Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Nucleosides isolated from Ophiocordyceps sinensis inhibit cigarette smoke extract-induced inflammation via the SIRT1–nuclear factor-κB/p65 pathway in RAW264.7 macrophages and in COPD mice
Authors Sun X, Dong Z, Li N, Feng X, Liu Y, Li A, Zhu X, Li C, Zhao Z
Received 28 April 2018
Accepted for publication 8 June 2018
Published 10 September 2018 Volume 2018:13 Pages 2821—2832
DOI https://doi.org/10.2147/COPD.S172579
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chunxue Bai
Xiao Sun,1 Zhonghua Dong,1 Nan Li,1 Xiuli Feng,1 Yan Liu,1 Ang Li,1 Xiaosong Zhu,1 Chunyan Li,1 Zhongxi Zhao1–3
1School of Pharmaceutical Sciences, Shandong University, Jinan, People’s Republic of China; 2Shandong Engineering and Technology Research Center for Jujube Food and Drug, Jinan, People’s Republic of China; 3Shandong Provincial Key Laboratory of Mucosal and Transdermal Drug Delivery Technologies, Shandong Academy of Pharmaceutical Sciences, Jinan, People’s Republic of China
Background: Ophiocordyceps sinensis (C. sinensis) extracts have been found to have a therapeutic effect on patients with chronic obstructive pulmonary disease (COPD). Silent information regulator 1 (SIRT1) plays an important role in the regulation of inflammatory mediators and correlates with lung function and COPD exacerbations. The objective of this work was to explore the anti-inflammatory effect and preliminary pathways of nucleosides from cultured C. sinensis on RAW264.7 macrophages and COPD mice.
Materials and methods: The nucleosides were extracted from cultured C. sinensis powder and further purified by macroporous resin D101 and glucan G10 columns. Inflammation and oxidative stress models in RAW264.7 macrophages and in mice were established by injection of cigarette smoke extract (CSE). We then examined how the isolated nucleosides regulated the production of the associated inflammatory mediators in vitro and in vivo by enzyme-linked immunosorbent assay, reverse transcription polymerase chain reaction, and Western blot.
Results: The nucleosides inhibited inflammatory mediator expression of tumor necrosis factor-α, interleukin-6, interleukin-1β, and nitric oxide in both the CSE-stimulated RAW264.7 macrophages and mice. Moreover, the nucleosides elevated SIRT1 activation and suppressed nuclear factor-κB (NF-κB)/p65 activation in vitro and in vivo. Nucleoside treatment significantly decreased the levels of the inflammatory mediators in the bronchoalveolar lavage fluid (BALF) and serum of the CSE-induced mice. The nucleosides also altered the recruitment of inflammatory cells in BALF and improved characteristic features of the lungs in the CSE-induced mice.
Conclusion: These results show that the nucleosides suppressed COPD inflammation through the SIRT1–NF-κB/p65 pathway, suggesting that the nucleosides may be partly responsible for the therapeutic effects of cultured C. sinensis on COPD patients.
Keywords: Ophiocordyceps sinensis, nucleosides, COPD, anti-inflammatory, SIRT1, NF-κB/p65
Introduction
Chronic obstructive pulmonary disease (COPD) is an incurable but preventable respiratory disease that is expected to become the third most common cause of death worldwide by 2020.1–3 The strongest and most common risk factor for COPD is exposure to cigarette smoke, which leads to the major pathologies of COPD, including emphysema (alveolar wall destruction with airspace enlargement), inflammation, protease/anti-protease imbalance, apoptosis, and oxidative stress.4–6 Cigarette smoke also activates resident cells in the lung (particularly epithelial cells and alveolar macrophages) to generate chemotactic molecules that recruit additional inflammatory cells (neutrophils, monocytes, and lymphocytes) into the lung, which in turn perpetuates inflammation and oxidative stress in the lung.7–9 Inflammatory cells play an important role in inflammation by production of inflammatory mediators, such as nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β). Collectively, these events lead to a vicious cycle of persistent inflammation accompanied by chronic oxidative stress, which leads to disturbances in the protease/anti-protease balance and defects in tissue-repairing mechanisms in the lung cells, and contributes to the severity and progression of COPD.
