Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 11
Nocturnal blood pressure decrease in patients with chronic kidney disease and in healthy controls – significance of obstructive sleep apnea and renal function
Authors Hornstrup BG , Gjoerup PH, Wessels J, Lauridsen TG, Pedersen EB, Bech JN
Received 7 June 2018
Accepted for publication 24 August 2018
Published 8 November 2018 Volume 2018:11 Pages 279—290
DOI https://doi.org/10.2147/IJNRD.S176606
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Bodil G Hornstrup,1 Pia H Gjoerup,2 Jost Wessels,2 Thomas G Lauridsen,1,2 Erling B Pedersen,1 Jesper N Bech1,2
1University Clinic in Nephrology and Hypertension, Holstebro Hospital and Aarhus University, Holstebro, Denmark; 2Department of Medicine, Holstebro Hospital, Holstebro, Denmark
Background: Chronic kidney disease (CKD) is often associated with a blunted nocturnal BP decrease and OSA. However, it is not fully clear whether a relationship exists between reduction in renal function and obstructive sleep apnea (OSA) on the one hand and relative nocturnal BP decrease in CKD patients on the other. The aim of this study was to investigate the association between nocturnal BP decrease and renal function, the degree of OSA, vasoactive hormones, and renal sodium handling in CKD3-4 patients and healthy age-matched controls.
Methods: We performed brachial and central 24-hour ambulatory BP measurement and CRM in 70 CKD3-4 patients and 56 controls. In plasma, we measured renin, AngII , aldosterone, and vasopressin. In urine, 24-hour excretion of sodium, protein fractions from the epithelial sodium channel (u-ENaCγ), and the AQP2 water channels (u-AQP2) were measured.
Results: CKD patients had lower relative nocturnal BP decrease than controls: brachial (10% vs 17%, P=0.001) and central (6% vs 10%, P=0.001). Moderate-to-severe OSA was more frequent in patients (15 vs 1%, P<0.0001). Neither the presence of OSA nor eGFR were predictors of either brachial or central nocturnal BP decrease. CKD3-4 nondippers were more obese, had higher HbA1c level, and more often a history of acute myocardial infarction than CKD3-4 dippers (P<0.05).
Conclusion: CKD3-4 patients had lower brachial and central nocturnal BP decrease than healthy controls. OSA and eGFR were not associated with nondipping in CKD patients or healthy controls. Nondipping in CKD3-4 was associated with obesity, diabetes, and cardiovascular disease.
ClinicalTrials.gov ID: NCT01951196.
Keywords: chronic kidney disease, nocturnal BP decrease, hypertension, OSA, central blood pressure
Plain language summary
When going to sleep, normal blood pressure regulation in humans involves a 10 to 20% decrease in blood pressure (BP), and lack of this is related to a higher risk of cardiovascular disease, ie stroke, heart failure, and renal disease.
Nocturnal BP decrease is often absent in patients with chronic renal disease and in patients with obstructive sleep apnea (OSA). However, whether this is because of the renal function or OSA in itself is not yet fully known. Hence, the aim of this study was to analyze this in a population of patients with chronic renal disease compared with healthy controls.
We found that patients with chronic renal disease had blunted nocturnal BP decrease compared with healthy controls. Blunted nocturnal BP decrease was not related to renal function or OSA, but instead to obesity, diabetes and cardiovascular disease. Moreover, patients with blunted nocturnal BP decrease received more antihypertensive medication and had been treated for hypertension for a longer period than the patients with normal decrease. In conclusion, absence of normal nocturnal BP decrease was not associated to renal function or OSA. In patients with chronic renal disease, it was associated to life style factors, such as obesity and diabetes.
Introduction
In normal BP regulation, there is a nocturnal BP decrease of 10%–20% of daytime BP.1 Blunted nocturnal BP decrease is a known predictor for cardiovascular disease (CVD) related to poorer cardiovascular outcome, and it can predict cardiovascular events independently of 24-hour BP levels.2–4 CKD is associated with an increased risk of cardiovascular disease even in the early stages of CKD.5–7 Nondipping hypertension is a predominant BP phenotype in patients with CKD with increasing prevalence as renal function declines.8–10 Hence, the pathophysiological mechanisms behind this are of considerable interest.
