvement is denoted by a score of +3, which indicates that compared with before the treatment, the number of hairs in the alopecia area tended to be normal. A moderate improvement is denoted by a score of +2, indicating that significant hair regeneration can be observed at the alopecia area, while a mild improvement is denoted by a score of +1, indicating significant vellus hair growth in the alopecia area. No change is indicated by a score of 0, meaning there is no change following treatment. Meanwhile, mild aggravation is denoted by a score of −1, indicating that the area of hair loss increased significantly following treatment, while moderate aggravation is denoted by a score of −2 and marked aggravation by a score of −3. Here, the percentages of +1, +2, and +3 points were totaled to serve as the effective rate.
After more than 12 weeks of treatment, the hair on the patient’s fixed area was counted using the hair mirror technique.
Statistical Analysis
The statistical analysis was conducted using SPSS 27.0 software for data entry and collation, while the χ2 test, Wilcoxon test, paired t-test, and single-factor analysis of variance test were used for the analysis. A P-value of <0.01 was considered to be statistically significant.
Results
Treatment Course and Basic and Specific Rating
The 83 patients underwent the treatment for between 1 and 28 weeks, with 11 undergoing treatment for 1–3 weeks, 60 for 4–12 weeks, and 12 for more than 12 weeks. There were 69 cases with a moderate BASP rating, with the patients undergoing 4–12 weeks of treatment accounting for 76.8% and those undergoing 12 weeks or more of treatment accounting for 8.7%. A total of 14 cases involved a severe BASP rating, with the patients undergoing 4–12 weeks of treatment accounting for 92.8% and those undergoing 12 weeks or more of treatment accounting for 50%. The number of completed treatments between the moderate and severe BASP rating groups entailed a statistically significant difference (P = 0.002, P < 0.01). (Figure 1)
 |
Figure 1 BASP rating and weeks of treatment. The χ2 test was used for data analysis. **p < 0.01 compared with severe BASP rating groups. |
Efficacy Evaluation, Treatment Course, and Basic and Specific Rating
Efficacy Evaluation
The evaluation using the seven-point method for 12 weeks of treatment with the 83 patients indicated a 100% response rate. Shown as Table 1, marked improvement rate was up to 19.3% while mild and moderate improvement rate were 42.1% and 38.6%, respectively. Additionally, these cases with a score of 0 or less were not listed.
 |
Table 1 Efficacy Evaluation of 7-Point Method for 1–12 Weeks of Treatment |
Efficacy Evaluation and Treatment Course
According to the seven-point method for evaluating the efficacy after 12 weeks of treatment, the treatment lasted for 8 > week number ≥4, and the moderate or above improvement accounted for 17.9%; 12 > week number ≥8, moderate or above improvement accounted for 96.8%; week number ≥12, moderate and above improvement accounted for 100%. There were no statistical differences between the groups with 4 > week number ≥1 and 8 > week number ≥4 (P > 0.01), but there were significant differences among the other groups.
A total of 12 patients were treated for more than 12 weeks, and their number of hair roots was measured using a hair microscope. As shown in Table 2, the number of hair roots of patients increased to (148.67±11.15) roots after combined treatment for 16 weeks and a continuous increase of the number of hair roots in patients could be found with the extension of treatment time. Additionally, following treatment for more than 12 weeks, the evaluation of the hair root count procedure indicated a statistical difference in hair root count during the period of week 20–week 24 (P < 0.01) .(Table 2 and Figure 2)
 |
Table 2 Number of Hair Roots Measured by Hair Mirror Between 16 and 24 Weeks of Treatment |
 |
Figure 2 Relationship between treatment times and total efficacy evaluation. Wilcoxon test was used for data analysis. **p < 0.01 represented significant difference between the compared two groups. |
Efficacy Evaluation and Basic and Specific Rating
In the evaluation of the efficacy of the treatment after 12 weeks among the 83 patients, there was no significant difference between the moderate and severe BASP rating groups (P > 0.01) (Table 3 and Figure 3).
 |
Table 3 Relationship Between Disease Severity and Efficacy Evaluation in Each Treatment Times Group |
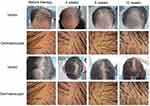 |
Figure 3 Comparison among patients with alopecia in terms of head Top hair volume and dermoscopy during treatment. |
Adverse Reactions
During the observation period, the main adverse reactions observed among the patients were pain or desquamation in the microneedle treatment area, while most of the pain subsided naturally within 4–6 hours. In terms of desquamation, measures such as washing the hair with warm water and adding specific scalp repair products could be administered to improve the condition. During this procedure, no allergic reactions were observed among the patients.
