Back to Journals » International Journal of Nanomedicine » Volume 15
Novel Silver-Platinum Nanoparticles for Anticancer and Antimicrobial Applications
Authors López Ruiz A, Bartomeu Garcia C , Navarro Gallón S , Webster TJ
Received 9 June 2018
Accepted for publication 23 August 2018
Published 15 January 2020 Volume 2020:15 Pages 169—179
DOI https://doi.org/10.2147/IJN.S176737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Carlos Rinaldi
Aida López Ruiz, 1 Caterina Bartomeu Garcia, 2 Sandra Navarro Gallón, 2, 3 Thomas J Webster 2
1Department of Chemical Engineering, Universitat Rovira i Virgili, Tarragona, Spain; 2Department of Chemical Engineering, Northeastern University, Boston, MA, USA; 3Facultad de Ciencias Exactas y Naturales, Universidad de Antioquia, Medellin, Colombia
Correspondence: Aida López Ruiz
Department of Chemical Engineering and Materials Science, New Jersey Institute of Technology, University Heights, 148 Chestnut Ave, Jersey City, NJ 07306, USA
Tel +1 2018785235
Email [email protected]
Thomas J Webster
Department of Chemical Engineering, Northeastern University, 360 Huntington Avenue, Boston, MA 02115, USA
Tel +1617 373 6585
Email [email protected]
Background: Cancer is a disease with an enormous worldwide impact. One of the fatal complications in cancer patients are bacterial opportunistic infections. The use of chemotherapeutic drugs made cancer remission more frequent and prolonged patient survival, but, increased the risk of infections.
Purpose: Address the current problem with growing pandemic cancer and considering high risks of complications with bacterial infections, the present study synthesized novel dendritic assembly of silver (Ag)-platinum (Pt) nanoparticles.
Methods: Nanoparticles were characterized by TEM analysis, and the composition was confirmed by EDX. Bacterial studies were performed for Gram-positive Staphylococcus aureus, Gram-negative Pseudomonas aeruginosa and Gram-negative multi-drug resistant Escherichia coli. Cell experiments were performed with two different cancer cell lines, glioblastoma and melanoma to determine anticancer activity. Finally, cytotoxicity with fibroblast was tested.
Results: The TEM analysis of silver-platinum (AgPt) nanoparticles showed dendrimer shape nanoparticles with a mean size of 42 ± 11nm. Elemental composition was analyzed by EDX, confirming the presence of both Ag and Pt metals. The synthesized nanoparticles significantly inhibited the growth of medically important pathogenic, Gram-positive Staphylococcus aureus, Gram-negative Pseudomonas aeruginosa and Gram-negative multi-drug resistant Escherichia coli. Bactericidal effect of AgPt nanoparticles had greater effectiveness than silver nanoparticles. MTS assay revealed a selective and dose-dependent anticancer activity of AgPt nanoparticles over cancer cell lines glioblastoma and melanoma in the 10– 250 μg/mL concentration range. Cytotoxicity experiments with fibroblast cells showed no side effects of nanoparticles against healthy cells at a range of concentrations from 10– 50 μg/mL.
Conclusion: The newly synthesized AgPt nanoparticles have a promising future as a potent anticancer agent with antibacterial properties.
