Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 14
Noninfectious, Severe Cryoglobulinemic Vasculitis with Renal Involvement – Safety and Efficacy of Long-Term Treatment with Rituximab
Authors Leśniak K, Rymarz A , Lubas A , Niemczyk S
Received 29 April 2021
Accepted for publication 30 June 2021
Published 16 July 2021 Volume 2021:14 Pages 267—277
DOI https://doi.org/10.2147/IJNRD.S315388
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Pravin Singhal
Ksymena Leśniak, Aleksandra Rymarz, Arkadiusz Lubas, Stanisław Niemczyk
Department of Internal Medicine, Nephrology and Dialysis, Military Institute of Medicine, Warsaw, Poland
Correspondence: Ksymena Leśniak
Department of Internal Medicine, Nephrology and Dialysis, Military Institute of Medicine, Warsaw, Poland
Email [email protected]
Background: The management of nonviral cryoglobulinemic vasculitis (CV) has not been established yet. Randomized control trials are challenging to perform because of the rarity of the disease. The most promising biological therapy is rituximab (RTX), an anti-CD 20 monoclonal antibody. The aim of the study was to assess rituximab treatment’s safety and effectiveness in patients with severe noninfectious cryoglobulinemic vasculitis.
Materials and Methods: We retrospectively reviewed 8 courses of RTX treatment in three patients with severe noninfectious CV. In 2 patients, the indication for the start of RTX therapy was the relapse of the disease despite the maintenance treatment, for the third patient, it was the first-line therapy.
Results: Clinical, renal, and immunologic efficacy was observed in all evaluable RTX courses. We found a significant decrease of cryoglobulins in the 3-rd month from RTX treatment. However, 5 clinical relapses occurred and two patients experienced severe adverse events (SAEs) after RTX therapy. Patients with SAEs were relatively older and had a longer duration of disease. Lower levels of hemoglobin, C3 component of complement and eGFR as well as higher rheumatoid factor (RF) concentration were observed before RTX treatments complicated with SAEs.
Conclusion: Data from our observation show the efficacy of rituximab in the refractory, nonviral cryoglobulinemic vasculitis with a severe course of the disease. However, the therapy is associated with the risk of SAEs, especially in elderly patients with kidney failure and significant immunologic alterations.
Keywords: cryoglobulinemia, rituximab, systemic vasculitis
Background
Cryoglobulinemic vasculitis is a systemic vascular inflammation characterized by the proliferation of B cell clones that produce pathogenic immunoglobulins, precipitating in vitro at temperatures below 37°C and dissolve upon rewarming.1 Concerning immunochemistry, cryoglobulins are usually divided into three subsets according to the classification by Brouet et al.2 In type I cryoglobulins are monoclonal immunoglobulins always associated with B-cell lymphoproliferative diseases. Mixed cryoglobulinemia (MC), containing both type II (mix of polyclonal IgG with monoclonal IgM with rheumatoid factor activity) and type III (polyclonal IgG and polyclonal IgM with rheumatoid factor activity) cryoglobulinemia. Mixed cryoglobulinemia is associated with infections, especially hepatitis C virus (HCV) infection (70–90%), while B cell lymphoproliferative disorders, autoimmune disorders [mainly primary Sjögren’s syndrome (SS), systemic lupus erythematosus (SLE)] and “essential MC” account for 10–30% of incidents.3–5 Clinical symptoms range from mild MC (purpura, arthralgia, and asthenia) to more severe manifestations such as neurological, renal, hepatic, or life-threatening widespread vascular involvement.6–9 MC is also associated with an increased possibility of developing B-cell non-Hodgkin lymphoma (NHL).10
Renal involvement (RI) has been reported in 18–40% of patients with cryoglobulinemic vasculitis.4,7,10 In patients with cryoglobulinemic vasculitis, RI is one of the worse prognostic factors, and death occurs more frequently than in individuals without RI.7,10,11 Renal involvement occurs 2.6–4 years after the diagnosis of cryoglobulinemia.12,13 The risk factors for kidney involvement are mainly: type II cryoglobulinemia, high serum cryoglobulin levels, the presence of monoclonal IgG kappa. Proposed biological risk factors for death or dialysis in patients with MC glomerulonephritis are: cryocrit levels higher than 10%, C3 concentration lower than 54mg/dl, serum creatinine level above 1.5mg/dl.12–14
