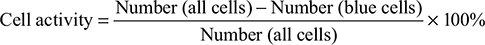Back to Archived Journals » Journal of Neurorestoratology » Volume 6
Neurorestorative clinical application standards for the culture and quality control of neural progenitor/precursor cells (version 2017)
Authors Feng S, Xiao J, Han F, Chen L, Gao W, Mao G, Huang H
Received 1 August 2017
Accepted for publication 29 May 2018
Published 22 August 2018 Volume 2018:6 Pages 65—68
DOI https://doi.org/10.2147/JN.S147917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Hari Shanker Sharma
Shiqing Feng,1,* Juan Xiao,2,3,* Fabin Han,4 Lin Chen,5 Wenyong Gao,2,3 Gengsheng Mao,2 Hongyun Huang2,3
On behalf of Neurorestoratology Professional Committee of Chinese Medical Doctors Association (Chinese Association of Neurorestoratology)
1International Science and Technology Cooperation Base of Spinal Cord Injury, Department of Orthopedics, Tianjin Medical University General Hospital, Tianjin 300052, People’s Republic of China; 2Institute of Neurorestoratology, The General Hospital of Chinese People’s Armed Police Forces, Beijing 100039, People’s Republic of China; 3Cell Therapy Center, Beijing Hongtianji Neuroscience Academy, Beijing 100043, People’s Republic of China; 4The Institute for Tissue Engineering and Regenerative Medicine, Liaocheng University/Liaocheng People’s Hospital, Liaocheng 252000, People’s Republic of China; 5Department of Neurosurgery, Tsinghua University Yuquan Hospital, Beijing 100040, People’s Republic of China
*These authors contributed equally to this work
Abstract: In order to promote the clinical use of neural progenitor or precursor cells for treating neurological diseases and damage, we need to standardize culture procedures for these cells. The Chinese Association of Neurorestoratology put forward these standards for training operators, standardized use and management of materials and equipment, standardized isolation and culture for neural progenitor/precursor cells, and the standardized management in preservation, transport, and related safe operation procedures of the neural progenitors. These cultures and quality control standards also include the Good Manufacturing Practice environment, routine maintenance as well as the monitoring and reporting of the clinical-grade neural progenitor cells. The aim of these standards is to improve the therapeutic efficacy and minimize the possible side effects from lake of quality control.
Keywords: standardization, cell culture, quality control, neural progenitor cells, neural precursor cells, neurorestoratology
Introduction
Neural progenitor/precursor cells have the ability to proliferate and differentiate into neurons, astrocytes, and oligodendrocytes.1 These cells are present in the fetal brain and spinal cord, especially in the striatum, hippocampus dentate gyrus, and ependymal, where they have stronger proliferation and differentiation abilities1–8 and can be isolated and cultured in vitro for subsequent clinical therapeutic application.9 Even though many clinical explorations of neural progenitor/precursor cell application have been done, there have been no standardized guidelines for the culture and quality control of these cells to date.10–21 The lack of standardization has posed a significant challenge for the clinical translation of progenitor/precursor cells for neurorestorative cell therapy. In order to promote clinical safety and efficacy of cell therapies using neural progenitor/precursor cells, the Chinese Association of Neurorestoratology has developed the following set of guidelines, which focus on the clinical application of neural progenitor cells. Thus, the therapeutic efficacy for neurorestoratology can be improved with these guidelines to standardize the application of neural progenitor/precursor cells.
Standardized training and instruction for operators
At least one senior scientist/manager with a professional-level qualification should be in charge of the laboratory. The operator engaged in cell preparation should be systematically and professionally trained by laboratory staff with a cell biology background.
Basic standardized training items include knowledge learning (cell growth characteristics, growth process, application of cell culture, etc), skills development (cell culture, cryopreservation, resuscitation, transportation, etc), and familiarity with applicable rules and regulations (details in below).
Training methods include centralized teaching, practical training led by teachers, small seminars, symposiums, conferences, etc.