Keywords: silver, platinum, nanoparticles, antimicrobial, anticancer, multi-drug resistant bacteria
Background
Cancer is a disease with an enormous worldwide impact. According to the National Cancer Institute (NCI), in 2016 around of 1.700.000 new cases of cancer were diagnosed in the United States, and 595,690 people died because of this disease. One of the fatal complications in cancer patients are bacterial opportunistic infections. The use of chemotherapeutic drugs made cancer remission more frequent and prolonged patient survival, but, increased the risk of infections.1 One example is the case of hepatocellular carcinomas, which is the most common type of primary liver cancer, where up to 80% of these cases are associated with chronic infections.2
The discovery of antibiotics is arguably one of the most important and revolutionary events in the history of medicine. However, their recent overuse has led to the growing prevalence of antibiotic-resistant bacteria.3–6 In fact, the Centers for Disease Control and Prevention (CDC) has also predicted more deaths from microorganisms than from all cancers combined by 2050. With the growing prevalence of acquiring bacterial infections in hospitals, especially in immunocompromised patients, such as those with cancer, there remains limited treatment options to combat these bacteria.7
Gram-negative bacteria are of particular interest because they are resistant to numerous different antibiotics and drugs (antibiotics containing β-lactum, such as penicillin, ampicillin, and methicillin).4,8 The emergence of multi-drug resistant (MDR) Gram-negative bacteria has affected the practice of almost every field of medicine.9 Therefore there is a need for the same material to be both antibacterial and anticancer.10 Consequently, there is a substantial need to develop new ways to treat cancer as well as infections caused by antibiotic resistant bacteria.3
Nanotechnology is a multidisciplinary field in chemistry, engineering, biology and medicine. There are a lot of different applications such as cancer, for both detection or treatment, and also combatting multi-drug resistance.11 To address the growing threat of antibiotic resistant bacteria and improve the treatment of cancer diseases, nanotechnology with biomedical applications is a very promising strategy to solve these problems.12 To combat drug-resistant bacteria, nanomaterials with antimicrobial effects can be used, such as silver, copper, selenium, palladium, and titanium dioxide.13,14 The antimicrobial activity of the nanoparticles is based on their small size and high surface area.15 Their small size and high surface area allow nanoparticles, to penetrate biofilms as well as bacteria cell walls, influencing intracellular mechanisms. On the other hand, nanoparticles can be used for cancer treatment through the incorporation of hydrophilic polymers that create a stealth surface for opsonization.11
The composition chosen for the presently fabricated nanoparticles is an alloy of silver and platinum. This composition was selected due to the high antimicrobial effect of silver and for the anticancer properties of platinum.16,17 The antibacterial activity of silver nanoparticles is based on the penetration of the cell membrane, which suppress the respiratory enzyme.18 Additionally, nanoparticles interfere with the DNA inside the bacteria cells, preventing the replication and transcription of the genetic material.19 On the other hand, platinum has a remarkable anticancer activity.20 For example, the use of cisplatin is further studied as a chemotherapeutic drug.21 The mechanism involve the binding to the DNA producing cell death by apoptosis or necrosis.22 Also, platinum can be used for imaging, Pt(II) complexes have photostability for multiphoton excitation in cells.23 That means, that using low-energy light in the visible region enhances imaging depths.23 However, drugs like cisplatin are not selective for cancer cells, and mammalian cells are also affected.24 For these reasons, platinum nanoparticles are a better option due to their lower toxicity and imaging characteristics.17
Materials and Methods
Chemicals and Reagents
Silver nitrate, Potassium tetrachloropalatinate (II), Ascorbic acid, Sodium chloride, Brij58, Tryptic soy broth (TSB), Fetal bovine serum (FBS), Penicillin-streptomycin solution, Trypsin and LIVE/DEAD™ Viability/Cytotoxicity Kit, were purchased from Sigma-Aldrich, United States. Dulbecco’s Modified Eagle’s Medium (DMEM) and Fluorescent solution BacLight™ bacterial viability kit, were obtained from Thermo Fisher, United States. CellTiter 96® AQueous One Solution Cell Proliferation assays (MTS) was acquired from Promega, United States. All chemicals were used as received.
Mammalian Cells and Bacteria Strains
S. aureus (12600), Gram-negative P. aeruginosa (27853) and multi-drug-resistant E. coli (#BAA-2471) were purchased from American Type Culture Collection (ATCC). Also, human dermal fibroblasts cells (HDF) (Detroit 551 - CCL-110), melanoma cells (#A375) and glioblastoma cells (U 87) were purchased from ATCC.
Characterization
Synthesis and Characterization of the Nanoparticles
The methodology for synthesizing the nanoparticles was adapted from Shim et al.25 As described below, the morphology, structure, and size of the nanoparticles was characterized using Transmission electron microscopy (TEM) (JEM 1010, JEOL, United States) with an acceleration voltage at 80Kev. The composition of the nanoparticles was measured by Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray analysis (EDX) (Hitachi S-4800, Hitachi, Japan). The charge of the nanoparticles was measured by Zeta potential using Dynamic Light Scattering (DLS) (90Plus Zeta, Brookhaven Instruments, Holtsville, NY).