OSA is characterized by nocturnal pauses in breathing caused by collapsing upper airways. OSA is associated with nondipping BP, seen in both hypertensive and normotensive populations.11,12 It is a common comorbidity in patients with CKD.13 It is, however, unclear if the presence of OSA is a determinant for blunted nocturnal BP decrease in CKD.
Noninvasive estimates of CASP provide more precise information on the stage of arteriosclerosis and presence of CVD-related end organ damage than brachial BP and may be a better predictor of cardiovascular risk.14,15 Yet, determinants for 24-hour CASP in CKD patients are not known.
We hypothesized that nocturnal BP decrease, brachial and central, was lower in a population of patients with CKD3-4 (ie, eGFR, 15–59 mL/minutes) than in healthy controls, that the nocturnal decrease was independently determined by eGFR and OSA, and that abnormal plasma levels of vasoactive hormones, abnormal renal sodium handling, or sodium balance were mechanisms involved in brachial nondipping. We aimed to evaluate risk markers for CVD as determinants for central daytime and nocturnal BP patients with CKD.
In CKD3-4 patients and healthy controls, we measured: 1) 24-hour brachial and central BP; 2) renal function as eGFR; 3) prevalence of OSA (CRM); 4) plasma levels of vasoactive hormones; renin (PRC), p-AngII, aldosterone (p-Aldo), and vasopressin (p-AVP); and 5) urinary excretion of sodium (u-Na), epithelial sodium channel (u-ENaCγ), and of AQP2 (u-APQ2).
Materials and methods
Design
The study was designed as a case–control study comparing patients with CKD3-4 (ie, eGFR 15–59 mL/minutes/1.73 m2) and healthy controls.
Study settings
The study was conducted at the University Clinic in Nephrology and Hypertension, Aarhus University and Holstebro Hospital, the Renal Outpatient Clinic, Department of Medicine, and the Sleep Apnea Clinic.
Participants
Patients
CKD3-4 patients were recruited from the Renal Outpatient Clinic, Department of Medicine, Holstebro Hospital.
The inclusion criteria for patients were men and women, aged 18–80 years, and eGFR 15–59 mL/minutes/1.73 m2. All origins of renal disease were accepted, including diabetic nephropathy. The exclusion criteria were unwillingness to participate, treatment for OSA, increased plasma metanephrine, malignant disease, liver disease (alanine-aminotransferase >200), uncompensated heart failure or atrial fibrillation, severe chronic obstructive lung disease (FEV1 less than 50% of expected), drug or alcohol abuse (more than 21 drinks per week for males and 14 for females), breast-feeding or pregnancy, and difference in BP between right and left arm above 10/10 mmHg. The withdrawal criteria were lack of completion of participation, lack of compliance, or development of exclusion criteria,
Controls
Subjects were invited to participate, if they had normal home BP at participation in a population study in Holstebro County.16 The inclusion criteria were both males and females aged 55–70 years, and BMI in the range of 18.5–30 kg/m2.
The exclusion criteria were the same as for patients, and in addition: 24 hours brachial ambulatory BP monitoring (ABPM) ≥130/80 mmHg; history of or clinical signs of disease in heart, lungs, kidneys, liver, or endocrine organs; clinically important abnormal laboratory tests (creatinine, leucocytes); diabetes mellitus; clinically important abnormalities in electrocardiogram; smoking; and medical treatment. The withdrawal criteria were identical to those for patients.
Number of subjects
The minimal relevant difference in relative nocturnal BP decrease on 24-hour BP from day to nighttime was 5% with SD 8%. Using a significance level of 5% and a power of 90%, it was calculated that the number of subjects in each group should be 54.
Effect variables
The primary effect variable was the difference in brachial nocturnal systolic BP decrease between CKD3-4 patients and healthy controls.