Mechanistic studies show that silent information regulator 1 (SIRT1) is involved in the pathogenesis of COPD, with decreased levels in bronchial epithelial cells (Beas-2B) and mouse lungs exposed to cigarette smoke extract (CSE), as well as in the lungs and blood cells of COPD patients.9,10 SIRT1 belongs to the class III histone/protein deacetylases, which possess anti-inflammatory, anti-aging/senescence properties.9,11 SIRT1-mediated protection in response to CSE-induced lung inflammatory response and injury is linked to the activation of nuclear factor-κB (NF-κB)/p65.9,11,12 NF-κB/p65 represents an essential family of transcription factors, which are involved in regulating the expression of numerous genes in the immune response, such as TNF-α, IL-6, and IL-1β.8,13 Therefore, increasing SIRT1 and decreasing NF-κB/p65 activation have potential applications in anti-inflammatory effects of COPD.
The current main pharmacotherapies in COPD include inhaled corticosteroid, long-acting β2-adrenoceptor agonists (LABAs), long-acting muscarinic antagonists, and phosphodiesterase 4 (PDE4) inhibitors.13,14 Drugs such as antitussives, mucolytics, and bronchodilators that are used only to relieve the symptoms of bronchitis do not affect or reverse the pathological progress of COPD. PDE4 inhibitors such as roflumilast can only treat COPD inflammation. All of these first-line COPD medicines have been shown to have severe side effects in addition to their limited efficacy. Therefore, novel drugs with multi-targeted properties are needed to effectively combat the complex pathological course of COPD and eventually lead to a complete cure.
Medicinal plants are traditionally the most widely used medications for treating various human diseases throughout the world. In terms of COPD treatment, various plants have been used in many countries for thousands of years.15,16 Among the many medicinal plants used in COPD therapy, medicinal mushrooms such as Ophiocordyceps sinensis (formerly known as Cordyceps sinensis [C. sinensis]) have shown some promising clinical results for COPD treatment. Ophiocordyceps sinensis, or Dong Chong Xia Cao (winter worm and summer grass) in Chinese, is a mushroom that grows on caterpillars, and has been used as a traditional Chinese medicine. Cultured C. sinensis has also been widely used in medicinal preparations to solve the problems with availability and heavy metal contamination related to the natural species. Ophiocordyceps sinensis has been shown to possess various therapeutic functions, including anti-cancer, anti-diabetic, anti-inflammatory, immunomodulatory, and anti-oxidant effects.17–19 Numerous studies have shown that the C. sinensis extracts have a therapeutic effect on patients with COPD, including improvement of symptoms, quantity of life, and lung function.20,21 A mechanistic study suggests that the C. sinensis extract inhibits the senescence induced by CSE in human bronchial epithelial cells via the ROS/PI3K/AKT/mTOR signaling pathway.20
However, the precise active ingredients in C. sinensis and their therapeutic mechanisms responsible for COPD treatment are not clear. A number of studies on the active ingredients and underlying mechanism of C. sinensis for COPD therapy have been initiated in our laboratory. Ophiocordyceps sinensis is rich in nucleosides, polysaccharides, sterols, proteins, amino acids, and polypeptides.20 The nucleosides are recognized as the main bioactive components in C. sinensis, and are used as chemical markers for quality control of C. sinensis.22,23 The objective of this work was to explore the anti-inflammatory effect and preliminary pathways of nucleosides from cultured C. sinensis on RAW264.7 macrophages and COPD mice stimulated by CSE. Inflammatory mediators such as TNF-α, IL-6, IL-1β, and inducible nitric oxide synthase (iNOS) in the CSE-stimulated RAW264.7 macrophages and mice were measured to explore the anti-inflammatory effects of the isolated nucleosides on COPD. In addition, the SIRT1–NF-κB/p65-dependent inflammation cascades in CSE-induced RAW264.7 cells and Balb/c mice were determined. The current study may provide valuable information on the determination of the structure–efficacy relationship and further optimization of more effective remedies with few side effects based on C. sinensis.