Antibacterial Activity in vitro
The antibacterial effect of the nanoparticles was tested with three different kinds of bacteria Gram-positive S. aureus, Gram-negative P. aeruginosa and multi-drug-resistant E. coli. The first step was incubating a single colony of bacteria selected from cultured agar plates with TSB media for 8 hrs at 37 °C in a shaking incubator. After this period of time, the concentration of bacteria was determined by optical absorbance measurements at 600 nm (OD600) with a Spectrophotometer (SpectraMax Paradigm, Molecular Devices, Silicon Valley, CA) and then was diluted to obtain a final bacteria density of 105 CFU/mL.
For the bacteria growth curve, different concentrations of nanoparticles (10, 25, 50 and 75 µg/mL) were added to a 96-well plate containing 100 µL of bacteria suspension with TSB. The plate was then incubated at 37 °C inside a spectrophotometer. Bacteria measurements were taken every 2 mins for 24 hrs with an optical density (OD) 600, and repeated three times three.
For colony counting, nanoparticles were used at a 10 µg/mL concentration and were added into a 96-well plate with 100 µL of a bacteria suspension. The plate was incubated at 37 ºC for 8 hrs inside the incubator until the bacteria reached the exponential growth phase. To prepare the agar plates, different dilutions were performed, and once the agar plate was labeled with the concentration of bacteria in every region, 30 µL was introduced into each section of the plate. Then, the plates were incubated for 9 hrs at 37 °C and measured for colony unit formation. All the experiments were repeated three times.
Cytotoxicity
To determine the cytotoxicity of nanoparticles to healthy human cells, MTS assays were performed with HDF. The cells were cultured with DMEM, complemented with 10% FBS and a 1% penicillin-streptomycin solution. For the incubation, the cells were cultured at 37 °C in a 75 mL flask. After the cells reached 80% confluence, they were detached from the surface using trypsin 0.05% for 5 min. The cells were collected by centrifugation (Centrifuge 5804 R, Eppendorf, Germany) at 222 rcf for 5 min.
MTS were performed for 1, 3 and 5 days. Cells were cultured in a 96-well plate at a concentration of 50,000 cells/mL and then were incubated for 24 hrs at 37 °C. After the incubation, the media was replaced with different concentrations of nanoparticles (10, 25, 50, 75, 100, 150, 200 and 250 µg/mL) in cell media and incubated for 1, 3 or 5 days. After incubating for the time required (the media was not changed between these days), the viability of the cells was determined by removing media and adding the MTS solution, which was then incubated for 3 hrs at 37 °C. To determine cell viability, the absorbance of every well was measured at a wavelength of 490 nm using a spectrophotometer. All experiments were performed three times three (nine times in total).
Cancer Cell Assays
To determine the cancer cell response to the nanoparticles, MTS assays were performed with two different kind of cancer cells, melanoma and glioblastoma. The cultured method used was the same for healthy cells. The cells were cultured with DMEM, complemented with 10% FBS and a 1% penicillin-streptomycin solution.
MTS assays were performed for 1, 3 and 5 days. Cells were cultured in a 96-well plate at a concentration of 50,000 cells/mL and then were incubated for 24 hrs at 37 °C. After the incubation, the media was replaced with different concentrations of nanoparticles (10, 25, 50, 75, 100, 150, 200 and 250 µg/mL) in cell media and incubated for 1, 3 or 5 days. After incubating for the time required (the media was not changed between these days), the viability of the cells was determined by removing media and adding the MTS solution, which was then incubated for 3 hrs at 37 °C. To determine cell viability, the absorbance of every well was measured at a wavelength of 490 nm using a spectrophotometer.