The treatment of mixed cryoglobulinemia is still largely empirical, but the rational therapeutic approach to patients with severe or life-threatening manifestations need urgent intervention to suppress immune complex formation. This is accomplished with immunosuppressive therapy (IST), which is used in other systemic vasculitides. The severe form includes skin ulcers, glomerulonephritis, progressive neuropathy, while life-threatening instance affects rapidly progressive glomerulonephritis (RPGN), alveolar bleeding, central nervous system (CNS) involvement, and intestinal ischemia. IST is primarily based on high-dose corticosteroids, cyclophosphamide, rituximab, and plasmaphereses. Both plasma exchange and plasmapheresis remove cryoglobulins from the circulation, thereby interrupting the immune-complex-mediated pathogenesis of cryoglobulinemic vasculitis. These interventions are useful in patients with an immediate life-threatening disease and for those with hyperviscosity syndrome.15
A vast amount of reports have reported the efficiency of anti-B cell therapy with rituximab for refractory HCV-associated MC despite antiviral therapy,16–22 as well as for noninfectious MC vasculitis.11,23–25 The rarity of the disease causes that data from randomized controlled trials (RCTs) are lacking, and the management of nonviral cryoglobulinemic vasculitis has not been defined yet, although relapses remain a major problem.
The aim of our study was to assess the effectiveness and safety of long-term treatment with rituximab in patients with severe noninfectious cryoglobulinemic vasculitis with renal involvement.
Materials and Methods
A retrospective analysis of all patients with cryoglobulinemic vasculitis (CV) without anti-HCV antibodies and HCV-RNA treated with rituximab were performed. The patients underwent treatment in the Nephrological Department between April 2013 and October 2020.
Patients included into our analysis fulfilled following criteria: 1) positive serum cryoglobulin with cryoglobulin total concentration >0.05g/L 2) symptoms of cryoglobulinemic vasculitis, 3) absence of anti-HCV, anti-hepatitis B virus (HBV), and anti-human immunodeficiency virus (HIV) antibodies and 4) rituximab therapy of cryoglobulinemic vasculitis.
The clinical data consisted of age, sex, neurologic involvement (peripheral neuropathy confirmed by electrophysiological tests, central nervous system (CNS) vasculitis demonstrated by magnetic resonance imaging (MRI), cranial nerve involvement), skin involvement (purpura, distal ulcers or Raynaud’s phenomenon), arthralgia, gastrointestinal vasculitis (abdominal pain, liver abnormalities, gastrointestinal ulcers with or without bleeding in endoscopy), pulmonary involvement (pulmonary hemorrhage without edema, adult respiratory distress syndrome, infectious pneumonia, lung cancer, or granulomatous disease). Renal involvement was defined using the following criteria: proteinuria >0.5g/24h, nephrotic range proteinuria >3g/24h, hematuria >10 red blood cells/mm3, renal failure with an estimated glomerular filtration rate (eGFR) <60mL/min/1.73m2).14 eGFR was achieved using the Modification of Diet in Renal Disease formula (MDRD).26
Laboratory tests which were performed included: serum cryoglobulin level, rheumatoid factor (RF), C3, C4 components of complement, protein electrophoresis, creatinine level, alanine aminotransferase (ALT), albumin, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT), hemoglobin (Hb) level, amount of white blood cells, serum IgG and IgM levels, HbsAg, anti-HCV antibodies, and HCV RNA, and urinalysis to screen for hematuria and a 24-hour urine protein examination. Clinically required organ involvement (skin, kidney, liver, bone marrow) was further investigated using ultrasonography or bio-optical tissue examination.
All blood samples were collected with pre-warmed syringes and tubes, allowed to clot, and centrifuged at 37 °C for serum separation, according to previously published recommendations.27
The presence of cryoglobulins was determined based on the loss of immunoglobulins and C3 and C4 fragments of the serum complement after 48 hours of incubation at 4°C. Immunoglobulin and complement fragments concentration were measured by nephelometry on a BN II analyzer. The results were reported quantitatively and as a percentage loss of serum components at 4° C.