The goal of this training is to master the professional knowledge of neural progenitor/precursor cells, training processes, and control procedures.
The standardized management system includes filing and arranging of the original records and work records, and operators’ need to comply with the Current Good Manufacturing Practice laboratory maintenance standard operating procedures and complete operating records.
Standardized use and management of materials and equipment
Cell culture laboratories are managed by professionals who are responsible for the maintenance of the equipment, regular calibration, personnel training, normal operation of the clean room, and the establishment and management of the quality certification files of the equipment.
CO2 incubator
The temperature should be kept consistently at 37°C, and the concentration of CO2 should be maintained at 5%.
Centrifuge
Objects should be kept for centrifugation in balance. Testing of the product and assessment of the supernatant for cell loss are needed.
Water bath
Distilled water should be changed once a month, and instrument disinfection solution should be added.
Inverted microscope
Power should be turned off and the microscope should be covered after use; and the lens should be cleaned after use of oil mirror.
Super-clean bench or biological safety cabinets
Operation guidance for procedures should be strictly followed.
To determine whether an instrument is operating appropriately, turn off the instrument power supply and complete the usage records after completion of the test.
Neural progenitor/precursor cell culture process
Donor selection
Donor selected from fetuses aborted at 12 weeks of gestation without brain malformations and no history of infectious disease, such as HIV-1, HIV-2 hepatitis B, hepatitis C, and syphilis. Water bag abortion is the best technique (mothers and their families are informed and they sign the informed consent forms, a procedure approved by the hospital ethics committee).22 Samples are kept in a sterile bag in the freezer and are delivered to the laboratory.
Cell culture medium
The complete DMEM:F12 (1:1) medium optimized for culturing neural progenitor/precursor cells should include antibiotics, 1% N-2 supplement, 2% B-27 supplement, leukemia inhibitory factor 10 ng/mL, epidermal growth factor 20 ng/mL, fibroblast growth factor 20 ng/mL, glutamine; 0.25% trypsin, and fetal bovine serum.
All materials must meet the quality standards of sterile, nonpathogenic microorganisms and endotoxins (bacteria ≤200 CFU/g, mold ≤50 CFU/g, endotoxins ≤10 EU/mL).
Neural progenitor/progenitor cell preparation procedures
The fetal remains should be disinfected using alcohol. The fetal scalp of head must be cut, the skull should be removed, and the dura mater should be peeled. Pieces of tissue in the subventricular zone (~1 cm3) are put into a sterile petri dish.
The sample tissue on a sterile culture dish should be transferred to the clean bench with DF12 rinse for three times and minced into small pieces with ophthalmic scissors. The broken-down pieces of tissue should be taken into a 15 mL sterile centrifuge tube and then 5 mL of DF12 culture medium should be added. The suspension should be aspirated and filtered through a 200 mesh filter to make a single-cell suspension.
The cell suspension should undergo centrifugation at 1,000 rpm/minute for 5 minutes. The supernatant should be removed and the cell pellet should be resuspended in PBS and centrifuged twice. The supernatant should be discarded after the final centrifugation. Then the optimized medium (as described in “Cell culture medium” section) is added for culturing the neural progenitor/precursor cells. After cell counting, cells are plated at a concentration of 2 × 105 cells/75 cm2 flask. After 24 hours, the cells are examined under a microscope to find out if they are contaminated with bacteria and the cells are passaged every 7–10 days. If cells in a flask are found to be contaminated with bacteria, the flask with those cells must be discarded. Each passage of the cells should be labeled, recorded, and stored. After the cells are passaged for 2–3 times, they can be used for clinical application.
Procedures for cryopreservation and recovery of neural progenitor/precursor cells
The cells can be cryopreserved when they have fully filled the culture flasks. The medium is discarded and 4 mL of DF12 is added to each flask to wash the cells. Again, 4 mL of DF12 is added to each flask and the cells are dissociated with a thick elbow straw from one side to another, avoiding the formation of bubbles. Cells are collected after centrifugation at 1,000 rpm/minute for 5 minutes. Then, 2 × 106 cells are added to 1 mL of freezing medium (10% dimethyl sulfoxide, 50% fetal bovine serum, and 40% optimized medium) and gently mixed with a thick elbow straw; then, the cell suspension is added to the Cryovials, the cell and tissue number is labeled, and the storage records are completed.