Secondary effect variables were brachial 24-hour BP, central 24-hour BP, PRC, p-Aldo, p-AngII, p-AVP, u-ENaCγ, u-AQP2, and 24-hour urinary albumin excretion (u-albumin).
Blood pressure
With the cuff placed on the right upper arm, 24-hour ABPM was measured using an oscillometric device, A&D TM-2430 (A&D Company Limited, Tokyo, Japan). Appropriate size cuff was selected after measuring the circumference of upper arms. Twenty-four-hour ambulatory CASP was measured using applanation tonometry by BPro Health Stat (BPro, HealthSTATS, Singapore). This method has previously been validated.17 The BPro device was placed on the left wrist and calibrated with the mean of the last three of four BP measurements on the left arm with the A&D device, used for 24-hour measurement on the same subject.
BP was measured by the A&D device every 15 minutes and every 30 minutes during daytime (fixed at 6 am to 11 pm) and overnight (11 pm to 6 am), respectively, and every 15 minutes by the BPro device for 24 hours. ABPM were considered adequate if there were 21 or more recordings in all; 14 or more daytime and seven or more nighttime recordings.
Bilateral brachial BP were recorded using a semiautomatic oscillometric device, Omron 705IT (Omron Matsusaka CO, Ltd., Matsusaka City, Japan), with the subject sitting in the upright position after a minimum of 10 minutes rest.
Brachial 24-hour BP of >130 mmHg systolic and/or >80 mmHg diastolic was defined as hypertension, and relative nocturnal systolic BP decrease of ≤10% was defined as nondipping. Both these definitions are according to most recent guidelines from the European Society of Hypertension/ European Society of Cardiology.18
Sleep apnea
Embletta Gold (Natus Medical Incorporated, CA, USA) was used for ambulatory CRM. The RemLogic-E software was used for analyzing and storing data. The sleep report was generated from sleep time, which was generated from continuous recordings from a nasal pressure transducer (air flow), microphone recordings (snoring), a pulse oximeter (arterial oxygen saturation), thoracic and abdominal impedance belts (respiratory effort), and sensors (body position). Cessation of nasal airflow and drop of the signal below 10% of the reference amplitude for an interval of 10 seconds was defined as apnea. A reduction of the signal below 70% of the reference amplitude for an interval of 10 seconds followed by a desaturation event no later than 20 seconds after the start of the event was defined as hypopnea. An oxygen desaturation by at least 4% was detected as an oxygen desaturation event. All events (apnea, hypopnea, and desaturation) lasting longer than 120 seconds were excluded.
The sum of apneas and hypopneas per hour of registered sleep defined the AHI. Oxygen desaturation events per hour of sleep defined the oxygen desaturation index (ODI). AHI of ≥5 defined OSA, whereas AHI of ≥15 defined moderate-to-severe OSA. Definitions above are according to the recommendations from The American Academy of Sleep Medicine.19
Experimental procedures
Patients
Eligible subjects were informed about the project at planned control in the Renal Outpatient Clinic, Holstebro Hospital, and if they were interested, an information meeting was planned. They received written and oral information about the study, and after written consent, further participation was planned. By questionnaire, knowledge of the subject’s medical history, smoking habits, and alcohol intake was obtained. Medicine prescriptions were obtained from the Electronic Patient Record, and by ESS symptoms of sleep disorder were reported.
After a minimum of 10 minutes of rest, bilateral office BP was measured, and after 20 minutes of rest in supine position, blood samples were drawn. ABPM, brachial and central, was completed the following day. The subjects were instructed to collect a 24-hour urine sample. The subjects were instructed to collect a 24-hour urine sample and returned it within 4 hours after completion.
The ambulatory CRM was applied by the subject after instructions at night, independently of the ABPM. Participation in this project was completed within one month from the time the written consent was provided.