The remaining losses of protein fractions were corrected by performing proteinograms twice at 37 ° C and 4 ° C (electrophoresis method using agarose gel on an InterLab G26 analyzer). In order to make proteinograms, it was necessary to determine total protein (colorimetric method - biuret reaction) and albumin (colorimetric method - bromocresol green method). Total protein and albumin were measured on a Cobas c 501 analyzer.
Most expert centers consider cryoglobulin’s value to be clinically significant >0.05g/L on two tests.2,4
In each case of CV, the recently proposed classification criteria were fulfilled.28
The diagnosis of primary SS was made on the basis of American-European Consensus Group classification criteria.29
Premedication with 100 mg of intravenous methylprednisolone, clemastinum and acetaminophen was administered before each infusion of RTX. Individuals with a follow-up longer than 3 months were assessed for the efficacy of rituximab by means of clinico-serological parameters.
The response to therapy were evaluated by comparing clinical and immunological parameters prior to rituximab infusion and at 3-month follow-up.
The clinical response to treatment was defined by evaluating main clinical factors such as cutaneous involvement (improvement or absence of purpura or ulcerative lesions), peripheral neuropathy (clinical and/or electrophysiological abnormalities), and absence of arthralgia or arthritis. A renal response was defined by a proteinuria <0.5 g/d, and/or the disappearance of hematuria and/or an improvement of eGFR >20% in case of baseline eGFR <60 mL/min/1.73m2. A complete clinical response (CR) was defined as a disappearance of baseline main clinical manifestations. A partial response (PR) was defined as an improvement of the baseline clinical manifestations (at least 50% of baseline). Patients without response or with an insufficient response to treatment were classified as nonresponders (NR).11
For immunological parameters, a complete response was defined by the disappearance of serum cryoglobulins, while a partial response was defined as a > 50% decrease in the baseline cryoglobulin level. In patients with abnormally reduced C4 (below the lower limit of normal range), the complete response was referred to the C4 normalization and the partial response to increased C4 levels without normalization. Relapse was interpreted as the reoccurrence of clinical symptoms of vasculitis.11
Severe adverse events attributable to rituximab were defined as an adverse event occurring in the infusion sessions or within the first twelve months following the treatment and that needed hospitalization and/or intravenous antibiotics administration in case of infection or resulted in death.11
All patients signed an informed consent form for the treatment procedures. The study design was accepted by Military Institute of Medicine Bioethics Committee (approval number 37/WIM/2018). The study was conducted in accordance with the Declaration of Helsinki.
Statistics Methods
Statistical analysis was conducted using STATISTICA v.12 software (StatSoft, Cracow, Poland). Results were presented as mean with standard deviation for variables with the distribution not different from the normal, otherwise, in the form of a median with interquartile range (IRQ). Differences between groups were tested with the use of the t-test and Mann–Whitney test depending on the fulfillment of an assumption of the normal distribution. Differences between paired variables were estimated using paired t-test or Wilcoxon’s test, accordingly. We performed ROC analyses to identify the best cut-off point value that could help recognize worse treatment outcomes. The statistical results were considered significant if the value of the type I error α < 0.05.
Results
Clinical characteristics of the investigated patients are presented in Table 1.
 |
Table 1 Clinical Characteristics of Patients with Cryoglobulinemic Vasculitis (CV) Before First Rituximab Therapy |
Individual characteristics of patients with cryoglobulinemic vasculitis (CV) treated with rituximab are presented in Table 2.
 |
Table 2 Individual Characteristics of Patients with Cryoglobulinaemic Vasculitis (CV) Treated with Rituximab |
Clinical Course
Clinical manifestations included cutaneous involvement in 3 patients (Raynaud’s phenomenon 1/3, purpura 3/3, distal ulcers 1/3, fingers skin necrosis 1/3), arthralgia/ arthritis in 3 patients, renal involvement in 3 patients (microscopic hematuria 3/3, renal failure 2/3, proteinuria 2/3), gastrointestinal involvement in 3 patients (abdominal pain 3/3, liver abnormalities 1/3, gastrointestinal ulcers with or without bleeding in endoscopy 2/3) and CNS in 3 patients (CNS vasculitis 3/3 and cranial nerve involvement 1/3], peripheral neuropathy in 2 patients, pulmonary involvement in 1 case (pulmonary hemorrhage leading to respiratory failure). All of the patients had a severe presentation of cryoglobulinemic vasculitis (Figure 1). The underlying disorder associated with noninfectious mixed cryoglobulinemia were connective tissue disease in 1 patient (primary Sjögren syndrome) and “essential mixed cryoglobulinemia” in 2 patients. Two patients had hypogammaglobulinemia with hypo-IgG (319 ± 73 mg/dl) before rituximab administration and needed immunoglobulin infusion. Two patients were treated with therapeutic plasma exchange (three exchanges per week for two weeks) before RTX infusions. The indications for TPE were rapidly progressive glomerulonephritis (RPGN) and fingers skin necrosis with severe peripheral neuropathy.