Fluorescence Microscopy Cell Assays
As a qualitative measurement, live/dead bacteria and cell viability assays were performed using fluorescence microscopy (Zeiss inverted fluorescence microscope, SpectraMax, Molecular Devices, Silicon Valley, CA). For this, bacteria at a 1×106 CFU/mL concentration and fibroblasts at 50,000 cells/mL were incubated with different concentrations of nanoparticles for 24 hrs. After 24 hrs, the plate was centrifuged to remove the supernatant and 100 µL of 0.85% NaCl was added (two times). Immediately thereafter, 1.25 µL of each fluorescent solution dye, Calcein and EthD-1, from the BacLight™ bacterial viability kit or LIVE/DEAD™ Viability/Cytotoxicity Kit in 1 mL of a 0.85% NaCl solution was added to each well and kept in the dark for 15 min. Finally, the solution was centrifuged, washed with 0.85% NaCl, and resuspended for fluorescence microscopy.
Statistics
Each experiment was performed at least three times three. Data are presented as mean, standard error of the mean was calculated, and also two-tailed Student’s t-tests were used to evaluate differences between means, with a value of p-value < 0.05 considered statistically significant.
Results and Discussion
Characterization of Silver/Platinum Nanoparticles (AgPt)
Transmission electron micrographs of the synthesized AgPt nanoparticles revealed the formation of dendritic shape nanoparticles, classifying them as a dendritic assembly of silver and platinum nanoparticles (Figure 1). And a size distribution of 42.5 ± 11 nm with a polydispersity index (PDI) of 0.2588 was calculated by analysis of TEM images. To determine stability and charge of the nanoparticle dispersion, Zeta potential was acquired showing a value of −30 mV, suggesting that nanoparticles were negatively charged and moderately stable in solution.26 The elemental composition of AgPt nanoparticles was confirmed by EDX (Figure 2). Peaks at 2.1 confirmed the presence of platinum, while the peaks between 2.9 and 3.2 indicated the presence of silver. Also, peaks were detected from carbon (C) and oxygen (O), from the polymer Brij 58 and copper (Cu) from the grid.
 |
Figure 1 TEM images of AgPt nanoparticles, imaged at a voltage of 60kV and 25000x magnification. |
 |
Figure 2 EDX spectra of AgPt nanoparticles. Peaks at 2.01 indicate platinum while between 2.8 and 3.35 indicate silver. |
Anticancer Assays
MTS Assays
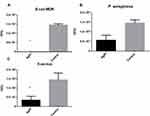
In this research, the cytotoxic effect of AgPt nanoparticles against two human cancer cells, melanoma and glioblastoma, was carried out in vitro by MTS assay. The results disclosed a high grade of cytotoxicity against melanoma cell line (Figure 3). Cytotoxicity increased with increase in concentration of AgPt nanoparticles. It is worth of mention that for concentrations from 10 to 50 µg/mL, the AgPt nanoparticles demonstrated a remarkable and statistically significant ability to reduce the viability of cancer cells (Figure 3). Although the results with melanoma and glioblastoma cells were quite similar, the effect of the nanoparticles was higher against glioblastoma cells, showing a decrease in cell viability around 60%, compared to control. The half maximal inhibitory concentration (IC50) calculated for AgPt nanoparticles against cancer cells was 50 µg/mL for the five days that the analysis lasted.