Storage procedures are as follows: 4°C ~45 minutes; −20°C ~1 hour; −80°C ~overnight; stored in a liquid nitrogen tank.
The cells are recovered in the following way: the Cryovials are removed from the liquid nitrogen tank, placed then into a water bath at 38.5°C as quickly as possible, and quickly swirled to accelerate thawing. The surface of the Cryovials is dried with sterile gauze. The cell suspension is aspirated into a 15 mL centrifuge tube containing 10 mL DF12 medium using a thick elbow straw and centrifuged for 5 minutes (1,000 rpm/minute). The supernatant is discarded, and the cell pellet is resuspended in a 15 mL of prewarmed DF12 medium for a second rinse; the mixture is then centrifuged for 5 minutes (1,000 rpm/minute). The supernatant is discarded and the cell pellet is resuspended in a 15 mL of prewarmed DF12 medium for a third rinse. The mixture is centrifuged for 5 minutes (1,000 rpm/minute), supernatant is discarded, the cell pellet is resuspended in a 15 mL of prewarmed culture medium, and cells are plated in the culture flask.
Neural progenitor/precursor cell quality control standards
Detecting exogenous factors in cell products
The current version of “Chinese Pharmacopoeia” related to biological products is followed in order to detect any exogenous bacteria, fungi, mycoplasma, and endotoxins.
Cell immunophenotype
Neural progenitor/precursor cells isolated from the subventricular zone are to be identified by using specific neural antigens including Nestin, neurofilament (NF), glial fibrillary acidic protein (GFAP), and NG2. The immunofluorescence staining or flow cytometry can be used to identify the immunophenotype. The approximate ratio among NF-positive neurons, NG2-positive oligodendrocytes, and GFAP-positive astrocytes should be 1:1.5:2.
Cell activity
Using placental blue staining, cell activity should reach more than 95%.
|
Tumorigenicity
No tumor formations have been reported after the transplantation of allogeneic neural progenitor/progenitor cells.
Cell quality control and management
Products must be double reviewed, and quality management records must be completed. Cells can be used for transplantation treatment only after the cell laboratory manager completes quality control testing and signs the record.
Clinical cell dosage
For the appropriate cell dosage, refer to the clinical cell therapy guidelines for neurorestoration.23,24
The temperature and time control of clinical cell transport
A biological safety box is used for transport after the preparation of cell products, and the temperature of biological safety box is 4°C. Cell products should be used for transplantation within 2 hours.25,26
Management of the clinical treatment level cell bank
To establish the clinical neural cell files and inventory management according to the cell sources, the generation of the culture, location in the liquid nitrogen tank, date of cryopreservation, and human leukocyte-antigen matching should be noted.
Summary
Formulation of clinical level neural progenitor/precursor cell preparation and quality control standards is essential to promote standardization of clinical neural repair treatment, and this standard is the minimum standard at present. This standard will be further optimized and improved in the future according to the progress of basic and clinical research.