Discussion
Currently, there are about 250 million people suffering from hair loss in China, 70% of whom are AGA. Therapeutic strategies for moderate to severe AGA usually included topical and oral medication, with a course of 1 year or more. Especially for female alopecia patients, the course of hair loss treatment is more than 1.5 years. The slow treatment efficiency and too long course of treatment remain major limitations of the treatment, which made high-efficiency and safe treatment methods urgent.
In the last decade, microneedles have been increasingly used in hair loss treatment due to its important part in enhancing transdermal delivery and improving dermal papillae stem cell proliferation.15–17 Since Dhurat et al conducted a landmark study of 100 AGA subjects and found a significant increase in hair volume with once weekly microneedle combined with twice daily 5% minoxidil as compared to minoxidil monotherapy over a 12-week period,12 more and more studies have been carried out and demonstrated that microneedle-based therapy could promote hair growth and enhance the permeability of local therapy. The above study evidences suggested that microneedle-based therapy is a safe and effective adjuvant therapy for AGA.7,18–20
There are two possible mechanisms for hair growth: regulation of the hair cycle and regeneration of new hair follicles.21 Microneedle therapy does not result in the creation of full-thickness wounds, and research has indicated that it involves microtrauma that stimulates the Wnt/β-catenin pathway, thereby promoting hair growth.22 Meanwhile, Kim et al17 found that repeated microneedle stimulation induced hair growth in their mouse model, generating increased expressions of Wnt3a, β-catenin, vascular endothelial growth factor (VEGF), and Wnt10b mRNA and protein, with both the Wnt/β-catenin signaling pathway and VEGF playing a role in promoting the growth cycle. As such, the main mechanism of microneedle-based treatment is potentially the regulation of the hair cycle rather than the regeneration of new hair follicles.
In fact, studies have found that microneedles work by releasing growth factors and activating dermal papilla-related stem cells. The slight physical trauma caused by the microneedles can induce collagen formation and form pore channels to enhance local drug penetration.23 In percutaneous collagen induction, the tissue response is highly dependent on the depth and density of the perforations, and different approaches of micro-acupuncture insertion will also trigger different skin physiological responses. Furthermore, the microneedles promote continuous epidermal thickening, normalize the polarity of the nuclei at the basal layer, reduce the degeneration of solar elastic tissue, and increase the collagen types I, III, and IV (a component of the basement membrane).24 Acupuncture can also destroy and revascularize an old and hardened cicatrix, while the migration and proliferation of fibroblasts can induce the formation of new blood vessels and new collagen.25
Meanwhile, microneedle-based therapy can promote drug delivery and increase the absorption of different compounds by the skin. In fact, the main limitation of topical drugs relates to poor percutaneous absorption. Here, the stratum corneum acts as a physical barrier to molecules with a molecular weight of >500 and is rich in lipids and ceramides, which limits the permeability of hydrophilic drugs.26 Microneedle therapy can induce mechanical changes in the keratinous barrier, forming micron-sized channels in the skin to transport a variety of molecules,27–29 including protein, and improving the absorption rate of macromolecules by up to 80%.30 Given that these microchannels naturally close after 10 min, leaving the epidermal barrier intact, postoperative infection does not occur.31 The microneedles also remove any scales and sebum residue from the infundibular portion of the hair follicles, allowing more effective penetration of the lipid product.31
Clearly, the use of microneedles for promoting hair regeneration does not only involve a drug delivery system, which would effectively present an efficacy threshold. In fact, the attendant mechanisms for inducing hair regeneration include the following:
- Initiation of the overexpression of hair growth-related genes (eg, VEGF, β-catenin, Wnt3a, and Wnt10b),16 regulation of the hair cycle, stimulation of the dermal papilla, and promotion of hair growth.11,30,32
- Damage of the integrity of the skin barrier, and the release of cytokines (IL-1α, IL-8, IL-6, TNF-α, and GM-CSF) together with neovascularization and keratinocyte migration.11
- Wound healing reaction.33,34 Following microneedle treatment, platelet-derived growth factors are released to increase the epidermal growth factor level and promote hair growth through the mechanisms of platelet activation and skin wound regeneration. The microenvironment of wound healing formed following microneedle treatment activates the stem cells in the hair follicle area. Neovascularization occurs in the area of the injury, which is beneficial for hair growth.12
- Transdermal route of administration to promote drug penetration and absorption.31,35
Following the administration of our combined treatment, the AGA patients had a 100% response rate after 1–12 weeks of treatment. In terms of improvement, with the increase in the number of treatments, the efficacy gradually became significant. Specifically, for the 44 patients who underwent treatment for eight weeks or more, the proportion of cases of marked improvement increased from 0% to 36.4%, indicating that patients with moderate to severe AGA should undergo the combined treatment for at least eight weeks. The thirteen patients who completed the treatment for 12 weeks or more had a marked improvement of 100%, again indicating the effectiveness of a sufficient course of the combined treatment. Eleven (91.7%) of the 12 patients who were treated for more than 12 weeks discontinued the treatment after achieving satisfactory results following 16–24 weeks of treatment, which indicates that the treatment course for the majority of moderate to severe AGA patients should be between 16 and 24 weeks. In the patients who received 20 weeks or more of treatment, the number of hair shafts had reached 150 or more by the end of the treatment, suggesting that moderate to severe AGA patients could obtain relatively satisfactory therapeutic effects with the combined treatment.