Live/Dead Assays
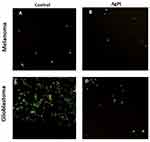
The proliferation of glioblastoma and melanoma cells was visualized under confocal microscopy after 1 day of treatment with AgPt nanoparticles and was compared with non-treated cells (control). The images obtained confirmed the anticancer activity of the nanoparticles. Although glioblastoma showed a significant reduction on cell number after treatment, the absence of cells stained in red can be due to detachment and loss of such dead cells during the processing of the samples (Figure 4). The results suggested that the nanoparticles not only killed cancer cells, but also prevented cell growth. A hypothetical mechanism of the anticancer activity of AgPt nanoparticles against glioblastoma and melanoma cells, could be related with the direct interaction of the nano-scaled particles with the cell surface, affecting and disrupting the cell membrane and affecting the normal development of the cell.27
Cytotoxicity
Human dermal fibroblasts are cells from the skin that are responsible for generating connective tissue and allowing the skin to recover from injury. This type of cells was selected due to the interest about to test AgPt particles as a novel treatment for skin cancer, but also, because HDF are commonly used as a model cell type for cytotoxicity tests with novel drugs. The results obtained from the treatment of HDF with AgPt nanoparticles, are presented in Figure 5. The analysis showed a high percentage of cell viability after treatment with concentrations below of 75 µg/mL. No significant differences were found between controls and treatments at 10, 25, and 50 µg/mL of AgPt. Higher concentrations of nanoparticles exert toxic effects over HDF cells. In addition, the viability of cells after 1, 3 and 5 days of treatment was quite similar, thus, it seems that prolongation on contact time with nanoparticles do not have an effect on toxicity. The IC 50 calculated for AgPt nanoparticles was 75 µg/mL, for all days measured. According to the results obtained from the anticancer and the cytotoxicity studies, it can be inferred that concentrations from 10 to 50 µg/mL of AgPt nanoparticles will have remarkable anticancer activity with no fibroblast cytotoxicity.
Antimicrobial Activity Assays
Bacteria Growth Curve Studies
To test the antibacterial effects of AgPt nanoparticles, different concentrations (10, 20, 50, and 75 µg/mL) were added to bacterial cultures and growth curves were generated. Two different nanoparticles were tested: AgPt and Ag (as a control) on the three previously mentioned bacteria. It was observed that AgPt nanoparticles inhibited the growth of each type of bacteria (Figure 6). Compared to Ag incubation, which served as a control, AgPt demonstrated significantly more antibacterial effects. On the other hand, for Gram-positive bacteria, the antibacterial effect was lower. This may be due to the thicker peptidoglycan layer in the cell wall of Gram-positive bacteria and, are thus, likely more difficult to kill. Furthermore, as the concentration of nanoparticles increased, the bacterial population decreased.
As a quantification of the OD measurements, a modified Gompertz model was used to assess the effects of nanoparticles on the bacterial growth curve:
(1)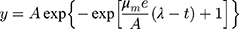
Where A is the asymptotic absorbance, µm is the maximum exponential growth rate, λ is the lag time, t is the time and y is the absorbance.
According to the Gompertz model curve fitting results, the calculated lag time was significantly higher as the concentration of nanoparticles increased. During lag phase conditions, bacteria are not able to divide and consequently the concentration of bacteria is constant due to the effect of nanoparticles. However, the maximum asymptotic absorbance was the reverse, as with higher concentrations of nanoparticles, lower A resulted. The maximum asymptotic absorbance determines bacteria maximum growth value, so, the use of nanoparticles decrease the final concentration of bacteria. With this value, it was demonstrated that the use of AgPt nanoparticles affected the bacteria growth, most impressively, for Gram-positive, Gram-negative and multi drug resistant bacteria.
Colony Counting
In agreement with the results obtained with growth curve assays, colony counting experiments (Figure 7) showed a remarkable antimicrobial effect even for the minimum concentration used (10 µg/mL). For P. aeruginosa, incubation with AgPt nanoparticles resulted in 60% viability. For multi-drug resistant E. coli, nanoparticle treatment showed a 1% viability. Finally, for S. aureus, incubation with AgPt nanoparticles resulted in a 12% viability. These experiments gave a confirmation of what was already observed with growth curve, with more accurate results. Collectively, these results concluded that AgPt nanoparticles are an effective antimicrobial treatment. The most likely killing mechanism that could take place over bacteria is the disruption on the bacterial cell membrane and the release of metal ions. Metal ions, but also nanoparticles are able to cross membranes due to their small size and high surface, thereby interrupting a series of intracellular processess.28 According to the results obtained from the bacterial assays and the cytotoxicity studies, it can be inferred that concentrations from 10 to 50 µg/mL of AgPt nanoparticles will have remarkable antibacterial activity with no fibroblast cytotoxicity.