 |
Figure 1 Clinical manifestation of CV patients. |
Safety
Infusion-related reactions were not observed. Two patients developed infectious complications represented by urinary tract infection (2 patients) and infectious pneumonia (1 patient). Two patients experienced severe adverse events (SAEs) in the form of infectious pneumonia (one patient) requiring hospitalization (two months after the first cycle of RTX treatment), and death (one patient) few days after the last dose of RTX in the first cycle. Patients with SAEs were relatively older (64.5 ± 2.1 vs 58.0 years) and had a longer duration of cryoglobulinemic vasculitis (5.5 ± 0.7 vs 1.0 years) than the patient without SAE (Table 2; Figure 2). Patients with SAEs had significantly lower Hb level (p= 0.035), C3 activity (p=0.012), eGFR (p=0.010) and higher RF (p=0.002) and serum creatinine level (p= 0.037). Patients with and without SAEs did not differ in inflammatory markers (Table 3). There was a trend to higher IgM loss in cryoglobulinemic test in SAEs group (p=0.062). There were no events of significant leucopenia and neutropenia after three months of RTX treatment, and these parameters did not differ in comparison to those before the treatment (7.33±1.75 vs 8.90±4.04; p = 0.081 and 5.20±1.80 vs 6.71±3.97; p = 0.099, respectively). Moreover, white blood cells (WBC) and neutrophils did not differ between SAE and non-SAE groups after 3 months of RTX treatment (8.96±0.21 vs 6.51 ±1.56; p = 0.193 and 6.91 ±0.51 vs 4.34 ±1.54; p = 0.476, respectively).
 |
Table 3 Comparison of Tests Between Groups of RTX Treatments in Patients with and without SAEs |
 |
Figure 2 Differences between patients with and without SAEs. |
Considering the role of immunologic factors in SAEs development, ROC analysis showed possible discriminatory properties of C3, RF, and IgM loss in predicting SAEs occurrence (Table 4; Figure 3).
 |
Table 4 ROC Analysis of Immunologic Tests in the Prediction of SAEs Occurrence |
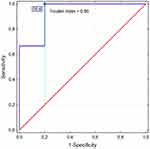 |
Figure 3 ROC diagram showing the discriminatory value of IgM loss in the identification of SAE. |
Effectiveness
Data for response to rituximab was obtained in 7 treatment courses for 2 of the 3 patients. We observed improvement in both patients 3 months after administration of rituximab (Table 2).
Considering all incidents of RTX treatment, we found a significant decrease of cryoglobulins in the 3-rd month from RTX administration (1.30 (1.20) vs 0.20 (0.70) g/L; p = 0.028) what corresponded with the lowering amount of IgM loss (43.6 ±16.6 vs 15.7 ±13.7%; p = 0.024). One patient experienced four relapses after 24 months (range 12–42 months) following the last rituximab course. Three of these clinical relapses retreated with rituximab in dose 1.0 g intravenous infusion two weeks apart, and complete or partial response was observed. Corticosteroids and MMF were used as maintenance therapy (Figure 4).
 |
Figure 4 Long-term treatment of cryoglobulinemic vasculitis. |
Discussion
All of our patients had a severe presentation of cryoglobulinaemic vasculitis and life-threatening in two cases. All had renal involvement with mean eGFR 46.6 ± 31.50 mL/min/1.73m2 before RTX administration. To compare, in CryoVas survey based on 242 cases mean eGFR was 69 ± 31 mL/min.24 On the other hand in AIR study mean eGFR was 70mL/min.11 In CryoVas study CNS, pulmonary and gastrointestinal tract were involved only in 2–5% of individuals. In AIR study none of the patients had a life-threatening presentation of cryoglobulinemia vasculitis. In the presented study, the mean cryoglobulin level was significantly higher (3.5 ± 1.7 g/L) than in CryoVas study (0.94 ±1.61g/L) or AIR study (0.77 g/L), which can be associated with severe clinical course of the disease.