Materials and methods
Chemicals
Dried cultured C. sinensis powder was a gift from Hangzhou Zhongmei East China Pharmaceutical Co. (Hangzhou, People’s Republic of China). All of the nucleoside standards were purchased from Aladdin Regents Co. (Shanghai, People’s Republic of China). Dulbecco’s Modified Eagle Medium and fetal bovine serum (FBS) were purchased from Biological Industries (Beit Haemek, Israel). Penicillin and streptomycin were purchased from Solarbio Biotechnology (Beijing, People’s Republic of China). NO, mouse TNF-α, and mouse IL-6 and IL-1β enzyme-linked immunosorbent assay (ELISA) kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, People’s Republic of China). Anti-GAPDH, anti-SIRT1, anti-NF-κB/p65, and anti-iNOS antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). Secondary antibodies were provided by ZSGB-BIO (Beijing, People’s Republic of China). All other chemicals were of analytical grade.
Preparation of the nucleosides
The dried cultured C. sinensis powder was mixed with distilled water (1:10 ratio of raw material to water, w/v) in a conical flask (500 mL) and ultrasound-assisted (ultrasonic power 100 W) extracted three times for 1 hour. Then, the merged suspension was centrifuged at 1,000 rpm, concentrated using a rotary evaporator, and freeze-dried (Alpha 1-2 LDplus; Christ, Osterode am Harz, Germany). The sample was redissolved in double-distilled water and purified with macroporous resin D101 and glucan G10 columns sequentially. The nucleosides were eluted with water at a flow speed of 1.0 mL/min, and the elute was collected with 10 mL in each tube. The nucleosides were determined by high-performance liquid chromatography (HPLC) and liquid chromatography–mass spectrometry. Finally, the nucleoside fraction was collected based on main peaks, freeze-dried, and stored at −20°C until used.
Separation and identification of the nucleosides in C. sinensis by HPLC
Analysis of the chemical composition of nucleosides was performed on an Agilent 1260 HPLC system equipped with a diode array (Agilent Technologies, Palo Alto, CA, USA). Separation was carried out using a Gemini-NX C12 column (4.6×150 mm, 5 μm; Phenomenex, Torrance, CA, USA), and the injection volume was 10 μL. The mobile phase consisted of solvents A (0.1% formic acid in water) and B (methanol) with gradient elution as follows: 0–19 minutes (5% B); 19–20 minutes (5%–15% B); 20–30 minutes (15% B). The flow rate was set at 0.4 mL/min and peaks of nucleosides were monitored at 260 nm.
Preparation of aqueous CSE
CSE was prepared as previously reported, with some modifications.12 In brief, three cigarettes (Taishan Brand, Shandong, People’s Republic of China) were burned and the smoke was collected by a vessel containing phosphate-buffered saline (10 mL) using a vacuum pump. This 100% CSE was adjusted to a pH of 7.4 and was sterile filtered through a 0.22 μm filter. The CSE was freshly prepared for each experiment and diluted with the culture medium containing 1% FBS immediately before use.
RAW264.7 cell culture
RAW264.7 cells, which were purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, People’s Republic of China), were cultured in Dulbecco’s Modified Eagle Medium supplemented with 100 U/mL of penicillin G, 100 μg/mL of streptomycin, and 10% (v/v) inactivated FBS. RAW264.7 cells were grown at 37°C in a humidified 5% CO2 incubator.
Determination of NO, TNF-α, IL-6, and IL-1β secretion
RAW264.7 cells (1×106 cells/well) were pre-incubated in 12-well plates for 24 hours at 37°C in a humidified incubator at 5% CO2. After cells had been cultured with or without 10% CSE in the absence or presence of nucleosides (25 and 50 μg/mL) for 24 hours, levels of NO, TNF-α, IL-6, and IL-1β in the culture supernatants were measured by an ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, People’s Republic of China) according to the manufacturer’s instructions.
RNA extraction and relative quantification using real-time polymerase chain reaction (PCR)
Total RNA was extracted using an RNAEasy kit according to the manufacturer’s instructions (Bioecon Biotec Co., Shanghai, People’s Republic of China). RNA purity was checked by measuring the OD260/280 of RNA samples. cDNA was synthesized through reverse transcription using M-MLV reverse transcriptase and oligo(dT) primer. The gene expression levels of SIRT1, TNF-α, IL-6, IL-1β, and iNOS were detected by real-time PCR. PCR amplification was performed in a 96-well plate in triplicate. Each reaction well consisted of 10 μL of 2× SYBR Green PCR Master mix, 0.5 μL forward and reverse primers, and 1 μL of template cDNA to obtain a final volume of 20 μL. The PCR analysis was performed using a Mastercycler ep realplex apparatus (Eppendorf, Germany). After an initial denaturation step for 3 minutes at 95°C, the conditions for cycling were 39 cycles of 30 seconds at 95°C, 30 seconds at 60°C, and 15 seconds at 72°C. The primer sets for SIRT1, TNF-α, IL-6, IL-1β, iNOS, and GAPDH were purchased from Dingguo Changsheng Biotechnology Co. (Beijing, People’s Republic of China) and are listed in Table 1.
  | Table 1 Primer sets |
The relative expression level of each target gene was normalized against the GAPDH control using the 2−ΔΔCt method and further compared with its own control. All samples were studied in independent triplicate experiments.