Controls
All controls participated in another study by the same authors.20 All eligible participants were invited by letter. If necessary, a reminder was sent. Arrangements for information meeting was made by telephone or email. Study information was sent prior to the first meeting. The rest of the procedure was identical for patients and controls, except for the medical prescription part, since controls did not receive any medication.
Biochemical analyses
Blood samples were centrifuged for 10 minutes at 2,200×g at 4°C. Until assayed, plasma samples were kept frozen at –20°C (p-AngII) and –80°C (PRC, p-Aldo, and p-AVP), and urine samples at –20°C. Urinary and plasma osmolality were measured by freezing-point depression (A2O Advanced Automated Osmometer, Advanced Instruments, MA, USA).
PRC was determined by radioimmunoassay using a kit from CIS Bio International, Gif-Sur-Yvette Cedex, France. The minimal detection level was 1 pg/mL. The coefficients of variations were 14.5% (interassay) and 4.5% (intraassay).
P-Aldo was determined by radioimmunoassay using a kit from Demeditec Diagnostics GmbH, Kiel, Germany. The minimal detection level was 3.99 pmol/L. The coefficients of variations were 17.2% (interassay) and 12.6% (intraassay).
P-AVP and P-AngII were extracted from plasma with C18 Sep-Pak (Waters Corporation, Milford, MA, USA) and determined by radioimmunoassay (RIA) as previously described.21,22 The antibodies against AVP, a gift from Professor Jacques Dürr (Miami, FL, USA), were with a minimal detection level: 0.5 pmol/L, and coefficients of variation: 13% (interassay) and 9% (intra-assay). Antibodies against AngII were obtained from the Department of Clinical Physiology, Glostrup Hospital, Denmark. The minimal detection level was 2 pmol/L. The coefficients of variation were 12% (interassay) and 8% (intraassay).
U-AQP2 was determined by RIA as previously described.23,24 Rabbit anti-AQP2 antibodies were a gift from Professor Soren Nielsen and Professor Robert Fenton from the Water and Salt Research Center, Aarhus University, Denmark: the minimal detection level was 32 pg/tube and the coefficients of variation were 11.7% (interassay) and 5.9% (intraassay).
U-ENaCγ was measured by RIA as described previously.25,26 ENaCγ was synthesized and purchased by Lofstrand, Gaithersburg, Maryland, USA. The ENaCγ antibody was a gift from Professor Soren Nielsen and Professor Robert Fenton from the Water and Salt Center, Aarhus University: the minimal detection level was 35 pg/tube, and the coefficients of variation were 10% at a mean level of 338 pg/tube (interassay), 9% at a mean level of 743 pg/tube (interassay), 5.0% in the range 125–135 pg/tube (intraassay), and 5.6% in the range 290–380 pg/ tube (intra-assay).
Routine methods at the Department of Clinical Biochemistry, Holstebro Hospital, Denmark, were used to measure p-creatinine, p-HbA1c, p-cholesterols, and urinary concentrations of albumin, creatinine, and sodium. eGFR was calculated using the MDRD equation.
Statistical methods
Statistical analyses were performed using IBM SPSS statistics version 22.0.0 (IBM Corp., Armonk, NY, USA). All data were tested for normality and variance equality. The statistical level of significance was P<0.05 in all analyses.
Continuous variables were reported as means with SD if normally distributed and medians with interquartile range (25;75%) if nonnormally distributed. The t-test was used for parametric continuous variables and Mann–Whitney’s test for nonparametric continuous variables. Categorical variables were reported as percentages with number. Chi-squared test or Fisher’s exact test was used to test for association between two categorical variables depending on minimum expected cell count.
Univariate analyses were performed using Pearson or Spearman correlation depending on whether the data were normally distributed or not. Multiple regression analyses were made with dependent and independent variables, as mentioned in the specific analyses.
Ethics
The Regional Committees on Health Research Ethics, Denmark (j.no. M-2013-224-13), and the Danish Data Protection Agency (j.no.:1-16-02-353-13) approved this study. It was carried out in accordance with the Helsinki Declaration. Oral and written information about the project was given to all study participants, and prior to study enrollment, they provided informed written consent.