For the first time in the available literature, we present C3 component of complement and rheumatoid factor as potential prognostic markers of SAEs in the course of RTX therapy of severe, noninfectious cryoglobulinemic vasculitis. All of our patients had renal involvement, but subgroup with SAEs had lower eGFR 25.2 ±18.9 mL/min/1.73m2 vs 72.6 ±16.8 mL/min/1.73m2 without SAEs. Lower hemoglobin levels in the group with SAEs are also likely to be associated with renal failure. Similar to our study, in Cryo Vas survey and AIR study, authors noted a higher frequency of severe infections after RTX therapy in a subgroup of patients with GFR <60 mL/min.11,24 We did not find any information in other studies considering an association between the occurrence of SAEs after RTX therapy and C3 complement or RF concentrations. The only data related to CV was the work of Tarantino et al, who noticed that C3 plasma level lower than 54mg/dl is one of the independent risk factors for death or dialysis in essential MC glomerulonephritis.13
None of our patients were treated with high doses of steroids and nevertheless developed infections. Contrary to our study, Terrier et al observed an increased risk of infections in the patient treated with a high dose of steroids.11,24 Besides, we suppose that the patient who died experienced a disease flare after rituximab infusion. This kind of an adverse event has been reported in patients with HCV-related cryoglobulinemic vasculitis and could be related to complex formation between rituximab and RF.30 Sene et al observed that, following the infusion of rituximab, 6 out of 22 patients (27.3%) suffered from systemic drug reactions. A severe flare of MC vasculitis was found in 4 individuals (with the involvement of the heart, kidneys, gastrointestinal tract, nervous system, and skin) 1 or 2 days following rituximab administration. Following the initial 1.0 g infusion of rituximab two patients experienced serum sickness syndrome after 7 and 9 days. The patients with drug reactions manifested increased concentrations of mixed cryoglobulin (1.4 ± 0.82 g/L vs 0.71 ± 0.77 g/L; p=0.048) and decreased concentrations of C4 (0.02 ± 0.006 g/L vs 0.07 ± 0.07 g/L; p=0.02) in comparison to patients without drug reactions. Furthermore, more of them received high‐dose rituximab protocol (50% vs 6.25%; p=0.046). The authors recommend careful use of RTX if concentrations of mixed cryoglobulin serum are elevated (≥1.00 g/L) and concentrations of C4 serum are reduced (≤0.03 g/L). They advise using the low-dose RTX protocol (375 mg/m2 weekly for 4 consecutive weeks or even lower doses17) and plasma exchanges before infusion of rituximab in patients with high levels of cryoglobulins.30 Recently, Desbois et al reported that RTX-associated CV flares concerned more often patients with renal involvement (p = 0.0008) compared to patients without flare after RTX.31
Considering the occurrence of severe adverse events, our data showed the efficacy of rituximab with corticosteroids in the achievement of complete or partial clinical, renal and immunologic responses in refractory noninfectious CV patients. These observations remain in accordance with previously reported data,11,24 that showed the clinical, renal and immunologic response in 66% (38/58), 88% (15/17), and 84% (41/49), respectively.24 Frequent relapses in severe CV raise the question of maintenance therapy. Our data show the efficacy of long-term retreatment with RTX for relapsing nonviral CV. The survival of our patient on long-term rituximab treatment was 7.5 years. We introduced MMF as the maintenance therapy, which was safe with a steroid-sparing effect. Only a few large studies included the retreatment schedule of RTX for MC patients who relapse after the first cycle, but all of them concern HCV-related CV.21,32 Quartuccio et al reported the efficacy and safety of a repeated treatment of rituximab administered at MC vasculitis relapse (1.0 g intravenous infusion two weeks apart). Retreatment was effective in two-thirds of the patients, with complete remission in one-third (6/17, 35.3%), partial response in 5/17 (29.4%), and no response in 6/17 (35.3%). The mean survival of patients on long-term rituximab treatment was 7.6 years in 17 individuals.21 Contrary to our study Quartuccio et al registered only non-severe infections that occurred in 3/30 (10%) patients. Colantuono et al treated patients for relapsing CV with RTX at the reduced dosage of 250 mg/m2 administered twice in the one-week interval. They suggest that this treatment is efficacious and safe for the long-term management. Three patients experienced severe adverse reactions, in 2 of whom at the first cycle.32 Similar to our study they did not observe any increase in the number or quality of adverse events in patients receiving more than one rituximab treatment.