Experimental protocols
Male Balb/c mice weighing 18–22 g were purchased from Jinan Pengyue Experimental Animal Breeding Co. (Jinan, People’s Republic of China). The mice were housed in a temperature-controlled room (25°C±2°C) with relative humidity of 40%–70% on a 12-hour light/dark cycle, and had free access to a standard commercial diet and water. All animal care and experimental protocols were approved by the Animal Care and Use Committee of Shandong University, and were performed in accordance with the committee’s animal care and use guidelines. After acclimatization to laboratory conditions for 1 week, the mice were randomly divided into five groups (n=7) as follows: control group (C), model group (M), positive group (P, budesonide, 2 mg/kg), nucleosides (20 mg/kg), and nucleosides (40 mg/kg). The mice, except those in the control group, were injected intraperitoneally with CSE (0.3 mL) on days 0, 7, and 14. All the drugs were suspended in 0.5% carboxy methyl cellulose (CMC) (w/v) and administered orally (10 mL/kg) daily for 21 days. The control mice received 0.5% CMC only. All mice were killed on day 21.
Lung histological examination
The lung tissues were fixed with 4% formaldehyde phosphate buffer overnight, then dehydrated, paraffin embedded, sliced into 4 μm sections, and stained with H&E. The slides were observed under a morphometric microscope (Nikon, Japan) at 100× magnification to evaluate the morphological changes in the lungs.
Serum collection
The whole blood was collected using a retro-orbital method. The blood was centrifuged at 3,500 rpm for 10 minutes, and serum was obtained for analysis of NO, TNF-α, and IL-1β using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, People’s Republic of China).
Analysis of bronchoalveolar lavage fluid (BALF)
The lungs were lavaged via a cannula inserted into the trachea and then instilled with 2×1 mL aliquots of saline. All aliquots were collected and centrifuged at 2,000 rpm for 10 minutes at 4°C. The supernatants were obtained and stored at −80°C for further analysis of NO, TNF-α, and IL-1β using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, People’s Republic of China). The cells were resuspended in a PBS solution (100 μL) and counted using a hemocytometer. The cell differential was determined from an aliquot of the cell suspension by centrifugation on a slide and Wright–Giemsa (Solarbio Biotechnology, Beijing, People’s Republic of China) stain. Differential cell counts were calculated based on morphological criteria.
Western blot analysis
The RAW264.7 cells and lung tissues were homogenized in radioimmunoprecipitation assay lysis buffer, and then total proteins were extracted and determined using a bicinchoninic acid assay kit (Beyotime Institute of Biotechnology, Beijing, People’s Republic of China). Equal quantities of proteins were separated on 8%–12% sodium dodecyl sulfate–polyacrylamide gels and transferred on to polyvinylidene fluoride membranes (Millipore Corp., Bedford, MA, USA). Membranes were incubated with primary antibodies overnight at 4°C, followed by incubation with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibodies for 1 hour at room temperature. The optical densities were measured using an enhanced chemiluminescence detector. The relative optical densities of the bands were quantified using AlphaView SA software. All Western blot analyses were carried out at least three times.
Immunohistochemistry for the expression of SIRT1 and NF-κB/p65
The expression of SIRT1 and NF-κB/p65 in the lung tissues of mice was determined by immunohistochemistry. In brief, the lung tissue slice embedded with paraffin was deparaffinized and rehydrated. The antigen was retrieved using sodium citrate and with heat-induced retrieval. After blocking with goat serum, SIRT1 and NF-κB/p65 antibodies were separately applied overnight at 4°C. Then HRP-conjugated anti-rabbit antibody was applied, and the expression of SIRT1 and NF-κB/p65 was finally visualized using a 3,3′-diaminobenzidine detection kit. Images of SIRT1 and NF-κB/p65 were obtained and photographed under a microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis of the control and treatment samples was performed with either a Student’s t-test or an analysis of variance for multiple comparisons using Prism version 5.0 (GraphPad Software, La Jolla, CA, USA). Data are presented as mean ± SEM. Values of P<0.05 were considered statistically significant.