Results
Demographics
Seventy patients and 56 controls were included for analyses (see Figure S1). Table 1 displays clinical and laboratory data. Patients and controls were of the same age and had a similar distribution of gender. Patients had higher BMI, HbA1c, triglycerides, lower eGFR, total cholesterol, and HDL than controls. Patients received a mean of 2.6 (95% CI 2.3;3.0) BP-lowering drugs, 67% (n=47) received angiotensin-converting enzyme inhibitors or AngII antagonists, 70% (n=49) received diuretics (thiazides, loop diuretics, or aldosterone-antagonists), 18% (n=12) received aldosterone-antagonists, and 53% (n=37) received statin treatment.
Six patients (9%) had type 1 diabetes, 19 patients (27%) had type 2 diabetes, and 16 of these patients received ant-diabetic treatment.
Mean office SBP/DBP difference between right and left arm was –0.8±5.0/–0.3±3.8 mmHg (P=0.20/P=0.57) in CKD patients and –1,0±5.3/–1.2±3.9 mmHg (P=0.18/P=0.022) in the controls.
Laboratory and medical history data on the CKD3-4 patients allocated into dippers and nondippers are shown in Table S1. Nondippers were more obese and had higher HbA1c than CKD3-4 dippers, and they had been treated for hypertension for a longer period, received more antihypertensive drugs, and had more history of AMI. They did not differ with regard to the presence of OSA or mean SaO2, plasma levels of total cholesterol, HDL or triglycerides. Twelve dippers and 11 nondippers received antihypertensive treatment in the evening or at bedtime.
Brachial ambulatory BP monitoring
Table 2 shows results from 24 hours brachial ABPM. Compared with controls, CKD3-4 patients had higher 24-hour day and nighttime BP, and lower absolute nocturnal BP decrease (14 vs 21 mmHg, P=0.001) corresponding to a relative decrease of 10% vs 17% (P<0.0001). Hypertension was present in 61% (n=43) of patients. Nocturnal hypertension was seen in 63% (n=44) of CKD3-4 patients and 7% (n=4) of controls (P<0.0001). More patients than controls were nondippers.
Central aortic systolic pressure
Table 2 shows results from 24-hour ambulatory CASP monitoring. CASP measurements of 19 participants were excluded due to insufficient measurements in daytime (nine patients and two controls) or in nighttime (three patients and five controls), and thus, CASP data from 58 CKD3-4 patients and 49 controls were analyzed. Patients had higher 24-hour day and nighttime CASP. Absolute nocturnal CASP decrease was lower in patients than in controls, 8 vs 11 mmHg (P=0.012), corresponding to a relative decrease of 6% vs 10% (P=0.001). More patients than controls had nocturnal CASP decrease <10% (72% vs 49%, P=0.013). Both patients and controls had lower CASP than brachial BP, except for nocturnal BP in healthy controls.
Sleep examination
In Table 3, results from ESS and CRM are displayed. CKD3-4 patients had higher AHI (5.8 vs 1.8 events per hour, P<0.0001), ODI (7.2 vs 1.7 events per hour, P<0.0001), longer snoring time, lower mean oxygen saturation (SaO2), and more CKD3-4 patients were diagnosed with all-degree and moderate-to-severe OSA. Sleep-monitoring time in CKD and control group was 396 minutes (95% CI 378;415) and 401 minutes (95%CI 385;417), respectively.
Vasoactive hormones in plasma
Table 4 shows the results of plasma hormone levels; CKD3-4 had higher p-Aldo (261 vs 82 pmol/L, P<0.0001) and PRC (30.5 vs 6.1 ng/L, P<0.0001), no differences in AngII and AVP were seen. No significant differences existed in either plasma hormone levels between dippers and nondippers in either patients or healthy controls.