In the absence of RCTs in HCV-unrelated MC, treatment of nonviral cryoglobulinemic vasculitis has yet to be determined. RTX therapy is probably the greatest advance for the treatment of CV in the last years, but in our opinion, rituximab should be administered with caution in severe nonviral cryoglobulinemic vasculitis after analyzing clinical and biological risk factors SAEs.
There are some limitations of our study. The small sample size is one of them and is associated with the fact that nonviral CV is a rare disease, especially with the severe, life-threatening course. Nevertheless, the significant impact of considered variables in ROC analysis could be affected by the differences between patients. Thus, we can only hypothesize significant discriminatory properties of investigated parameters. Furthermore, the monocenter source of data may limit the validity of our results, which should be confirmed in further studies.
Conclusions
In conclusion, data from our study showed the efficacy of rituximab in refractory patients with severe noninfectious CV, but severe adverse events were also present. Lower hemoglobin levels, worse kidney function, lower C3, and higher RF concentrations can be considered as the potential risk factors of SAE. B-cell depletion with rituximab in induction or maintenance therapy in severe nonviral cryoglobulinemic vasculitis should be defined in randomized controlled trials.
Disclosure
The authors declare no conflicts of interest.
References
1. Ferri C. Mixed cryoglobulinaemia. Orphanet J Rare Dis. 2008;3(1):25. doi:10.1186/1750-1172-3-25
2. Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins: a report of 86 cases. Am J Med. 1974;57(5):775–788. doi:10.1016/0002-9343(74)90852-3
3. Ferri C, Greco F, Longombardo G, et al. Association between hepatitis C virus and mixed cryoglobulinemia. Clin Exp Rheumatol. 1991;9:621–624.
4. Trejo O, Ramos-Casals M, Garcia-Carrasco M, et al. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore). 2001;80(4):252–262. doi:10.1097/00005792-200107000-00004
5. Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol. 2002;55(1):4–13. doi:10.1136/jcp.55.1.4
6. Meltzer M, Franklin EC, Elias K, McCluskey RT, Cooper N. Cryoglobulinemia. A clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med. 1966;40(6):837–856. doi:10.1016/0002-9343(66)90200-2
7. Ferri C, Sebastiani M, Giuggioli D, et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33(6):355–374.
8. Ramos-Casals M, Robles A, Brito-Zeron P, et al. Life-threatening cryoglobulinemia: clinical and immunological characterization of 29 cases. Semin Arthritis Rheum. 2006;36(3):189–196. doi:10.1016/j.semarthrit.2006.08.005
9. Retamozo S, Díaz-Lagares C, Bosch X, Bové A, Brito-Zerón P, Gómez ME. Life-threatening cryoglobulinemic patients with hepatitis C: clinical description and outcome of 279 patients. Medicine (Baltimore). 2013;92(5):273–284. doi:10.1097/MD.0b013e3182a5cf71
10. Saadoun D, Sellam J, Ghillani-Dalbin P, et al. Increased risks of lymphoma and death among patients with non-hepatitis C virus-related mixed cryoglobulinemia. Arch Intern Med. 2006;166(19):2101–2108. doi:10.1001/archinte.166.19.2101
11. Terrier B, Launay D, Kaplanski G, et al. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French autoimmunity and rituximab registry. Arthritis Care Res (Hoboken). 2010;62(12):1787–1795. doi:10.1002/acr.20318
12. Roccatello D, Fornasieri A, Giachino O, et al. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis. 2007;49(1):69–82. doi:10.1053/j.ajkd.2006.09.015
13. Tarantino A, Campise M, Banfi G, et al. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int. 1995;47(2):618–623. doi:10.1038/ki.1995.78