Results
Identification of the nucleoside fraction by HPLC
The major compounds in the nucleosides were detected and identified by HPLC with a diode array detector. Figure 1 shows the typical HPLC chromatograms of the isolated nucleosides. Ten major compounds were initially identified by comparing the retention time and ultraviolet spectra of the sample peaks with corresponding standards. The retention times of the ten nucleosides and their concentrations in C. sinensis are shown in Table 2.
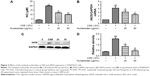
Nucleosides suppress CSE-induced NO production and expression of iNOS in RAW264.7 cells
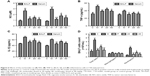
The non-lethal concentrations of 25 and 50 μg/mL were determined by measuring the RAW264.7 cell viability and used in the subsequent experiments. A concentrated CSE (100% CSE) and incubation time were determined in a preliminary study based on the induction of inflammatory responses in RAW264.7 cells and mice, as well as appropriate histopathological changes in mouse lungs. It was found that a ten-fold dilution (10% CSE) and incubation duration of 1 hour were optimal for the stimulation of RAW264.7 cells. In Figure 2A, incubation with 10% CSE has significantly increased the level of NO compared to the control group. Compared with the CSE-induced group, the isolated nucleosides significantly decreased the production of NO. To examine whether the decrease in NO production resulted from suppression of iNOS gene expression, we determined the levels of iNOS mRNA and protein in cells treated with 10% CSE in the presence of various concentrations of the isolated nucleosides. Reverse transcription polymerase chain reaction (RT-PCR) and Western blot analyses showed that the nucleosides decreased the levels of iNOS mRNA and protein in 10% CSE-stimulated cells in a concentration-dependent manner (Figure 2B–D).
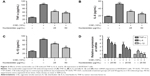
Nucleosides suppress CSE-induced TNF-α, IL-6, and IL-1β expression in RAW264.7 cells
The cells were pretreated for 1 hour with 10% CSE before the nucleoside treatment. After 24 hours, TNF-α, IL-6, and IL-1β expression were measured by ELISA kits and RT-PCR analysis. The results show that the CSE significantly increased the levels of TNF-α (Figure 3A), IL-6 (Figure 3B), and IL-1β (Figure 3C), in contrast to the control group, which was inhibited in a dose-dependent manner by co-treatment with nucleosides. As shown in Figure 3D, the RT-PCR analysis showed that nucleosides significantly decreased the TNF-α, IL-6, and IL-1β mRNA levels induced by 10% CSE.
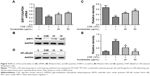
Nucleosides increase SIRT1 and decrease NF-κB/p65 in CSE-induced RAW264.7 cells
The RAW264.7 cells were pretreated for 1 hour with 10% CSE before treatment with the isolated nucleosides for 24 hours. RT-PCR and Western blot analysis showed that the CSE significantly decreased the SIRT1 activity, whereas the isolated nucleosides significantly increased both the SIRT1 protein and its mRNA (Figure 4A–C). Besides, CSE significantly increased the level of NF-κB/p65, whereas the control group was inhibited in a dose-dependent manner by co-treatment with the isolated nucleosides (Figure 4D and E).
Blocking SIRT1 increases NF-κB/p65 activation and attenuates the protective effects of nucleosides on CSE-induced RAW264.7 cells
Previous studies have shown that SIRT1 modulates the activation of NF-κB/p65, which contributes to inflammatory cytokine production.11,12 In this work, the RAW264.7 cells were pretreated with 10% CSE for 1 hour and subsequently treated with a specific SIRT1 antagonist, EX527 (10 μM), and/or nucleosides (50 μg/mL) for 24 hours. The results show that EX527 significantly reversed the suppressive effects of the nucleosides on the CSE-induced mRNA expression of TNF-α, IL-6, IL-1β, and iNOS (Figure 5A). Besides, Western blot results show that the nucleosides obviously down-regulated the expression of NF-κB/p65 and iNOS, and this suppressive effect by the nucleosides was then markedly reversed by the SIRT1 antagonist EX527 (Figure 5B and C). These results demonstrate that the inhibition of nucleosides in the CSE-induced RAW264.7 cells took place through the SIRT1–NF-κB/p65 pathway.