Univariate correlation analyses showed a negative association between p-AngII and brachial nocturnal BP decrease in patients (r= - 0.30, P=0.012). This was not seen in controls. This association was not present when excluding patients receiving aldosterone-antagonists or angiotensin-converting enzyme inhibitors or AngII antagonists. No association existed between brachial nocturnal BP decrease or CASP decrease and the other hormones (data not shown).
Albumin, sodium, ENaCγ, and AQP2 in urine
Table 4 shows that patients had higher 24-hour urinary excretion of albumin than controls. Twenty-four-hour u-Na did not differ significantly. Urinary excretion of AQP2 was higher in patients than in controls (158 vs 134 ng/µmol creatinine, P<0.0001). CKD nondippers had higher urinary excretion of AQP2 than CKD dippers (P=0.005). There was no significant difference between dippers and nondippers in the control group. When excluding patients receiving diuretics (n=49), the urinary excretion rate of AQP2 was no longer significantly different between patients and controls or between nondippers and dippers within the patient group. P-AVP correlated to u-AQP2 in both patients (r=0.389, P=0.001) and controls (r=0.338, P=0.011).
The urinary ENaCγ excretion was higher in patients than in controls (127 vs 108 ng/µmol creatinine, P=0.029). When excluding CKD3-4 receiving aldosterone-antagonists (n=12), the excretion rate was no longer significantly different between patients and controls (P=0.077).
Univariate correlation analyses showed the association between urinary excretion of AQP2 and both relative and absolute nocturnal BP decrease in patients (r=–0.277, P=0.02 and r=–0.254, P=0.034, respectively). This association was not found in the control group. Nor an association between urinary excretion of ENaCγ and relative and absolute nocturnal BP decrease was in either patients or controls (data not shown).
Determinants of brachial nocturnal BP decrease
Univariate correlation analyses in patients showed that relative nocturnal brachial BP decrease was associated with HbA1c (r=−0.34, P=0.004), BMI (r=−0.32, P=0.007), the number of BP-lowering drugs (r=−0.33, P=0.006), years of antihypertensive treatment (r=–0.28, P=0.02), and history of AMI (r=-0.38, P=0.001). No associations with eGFR or the presence of OSA were found. Identical analyses were performed in controls and no associations were found.
Regression analysis with relative nocturnal brachial BP decrease as a dependent variable and eGFR, OSA, HbA1c, and BMI as independent variables was performed (see Table 5). This regression model did not predict the association of eGFR, OSA, BMI, or HbA1c with nocturnal BP decrease for controls. In CKD patients, this model showed association between nocturnal BP decrease and BMI (β=−0.29, P=0.023) and HbA1c (β=−0.29, P=0.031).
Another regression analysis in patients with relative nocturnal BP decrease as the dependent variable and logarithmic transformed (ln) plasma hormone levels (p-AngII, p-renin, p-Aldo, p-AVP) as independent variables, showed R2 of 0.16, P=0.022. In this model, ln(AngII) was associated with nocturnal BP decrease (β=−0.38, P=0.005), whereas the other partial regression coefficients were nonsignificant (model not shown).
Determinant of nocturnal CASP decrease and nocturnal CASP decrease
Univariate correlation analyses showed no association of relative nocturnal CASP decrease and eGFR, presence of moderate-to-severe OSA, BMI, or HbA1c in either patients or controls. Regression analyses with nocturnal CASP decrease as the dependent variable and eGFR, presence of OSA, HbA1c, and BMI as independent variables did not predict nocturnal CASP decrease in either patients or controls (data not shown), and neither did the regression model with nocturnal CASP decrease and logarithmic transformed plasma hormone levels (p-AngII, p-renin, p-Aldo, p-AVP) as independent variables. Univariate correlations of daytime and nocturnal CASP are shown in Table 6. In CKD3-4 patients, smoking (previously and present vs nonsmoking) was associated with both daytime and nocturnal CASP; none of these associations were seen in controls. There was no association between daytime or nocturnal CASP and the following: age, male gender, BMI, HbA1c, eGFR, AHI, or 24-hour u-Na.