Acknowledgment
We sincerely thank Professor Priscilla Song (Washington University in St. Louis) for her editorial assistance in polishing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Richards LJ, Kilpatrick TJ, Banlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992;89(18):8591–8595. | ||
McKay R. Stem cells in the central nervous system. Science. 1997; 276(5309):66–71. | ||
Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. | ||
Cai Z, Yan Y, Tang Y, et al. Neural stem cells: promising therapy for Alzheimer’s disease. Chin Bull Life Sci. 2008;20(1):90–94. | ||
Zhao N, Zhong C, Hong Z. Neurogenesis in the adult subventricular zone. Chin J Clin Neurosci. 2007;15(01):96–99. | ||
Davis AA, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994;372(6503):263–266. | ||
Jiang X, Wang H, Chen L, et al. Culture and identification of neural stem/progenitor cells from human embryonic subependymal zone by adherent method. Prog Anat Sci. 2011;16(4):307–310. | ||
Gao W, Jiang X, Xiao J, et al. The cultivation, cryopreservation and identification of human embryonic striatum tissue neural stem/progenitor cells. Prog Anat Sci. 2015;(6):642–644. | ||
Bao J, Huang H. Definition of neural stem cells. Chin J Neurosurg. 2008;24(10):793–796. | ||
Moviglia GA, Moviglia-Brandolino MT, Varela GS, et al. Feasibility, safety, and preliminary proof of principles of autologous neural stem cell treatment combined with T-cell vaccination for ALS patients. Cell Transplant. 2012; 21(Suppl 1): S57–S63. | ||
Riley J, Glass J, Feldman EL, et al. Intraspinal stem cell transplantation in ALS: a Phase I trial, cervical microinjection and final surgical safety outcomes. Neurosurgery. 2012;71(2):405–416. | ||
Feldman EL, Boulis NM, Hur J, et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75(3):363–373. | ||
Mazzini L, Gelati M, Profico DC, et al. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med. 2015;13:17. | ||
Nelson PT, Kondziolka D, Wechsler L, et al. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160(4):1201–1206. | ||
Luan Z, Liu WP, Qu SQ, et al. [Treatment of newborns with severe injured brain with transplantation of human neural precursor cells]. Zhonghua Er Ke Za Zhi. 2011;49(6):445–449. | ||
Chen L, Xi H, Huang H, et al. Multiple cell transplantation based on an intraparenchymal approach for patients with chronic phase stroke. Cell Transplant. 2013;22(Suppl 1): S83–S91. | ||
Xi H, Chen L, Huang H, et al. Preliminary report of multiple cell therapy for patients with multiple system atrophy. Cell Transplant. 2013;22 (Suppl 1):S93–S99. | ||
Zhou Q, Zhang SZ, Xu RX, Xu K. Neural stem cell transplantation and postoperative management: report of 70 cases. Di Yi Jun Yi Da Xue Xue Bao. 2004;24(10):1207–1209. | ||
Guzman R, Schubert M, Keller-Lang D, Huhn SL, Curt A. Human neural stem cell transplantation in chronic SCI: interim results of a Phase I/II trial. Neurosurgery. 2013;60(Suppl 1):185. | ||
Shin JC, Kim KN, Yoo J, et al. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015;2015:630932. | ||
Curtis E, Gabel BC, Marsala M, Ciacci JD. A phase I, open-label, single-site, safety study of human spinal cord-derived neural stem cell transplantation for the treatment of chronic spinal cord injury. Neurosurgery. 2016;63(Suppl 1):168–169. | ||
Ren Y, Tian G, Wang H, et al. Quality specification of human embryo olfactory ensheathing cells from olfactory bulb. Chin J Tissue Eng Res. 2008;12(16):3156–3157. | ||
Chinese Medical Doctor Association of Neurorestoratology Professional Committee; Chinese Branch of the International Association of Neurorestoratology. Clinical cell therapy guidelines for neurorestoration (China version 2015). Chin J Cell Stem Cell. 2016;6:1–7. | ||
Huang H, Chen L, Zou Q, et al. Clinical cell therapy guidelines for neurorestoration (China version 2016). J Neurorestoratol. 2017;5:39–46. | ||
Jiang X, Xiao J, Ren Y et al. The influence of 4 degree centigrade conservation on cells activity of rats’ olfactory bulbs derived olfactory ensheathing cells. Prog Anat Sci. 2011;17(5):424–427. | ||
Gobbel GT, Kondziolka D, Fellows-Mayle W, Uram M. Cellular transplantation for the nervous system: impact of time after preparation on cell viability and survival. J Neurosurg. 2010;113(3):666–672. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.