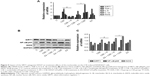
Nucleosides inhibit CSE-induced release of pro-inflammatory mediators in BALF and serum in mice
We measured the levels of pro-inflammatory mediators (NO, TNF-α, and IL-1β) in the BALF and serum in response to CSE interference by intraperitoneal injection in mice. The results show that the CSE-induced group had significantly increased levels of NO, TNF-α, and IL-1β, in contrast to the control group, and this increase was inhibited in a dose-dependent manner by co-treatment with the nucleosides (Figure 6A–C).
Nucleosides inhibit CSE-induced recruitment of inflammatory cells in BALF
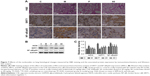
Male Balb/c mice were treated with CSE (0.3 mL) on days 0, 7, and 14, and then killed on day 21. The numbers of inflammatory cells in the BALF were measured by Wright–Giemsa staining. As presented in Figure 6D, the CSE challenge resulted in increased numbers of the total cell and neutrophil counts in the BALF compared with the control group. In contrast, compared with the model group, the nucleosides led to a significant reduction in the total and relative cell counts of neutrophils.
Nucleosides alleviate CSE-induced COPD through the SIRT1–NF-κB/p65 pathway in vivo
It has been shown that SIRT1 is involved in the pathogenesis of COPD, with decreasing levels in mouse lungs exposed to CSE,9,10 and SIRT1-mediated protection in response to CSE-induced lung inflammatory response and injury is linked to the activation of NF-κB/p65.9,12 As shown in Figure 7A, the lung parenchyma of the CSE-induced group (M) showed increased inflammatory cell infiltration, thickened small airways, and alveolar space collapse, whereas no apparent histological changes were detected in lung tissues of the control group (C group). Compared to the CSE-treated group (M group), these histopathological changes were improved in the positive (P group) and nucleoside-treated groups. In addition, immunohistochemistry and Western blot assays showed that CSE treatment increased the levels of iNOS and NF-κB/p65 protein, and decreased the levels of SIRT1 protein, whereas these changes were alleviated by the nucleoside treatment (Figure 7B and C), demonstrating the protective effect of the nucleosides isolated from C. sinensis on COPD models in vitro and in vivo through the SIRT1–NF-κB/p65 pathway.
Discussion
COPD, which is a chronic inflammatory disease of the airways, affects many individuals worldwide. It can cause severe morbidity and mortality if it is exacerbated. To date, the treatment strategy for COPD consists of the use of inhaled bronchodilators (eg, the long-acting β2-adrenoceptor agonist salmeterol), inhaled corticosteroids (eg, fluticasone), and long-acting anticholinergic agents (eg, tiotropium), all of which have limited efficacy and severe side effects. Thus, highly effective and less toxic COPD medications that can alleviate airway inflammation are urgently needed.
Ophiocordyceps sinensis has been shown to have a therapeutic effect on COPD patients. A large number of studies demonstrate that nucleosides, the major bioactive components from C. sinensis, have anti-oxidative, anti-inflammatory, and anti-tumor activities.22,24,25 However, few reported studies have investigated the effects of nucleosides on COPD. In this work, the anti-inflammatory effects of the isolated nucleosides from C. sinensis on CSE-induced COPD models both in vitro and in vivo have been examined. In brief, the levels of pro-inflammatory mediator such as NO, TNF-α, IL-6, and IL-1β were increased after CSE challenge in vitro and in vivo, whereas nucleoside treatment significantly attenuated the increase in these inflammatory mediators (NO, TNF-α, IL-6, and IL-1β). Furthermore, the nucleoside treatment impeded the recruitment of inflammatory cells in the BALF in CSE-treated mice. In addition, SIRT1 expression was reduced by the CSE challenge and markedly reversed by nucleoside treatment, whereas NF-κB/p65 expression was elevated by the CSE challenge and significantly reversed after nucleoside treatment. These results imply that the effect of the isolated nucleosides on airway inflammation induced by CSE may act via the SIRT1–NF-κB/p65 pathway.