Conclusion
In this study, by a 28-week drug intervention combined with electric microneedling treatment in 83 patients, we found that most patients could recover the number of hairs of that of their peers, ie, more than 150 mature hair, in 20–24 weeks of combined treatment. A few patients with more severe AGA could also keep hair volume consistently recovered by increasing the treatment time until the hair volume reached to their own satisfaction. The medication combined with electric microneedling strategy not only greatly improves the treatment efficacy and shortens the treatment course for patients with moderate to severe AGA, but also helps to increase the frequency of doctor–patient communication and optimize the doctor–patient relationship. In a nutshell, medication combined with the use of electric microneedles for moderate to severe AGA could effectively increase hair density and exhibit attractive advantages including simple operation and mild side effects, suggesting this combined treatment strategy has a wide application prospect.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Beijing Jishuitan Hospital. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Funding
2021 Beijing Jishuitan Hospital Natural Fund Cultivation Program (No.ZR202112).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang JZ. Guideline for diagnosis and treatment of androgenetic alopecia. J Clin Dermatol. 2014;43(03):182–186. Chinese. doi:10.16761/j.cnki.1000-4963.2014.03.025
2. Sheng RY, Yan Y, Linh Dang HH. Acupuncture for hot flashes: a literature review of randomized controlled trials conducted in the last 10 years. World J Tradit Chin Med. 2021;7:397–407. doi:10.4103/wjtcm.wjtcm_27_21
3. Park S, Kim IR, Baek KK, et al. Quality of life assessment in male patients with androgenetic alopecia: result of a prospective, multicenter study. Ann Dermatol. 2012;24(6):1630–1639. doi:10.1093/annonc/mds649
4. Sawant N, Chikhalkar S, Mehta V, Ravi M, Madke B, Khopkar U. Androgenetic alopecia: quality-of-life and associated lifestyle patterns. Int J Trichology. 2010;2:81–85. doi:10.4103/0974-7753.77510
5. Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136–141.e5. doi:10.1016/j.jaad.2017.02.054
6. Mccrudden MTC, McAlister E, Courtenay AJ, et al. Microneedle applications in improving skin appearance. Exp Dermatol. 2015;24:561–566. doi:10.1111/exd.12723
7. Faghihi G, Nabavinejad S, Mokhtari F, Fatemi Naeini F, Iraji F. Microneedling in androgenetic alopecia; comparing two different depths of microneedles. J Cosmet Dermatol. 2021;20(4):1241–1247. doi:10.1111/jocd.13714
8. Parajuli S, Paudel U. Microneedling for androgenetic alopecia not responding to conventional treatment. Our Dermatol Online. 2020;11:140–142. doi:10.7241/ourd.20202.5
9. Gowda BHJ, Ahmed MG, Sahebkar A, Riadi Y, Shukla R, Kesharwani P. Stimuli-responsive microneedles as a transdermal drug delivery system: a demand-supply strategy. Biomacromolecules. 2022;23(4):1519–1544. doi:10.1021/acs.biomac.1c01691
10. Gowda BHJ, Ahmed MG, Sanjana A. Can microneedles replace hypodermic needles? Resonance. 2022;27:63–85. doi:10.1007/s12045-022-1294-5
11. Ocampo‐Garza SS, Fabbrocini G, Ocampo‐Candiani J, Cinelli E, Villani A. Micro needling: a novel therapeutic approach for androgenetic alopecia. A review of literature. Dermatol Ther. 2020;33(6):e14267. doi:10.1111/dth.14267
12. Ms Sukesh DR, Avhad G, Dandale A, Pal A, Pund P, Pund P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5(1):6–11. doi:10.4103/0974-7753.114700
13. Hair Plastic and Cosmetic Surgery Committee of Aesthetic and Plastic Physicians Branch of China Medical Association. Guidelines for the treatment of androgenetic alopecia in Chinese people, Chin J Aesth Plast Surg. 2019;30(1):8–12. Chinese. doi:10.3969/j.issn.1673-7040.2019.01.001