14. Coliche V, Sarda MN, Laville M, et al. Predictive factors of renal involvement in cryoglobulinaemia: a retrospective study of 153 patients. Clin Kidney J. 2018;12(3):365–372. doi:10.1093/ckj/sfy096
15. Pietrogrande M, De Vita S, Zignego A, et al. Recommendations for the management of mixed cryoglobulinemia syndrome in hepatitis C virus‐infected patients. Autoimmun Rev. 2011;10(8):444–454. doi:10.1016/j.autrev.2011.01.008
16. Ferri C, Cacoub P, Mazzaro C, et al. Treatment with rituximab in patients with mixed cryoglobulinemia syndrome: results of multicenter cohort study and review of the literature. Autoimmun Rev. 2011;11(1):48–55. doi:10.1016/j.autrev.2011.07.005
17. Visentini M, Ludovisi S, Petrarca A, et al. A Phase II, single-arm multicenter study of low-dose rituximab for refractory mixed cryoglobulinemia secondary to hepatitis C virus infection. Autoimmun Rev. 2011;10(11):714–719. doi:10.1016/j.autrev.2011.04.033
18. De Vita S, Quartuccio L, Isola M, et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64(3):843–853. doi:10.1002/art.34331
19. Sneller MC, Hu Z, Langford CA. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64(3):835–842. doi:10.1002/art.34322
20. Visentini M, Tinelli C, Colantuono S, et al. Efficacy of low-dose rituximab for the treatment of mixed cryoglobulinemia vasculitis: Phase II clinical trial and systematic review. Autoimmun Rev. 2015;4(10):889–896. doi:10.1016/j.autrev.2015.05.013
21. Quartuccio L, Zuliani F, Corazza L, et al. Retreatment regimen of rituximab monotherapy given at the relapse of severe HCV-related cryoglobulinemic vasculitis: long-term follow up data of a randomized controlled multicentre study. J Autoimmun. 2015;63:88–93. doi:10.1016/j.jaut.2015.07.012
22. Roccatello D, Sciascia S, Baldovino S, et al. Improved (4 plus 2) rituximab protocol for severe cases of mixed cryoglobulinemia: a 6-year observational study. Am J Nephrol. 2016;43(4):251–260. doi:10.1159/000445841
23. Foessel L, Besancenot JF, Blaison G, et al. Clinical spectrum, treatment, and outcome of patients with type II mixed cryoglobulinemia without evidence of hepatitis C infection. J Rheumatol. 2011;38(4):716–722. doi:10.3899/jrheum.100898
24. Terrier B, Krastinova E, Marie I, et al. Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood. 2012;119(25):5996–6004. doi:10.1182/blood-2011-12-396028
25. Terrier B, Marie I, Launay D, et al. Predictors of early relapse in patients with non- infectious mixed cryoglobulinemia vasculitis: results from the French nationwide CryoVas survey. Autoimmun Rev. 2014;13(6):630–634. doi:10.1016/j.autrev.2013.11.006
26. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi:10.7326/0003-4819-130-6-199903160-00002
27. Damoiseaux J. The diagnosis and classification of the cryoglobulinemic syndrome. Autoimmun Rev. 2014;13(4–5):359–362. doi:10.1016/j.autrev.2014.01.027
28. De Vita S, Soldano F, Isola M, et al. Preliminary classification criteria for the cryoglobulinaemic vasculitis. Ann Rheum Dis. 2011;70:1183–1190.
29. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558. doi:10.1136/ard.61.6.554
30. Sène D, Ghillani-Dalbin P, Amoura Z, Musset L, Cacoub P. Rituximab may form a complex with IgM kappa mixed cryoglobulin and induce severe systemic reactions in patients with hepatitis C virus-induced vasculitis. Arthritis Rheum. 2009;60(12):3848–3855. doi:10.1002/art.25000
31. Desbois AC, Biard L, Sene D, et al. Rituximab associated vasculitis flare: incidence predictors and outcome. J Rheumatol. 2020;47(6):896–902. doi:10.3899/jrheum.190076
32. Colantuono S, Mitrevski M, Yang B, et al. Efficacy and safety of long-term treatment with low-dose rituximab for relapsing mixed cryoglobulinemia vasculitis. Clin Rheumatol. 2017;36(3):617–623. doi:10.1007/s10067-017-3552-6
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