Inflammation is thought to play an important role in the pathogenesis of COPD.26,27 It is characterized by the accumulation of inflammatory cells, such as neutrophils and mononuclear cells, and cytokines (eg, NO, TNF-α, IL-6, and IL-1β) are released in lung parenchyma to induce COPD aggravation.8,27,28 Exposure to CSE causes inflammatory responses due to production of the high levels of these cytokines. Inhibition of cytokine production or function severs a key mechanism in the control of inflammation.18 Nucleosides could regulate the release of inflammatory mediators in the immune response in macrophages.25,29 Consistent with the data in the literature, our results show that treatment with the isolated nucleosides significantly improved the histological structure in the lung tissue. They also demonstrate that the nucleosides significantly reduced pulmonary inflammation, as determined by the numbers of total cells and the proportion of neutrophils in the BALF. NO, a prevalent pro-inflammatory mediator, is produced by iNOS, and potential inhibitors of iNOS have been considered to be anti-inflammatory drugs.18 Our results also show that the isolated nucleosides inhibited significantly mRNA and protein expression of iNOS in the CSE-stimulated RAW264.7 cells and mice. These experiments demonstrate that the nucleosides inhibited the CSE-induced inflammation response in COPD.
SIRT1 regulates inflammation, aging, stress resistance, senescence, apoptosis/autophagy, and other cellular functions through deacetylation of transcription factors, histones, and coactivators, such as NF-κB/p53.30,31 Previous studies show that the level of SIRT1 can be reduced by cigarette smoke in the lungs of COPD patients and rats as well as in macrophage cells, which implicates an important role of SIRT1 in the pathogenesis of COPD.30 NF-κB is an important transcription factor responsible for transcription of pro-inflammatory genes, such as TNF-α, IL-6, and IL-1β.8,30,31 In this work, we demonstrated that the CSE challenge reduced the level of SIRT1, resulting in up-regulation of NF-κB/p65 and leading to an increased inflammatory response. The nucleoside treatment increased the reduced expression of SIRT1 and inhibited the inflammation in the CSE-induced RAW264.7 cells and mice. Moreover, the addition of the SIRT1 antagonist EX527 significantly alleviated the inhibitory effect of the nucleosides in the CSE-induced RAW264.7 cells. These results suggest that the isolated nucleosides inhibited CSE-induced inflammation responses through the SIRT1–NF-κB/p65 pathway in COPD.
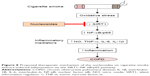
In conclusion, our results reveal that the isolated nucleosides from C. sinensis inhibited inflammation via the activation of SIRT1 and the deactivation of its downstream targets (NF-κB/p65) in RAW264.7 macrophages and COPD mice, the proposed scheme of which is shown in Figure 8. Our findings demonstrate that the isolated nucleosides can be expected to be one of the new potential candidates for use in clinical COPD therapy.
Acknowledgments
This work was supported by funds from the National “Major Science and Technology Project of Prevention and Treatment of AIDS, Viral Hepatitis, and Other Major Infectious Diseases” (grant no 2013ZX10005004), Major Project of Science and Technology of Shandong Province (grant no 2015ZDJS04001), Science & Technology Enterprise Technology Innovation Fund of Jiangsu Province (grant no BC2014172), and Small & Medium Enterprise Technology Innovation Project of Lianyungang City (grant no CK1333).
Disclosure
The authors report no conflicts of interest in this work.