14. Lee W-S, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57(1):37–46. doi:10.1016/j.jaad.2006.12.029
15. Sharma A, Surve R, Dhurat R, et al. Microneedling improves minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. J Biol Regul Homeost Agents. 2020;34(2):659–661. doi:10.23812/19-385-L-51
16. Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447(7142):316–320. doi:10.1038/nature05766
17. Kim YS, Jeong KH, Kim JE, Woo YJ, Kim BJ, Kang H. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28(5):586–592. doi:10.5021/ad.2016.28.5.586
18. Kumar MK, Inamadar AC, Palit A. A randomized controlled, single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11(4):211–216. PMID: 30886475; PMCID: PMC6371730. doi:10.4103/JCAS.JCAS_130_17
19. Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60(3):260–263. PMID: 26120151; PMCID: PMC4458936. doi:10.4103/0019-5154.156361
20. Jha AK, Vinay K, Zeeshan M, Roy PK, Chaudhary RKP, Priya A. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil. J Cosmet Dermatol. 2019;18(5):1330–1335. doi:10.1111/jocd.12864
21. Rahmani W, Sinha S, Biernaskie J. Immune modulation of hair follicle regeneration. NPJ Regen Med. 2020;5:9. PMID: 32411394; PMCID: PMC7214459. doi:10.1038/s41536-020-0095-2
22. Fakhraei Lahiji S, Seo SH, Kim S, et al. Transcutaneous implantation of valproic acid-encapsulated dissolving microneedles induces hair regrowth. Biomaterials. 2018;167:69–79. PMID: 29554482. doi:10.1016/j.biomaterials.2018.03.019
23. Nestor MS, Ablon G, Gade A, Han H, Fischer DL. Treatment options for androgenetic alopecia: efficacy, side effects, compliance, financial considerations, and ethics. J Cosmet Dermatol. 2021;20(12):3759–3781. PMID: 34741573. doi:10.1111/jocd.14537
24. Miot H. Percutaneous Collagen Induction with Microneedling. Springer International Publishing; 2021:59–67.
25. Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7(4):244–254. PMID: 27559496; PMCID: PMC4976400. doi:10.4103/2229-5178.185468
26. Pathoulas JT, Bellefeuille G, Raymond O, Khalid B, Farah RS. Energy-based devices for hair loss. Dermatol Clin. 2021;39(3):447–461. PMID: 34053597. doi:10.1016/j.det.2021.04.002
27. Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581–587. PMID: 15019747. doi:10.1016/j.addr.2003.10.023
28. Kaushik S, Hord AH, Denson DD, et al. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92(2):502–504. PMID: 11159258. doi:10.1097/00000539-200102000-00041
29. Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Current Topics in Microbiology and Immunology. Springer Berlin Heidelberg; 2009:369–393.
30. Emerson lima ML. Percutaneous Collagen Induction with Microneedling. Springer, Cham; 2021.
31. Serrano G, Almudéver P, Serrano JM, et al. Microneedling dilates the follicular infundibulum and increases transfollicular absorption of liposomal sepia melanin. Clin Cosmet Investig Dermatol. 2015;8:313–318. PMID: 26170707; PMCID: PMC4489818. doi:10.2147/CCID.S77228
32. Vañó-Galván S, Camacho F. New treatments for hair loss. Actas Dermosifiliogr. 2017. 108(3):221–228. English, Spanish. PMID: 28061966. doi:10.1016/j.ad.2016.11.010
33. Hackam DJ, Ford HR. Cellular, biochemical, and clinical aspects of wound healing. Surg Infect. 2002;3(Suppl 1):S23–S235. PMID: 12573037. doi:10.1089/sur.2002.3.s1-23
34. Fabbrocini G, Fardella N, Monfrecola A, Proietti I, Innocenzi D. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34(8):874–879. PMID: 19486041. doi:10.1111/j.1365-2230.2009.03291.x
35. Sasaki GH. Micro-needling depth penetration, presence of pigment particles, and fluorescein-stained platelets: clinical usage for aesthetic concerns. Aesthet Surg J. 2017;37(1):71–83. PMID: 27530764. doi:10.1093/asj/sjw120
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