Live/Dead Assays
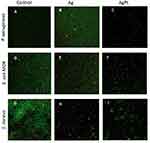
As a qualitative test, live/dead assays were performed with the nanoparticles at a 10 µg/mL concentration. As a confirmation of the growth curve experiments and colony counting, data from live/dead assays (Figure 8) demonstrate that the use of nanoparticles killed bacteria, due to the increase in the number of dead bacteria (red spots). Furthermore, the use of nanoparticles not only killed bacteria, but also did not allow them to grow, reducing the density of bacteria when using nanoparticles more than for the control. That means, that the nanoparticles are bacteriostatic agents that stop bacteria from reproducing but not necessarily kill all of them. Also, with this test it is possible to appreciate that AgPt nanoparticles are more effective than Ag nanoparticles alone, due to the difference in bacteria density after the treatment with both nanoparticles.
Conclusions
In conclusion, it has been successfully demonstrated the synthesis and applicability of new silver-platinum nanoparticles as anticancer and antibacterial agents. The characterization of the physicochemical properties has been carefully assessed. And biological experiments were performed to evaluate the antibacterial activity against three bacteria strains of medically relevance. The study, thus, showed that the newly AgPt nanoparticles possessed anticancer activity against melanoma and glioblastoma cells without being harmful to healthy human fibroblasts, while exhibiting at the same time antibacterial activity over Gram-positive, Gram-negative and multidrug-resistant bacteria.
The anticancer activity of such nanoparticles could be allied with the interaction between particles and cell membrane, preventing cancer spread or even killing the cancer cells. The selectivity of cytotoxicity observed over human cancer cells, compared with human healthy fibroblast, could be due to the targeting of cancer cells, via enhanced permeability and retention. Where nanoparticles tend to accumulate in tumor tissue, because its higher permeability compared to healthy tissue.27 In summary, the present nanoparticles could be a promising selective anticancer agent able to overcome the most important problem of most of the anticancer drugs, their toxicity against healthy cells. Furthermore, the inhibitory effect of AgPt nanoparticles over bacteria would allow the prevention of infections by opportunistic pathogens in immunocompromised patients. Furthermore, the effect of the presented nanoparticles against multi-drug resistant bacteria opens new ways of treatment for infections. Considering the interesting previous results, it is necessary further investigations conducted to in vivo research.
Acknowledgements
The authors would like to thank Northeastern University for the founding.
Author Contributions
ALR performed all the experimental part, and wrote the manuscript. CBG, SNG and TJW help in the data analysis and experimental design. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Miss Aida Lopez Ruiz reports a patent (The nanoparticles have patent pending from Northeastern University) 19815-473-INV17097. The authors declare no other competing interests in this work.
References
1. Dionigi R, Dominioni L, Campani M. Infections in cancer patients. Surg Clin North Am. 1980;60(1):145–159. doi:10.1016/S0039-6109(16)42040-2
2. Alibek K, Bekmurzayeva A, Mussabekova A, Sultankulov B. Using antimicrobial adjuvant therapy in cancer treatment: a review. Infect Agent Cancer. 2012;7:33. doi:10.1186/1750-9378-7-33
3. Mi G, Shi D, Herchek W, Webster TJ. Self-assembled arginine-rich peptides as effective antimicrobial agents. J Biomed Mater Res Part A. 2017;105(4):1046–1054. doi:10.1002/jbm.v105.4
4. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277–283.
5. Harris M. Pharmaceutical Microbiology. J of Med Ed 1964; 39(11):xix.
6. Antibiotic/Antimicrobial Resistance | CDC. Available from: https://www.cdc.gov/drugresistance/. Accessed April 25, 2017.
7. Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362(19):1804–1813. doi:10.1056/NEJMra0904124
8. Chatterjee T, Chatterjee BK, Majumdar D, Chakrabarti P. Antibacterial effect of silver nanoparticles and the modeling of bacterial growth kinetics using a modified Gompertz model. Biochim Biophys Acta. 2015;1850(2):299–306. doi:10.1016/j.bbagen.2014.10.022
9. Golkar Z, Bagasra O, Pace DG. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J Infect Dev Ctries. 2014;8(2):129–136. doi:10.3855/jidc.3573
10. MacDonald V. Chemotherapy: managing side effects and safe handling. Can Vet J. 2009;50(6):665–668.
11. Jabir NR, Tabrez S, Ashraf GM, Shakil S, Damanhouri GA, Kamal MA. Nanotechnology-based approaches in anticancer research. Int J Nanomedicine. 2012;7:4391–4408. doi:10.2147/IJN.S33838
12. Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomedicine. 2011;6:1463–1473. doi:10.2147/IJN.S22021
13. Jayawardena HSN, Jayawardana KW, Chen X, Yan M. Maltoheptaose promotes nanoparticle internalization by Escherichia coli. Chem Commun (Camb). 2013;49(29):3034–3036. doi:10.1039/c3cc40491a
14. Chen W-Y, Chang H-Y, Lu J-K, et al. Self-assembly of antimicrobial peptides on gold nanodots: against multidrug-resistant bacteria and wound-healing application. Adv Funct Mater. 2015;25(46):7189–7199. doi:10.1002/adfm.201503248
15. Ishihara M, Nguyen VQ, Mori Y, Nakamura S, Hattori H. Adsorption of silver nanoparticles onto different surface structures of chitin/chitosan and correlations with antimicrobial activities. Int J Mol Sci. 2015;16(6):13973–13988. doi:10.3390/ijms160613973
16. Jahromi EZ, Divsalar A, Saboury AA, Khaleghizadeh S, Mansouri-Torshizi H, Kostova I. Palladium complexes: new candidates for anti-cancer drugs. J Iran Chem Soc. 2016;13(5):967–989. doi:10.1007/s13738-015-0804-8
17. Bendale Y, Bendale V, Paul S. Evaluation of cytotoxic activity of platinum nanoparticles against normal and cancer cells and its anticancer potential through induction of apoptosis. Integr Med Res. 2017;6(2):141–148. doi:10.1016/j.imr.2017.01.006
18. Bragg PD, Rainnie DJ. The effect of silver ions on the respiratory chain of Escherichia coli. Can J Microbiol. 1974;20(6):883–889. doi:10.1139/m74-135
19. Li W-R, Xie X-B, Shi Q-S, Zeng H-Y, OU-Yang Y-S, Chen Y-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 2010;85(4):1115–1122. doi:10.1007/s00253-009-2159-5
20. Reedijk J. The mechanism of action of platinum anti-tumor drugs. Pure AppI Chem. 1987;59(2):181–192. doi:10.1351/pac198759020181
21. Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307–320. doi:10.1038/nrd1691
22. Fuertes MA, Alonso C, Pérez JM. Biochemical modulation of cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem Rev. 2003;103(3):645–662. doi:10.1021/cr020010d
23. Botchway SW, Charnley M, Haycock JW, et al. Time-resolved and two-photon emission imaging microscopy of live cells with inert platinum complexes. Proc Natl Acad Sci U S A. 2008;105(42):16071–16076. doi:10.1073/pnas.0804071105
24. Johnstone TC, Suntharalingam K, Lippard SJ. The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev. 2016;116(Ii):3436–3486. doi:10.1021/acs.chemrev.5b00597
25. Shim K, Kim J, Heo Y-U, et al. Synthesis and cytotoxicity of dendritic platinum nanoparticles with HEK-293 cells. Chem - an Asian J. 2017;12(1):21–26. doi:10.1002/asia.201601239
26. Hanaor D, Michelazzi M, Leonelli C, Sorrell CC. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J Eur Ceram Soc. 2012;32(1):235–244. doi:10.1016/j.jeurceramsoc.2011.08.015
27. Sharma A, Goyal AK, Rath G. Recent advances in metal nanoparticles in cancer therapy. J Drug Target. 2017;26(8):617–632. doi:10.1080/1061186X.2017.1400553
28. Lam SJ, O’Brien-Simpson NM, Pantarat N, et al. Combating multidrug-resistant gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat Microbiol. 2016; 1(11):16162. doi:10.1038/nmicrobiol.2016.162
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.