Discussion
The key finding of this study was significantly lower nocturnal BP decrease in CKD3-4 patients than in healthy controls, both using 24-hour brachial and central BP measurements. All-degree (AHI >5) and moderate-to-severe OSA (AHI >15) were more frequent in CKD3-4 patients. Neither brachial nor central nocturnal BP decrease was associated with eGFR or the presence of OSA. Nondipping was present in 44% of CKD3-4 patients and was associated with BMI, presence of diabetes, and history of cardiovascular disease.
As hypothesized, we demonstrated a lower relative nocturnal BP decrease in patients compared with healthy controls using 24-hour brachial BP measurements. This is in line with the findings from previous studies addressing the nondipping phenomenon in patients with CKD. These studies demonstrated that declining renal function was associated with lower nocturnal BP decrease and higher nondipping frequency.8,27,28 One of these studies, however, demonstrated the effect of eGFR on nocturnal BP decrease in a population of CKD1-5 patients.28 Another study, on the other hand, found no difference in eGFR between dippers and nondippers in a CKD2-4 population.10 Hence, the effect of renal function on nocturnal BP decrease is not clear.
In our CKD3-4 population, we did not find eGFR to be a determinant for nocturnal BP decrease using either univariate analysis or multiple regression analysis adding the presence of OSA, HbA1c, and BMI as independent variables. However, the present study confirms the well-known blunted nocturnal BP decrease and higher frequency of nondipping in CKD patients compared with healthy age-matched controls.
In the present CKD3-4 population, nocturnal BP decrease was associated with BMI and HbA1c in a both univariate and multiple regression analyses. Nondipping CKD3-4 patients were characterized by the increased presence of cardiovascular disease (AMI) and in need of more antihypertensive treatment than dipping counterparts. This is in line with the previous findings of associations between nondipping and classical cardiovascular risk factors such as BMI and diabetes status.8,29 The present study indicates that nocturnal BP decrease in our CKD3-4 patients was mostly associated with life-style factors such as BMI and HbA1c rather than decreasing renal function. Furthermore, the results from the present study indicate that nondipping status in CKD3-4 patients is associated with longer duration of hypertension treatment with more antihypertensive drugs. Even though our study did not find the effect of renal function on nocturnal BP decrease, a possible effect of renal function cannot be ruled out, as the life-style factors may have been superior to renal function in this population. However, as nondipping was seen associated with longer period of antihypertensive treatment, this finding underlines the importance of regular 24-hour BP measurements in patients with renal diseases.
In the present study, all-degree OSA and moderate-to-severe OSA were more frequent in CKD3-4 patients compared than in healthy controls. However, OSA was not shown to be a determinant for nocturnal BP decrease or nondipping in the CKD3-4 patients.
Previous studies have found the severity of OSA to be a determinant for nocturnal BP decrease in populations suspected of or diagnosed with OSA.12,30 We did not demonstrate this association between the severity of OSA (AHI) and nocturnal BP decrease in either patients or healthy controls. We used both univariate analysis and multivariate regression analysis to test for this association. However, several differences between the previous studies and ours may explain, why we did not confirm this association. The previous studies examined populations of slightly younger, primarily male patients suspected of, or diagnosed with, OSA. Moreover, these studies did not account for renal function, diabetic status, BP medication, or other risk factors. Hence, in the present study, the relative influence of OSA on BP regulation may have been diminished by the presence of other, and possibly stronger, predictors such as BMI and HbA1c.