References
Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD – implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis. 2014;9:1207–1224. | ||
Barnes PJ. Chronic obstructive pulmonary disease: a growing but neglected global epidemic. PLoS Med. 2007;4(5):e112. | ||
Trifilieff A, Ethell BT, Sykes DA, et al. Comparing the cardiovascular therapeutic indices of glycopyrronium and tiotropium in an integrated rat pharmacokinetic, pharmacodynamic and safety model. Toxicol Appl Pharmacol. 2015;287(1):9–16. | ||
He X, Li T, Kang N, et al. The protective effect of PRMT6 overexpression on cigarette smoke extract-induced murine emphysema model. Int J Chron Obstruct Pulmon Dis. 2017;12(12):3245–3254. | ||
Kato R, Mizuno S, Kadowaki M, et al. Sirt1 expression is associated with CD31 expression in blood cells from patients with chronic obstructive pulmonary disease. Respir Res. 2016;17(1):139. | ||
van Eeden SF, Sin DD. Oxidative stress in chronic obstructive pulmonary disease: a lung and systemic process. Can Respir J. 2013;20(1):27–29. | ||
Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. | ||
Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12(7):543–559. | ||
Sundar IK, Yao H, Rahman I. Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases. Antioxid Redox Signal. 2013;18(15):1956–1971. | ||
Kato R, Mizuno S, Kadowaki M, et al. Sirt1 expression is associated with CD31 expression in blood cells from patients with chronic obstructive pulmonary disease. Respir Res. 2016;17(1):139. | ||
Caruso R, Marafini I, Franzè E, et al. Defective expression of SIRT1 contributes to sustain inflammatory pathways in the gut. Mucosal Immunol. 2014;7(6):1467–1479. | ||
Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L567–L576. | ||
Zhai XT, Zhang ZY, Jiang CH, et al. Nauclea officinalis inhibits inflammation in LPS-mediated RAW 264.7 macrophages by suppressing the NF-κB signaling pathway. J Ethnopharmacol. 2016;183:159–165. | ||
Babu KS, Morjaria JB. Emerging therapeutic strategies in COPD. Drug Discov Today. 2015;20(3):371–379. | ||
Ram A, Balachandar S, Vijayananth P, Singh VP. Medicinal plants useful for treating chronic obstructive pulmonary disease (COPD): current status and future perspectives. Fitoterapia. 2011;82(2):141–151. | ||
Kim HP, Lim H, Kwon YS. Therapeutic potential of medicinal plants and their constituents on lung inflammatory disorders. Biomol Ther. 2017;25(2):91–104. | ||
Huang TT, Lai HC, Ko YF, et al. Hirsutella sinensis mycelium attenuates bleomycin-induced pulmonary inflammation and fibrosis in vivo. Sci Rep. 2015;5:15282. | ||
Kim KM, Kwon YG, Chung HT, et al. Methanol extract of Cordyceps pruinosa inhibits in vitro and in vivo inflammatory mediators by suppressing NF-kappaB activation. Toxicol Appl Pharmacol. 2003;190(1):1–8. | ||
Chiu CP, Liu SC, Tang CH, et al. Anti-inflammatory Cerebrosides from Cultivated Cordyceps militaris. J Agric Food Chem. 2016;64(7):1540–1548. | ||
Liu A, Wu J, Li A, et al. The inhibitory mechanism of Cordyceps sinensis on cigarette smoke extract-induced senescence in human bronchial epithelial cells. Int J Chron Obstruct Pulmon Dis. 2016;11(1):1721–1731. | ||
Chen X, May B, Di YM, et al. Oral Chinese herbal medicine combined with pharmacotherapy for stable COPD: a systematic review of effect on BODE index and six minute walk test. PLoS One. 2014;9(3):e91830. | ||
Zong SY, Han H, Wang B, et al. Fast simultaneous determination of 13 nucleosides and nucleobases in Cordyceps sinensis by UHPLC-ESI-MS/MS. Molecules. 2015;20(12):21816–21825. | ||
Li SP, Yang FQ, Tsim KW. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J Pharm Biomed Anal. 2006;41(5):1571–1584. | ||
Xie CY, Gu ZX, Fan GJ, Gu FR, Han YB, Chen ZG. Production of cordycepin and mycelia by submerged fermentation of Cordyceps militaris in mixture natural culture. Appl Biochem Biotechnol. 2009;158(2):483–492. | ||
Kumar V, Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009;616(1–3):7–15. | ||
Wang X-L, Li T, Li J-H, Miao S-Y, Xiao X-Z. The effects of resveratrol on inflammation and oxidative stress in a rat model of chronic obstructive pulmonary disease. Molecules. 2017;22(9):1529. | ||
Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122(8):2749–2755. | ||
Leclerc O, Lagente V, Planquois JM, et al. Involvement of MMP-12 and phosphodiesterase type 4 in cigarette smoke-induced inflammation in mice. Eur Respir J. 2006;27(6):1102–1109. | ||
Yang X, Li Y, He Y, et al. Cordycepin alleviates airway hyperreactivity in a murine model of asthma by attenuating the inflammatory process. Int Immunopharmacol. 2015;26(2):401–408. | ||
Hwang JW, Yao H, Caito S, Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med. 2013;61:95–110. | ||
Yao H, Rahman I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol. 2012;84(10):1332–1339. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.