In the present study, we found that CKD3-4 patients had higher 24-hour day and nighttime CASP and lower nocturnal CASP dipping than healthy age-matched controls. Smoking status was the only demonstrated predictor for daytime and nocturnal CASP in CKD3-4, whereas BMI, OSA, HbA1c or eGFR were not. No predictors were found in controls. Estimation of CASP has been used in order to obtain more knowledge of the cardiovascular risk profile.14,15 In 2014, Herbert et al collected materials for a large database of central BP measurements and established age, smoking and male sex as positive determinants for CASP in controls, and smoking as positive and male sex as negative determinants in hypertensive patients.31 As they did not include subjects with CKD, no references are available for this population. Moreover, they measured central parameters at one time point in a laboratory setting; hence, no determinants for nocturnal CASP were established. To our knowledge, the present study is the first to investigate possible determinants for nocturnal central BP. We did confirm the findings from Herbert et al regarding the influence of smoking on central BP.31 We did not find age to be a determinant; however, both groups in our study had narrow age spans. In the healthy controls, we did not ascertain any determinant for nocturnal CASP. However, the control group was homogenous with no known risk factors for CVD, except for a limited number of past smokers. Nor did we confirm any of the known risk factors for CVD (ie, BMI, gender, diabetes, and renal disease) to be determinants in the CKD3-4 group, which may be evidence of the more complex etiology of CVD associated with CKD.
In the present study, we found a negative association between AngII and nocturnal BP decrease in CKD patients. No associations between the other three vasoactive hormones were found. Previous studies on hypertensive populations reported opposing findings on the association between these hormones and nocturnal BP decrease.32,33 Our study does not clearly indicate whether abnormal plasma levels of vasoactive hormones are accountable for nondipping in CKD patients. Our CKD3-4 patients had higher plasma levels of aldosterone and renin compared with healthy controls. This is presumably due to treatment with angiotensin-converting enzyme inhibitors, AngII antagonists, and aldosterone-antagonists in the CKD3-4 group, as antihypertensive treatment was not discontinued while participating in this project.
We found that urinary excretion of AQP2 was associated with nondipping in CKD patients, not in healthy controls. We did not find association of sodium balance and renal handling of sodium on nondipping. We did, however, demonstrate higher renal excretion levels of AQP-2 and ENaCγ in patients with CKD3-4. AQP-2 plays an important role in vasopressin-regulated water reabsorbtion in the principal cell in the collecting ducts. In the present study, AQP-2 excretion rate was associated to AVP levels, which suggests altered handling of water in the nephron in the CKD3-4 population driven by vasopressin. Higher excretion level of ENaCγ in the patients is considered a sign of higher activity of the ENaC channels. By reabsorption of sodium and water and thereby enhancing the extracellular volume, this mechanism may contribute to high BP in this CKD3-4 population.34–37
In this study, patient and control populations were well-matched regarding age and gender. Brachial and central 24-hour BP was measured simultaneously. OSA screening was completed systematically.
Examination of patients without interference of antihypertensive treatment would require 3–4 weeks of discontinuation of all antihypertensive treatment. It is a weakness of the study that the patient population received their usual antihypertensive medication; however, we did not consider it ethically justified to discontinue all antihypertensive agents for this period of time. Moreover, discontinuation of antihypertensive medication would still not remove the effect of previous antihypertensive treatment on BP regulation.
The present study did not examine any aspect of BP regulation by the autonomic nervous system or endothelial function.
Conclusion
In summary, the blunted nocturnal BP decrease in CKD patients was not associated with either eGFR or the presence of OSA. Nondipping in CKD was associated with comorbidity and metabolic factors such as diabetes and BMI. Central daytime and nocturnal BP was only related to smoking, not to other CVD risk factors. Altered handling of sodium and water in CKD were not related to dipping status, but may contribute to the higher BP seen in CKD patients.
Abbreviations
ABPM, Ambulatory BP measurement
AHI, Apnea Hypopnea Index
Aldo, Aldosterone
AMI, Acute myocardial infarction
AQP2, Aquaporin 2
AngII, Angiotensin II
AVP, Arginine vasopressin
BMI, Body mass index
BP, Blood pressure
CASP, Central aortic systolic pressure
CKD, Chronic kidney disease
CRM, Cardiorespiratory monitoring
eGFR, estimated glomerular filtration rate
ENaCγ, Epithelial sodium channel γ
ESS, Epworth Sleepiness Scale
OSA, Obstructive sleep apnea
PRC, Plasma renin concentration
Acknowledgments
The authors greatly acknowledge the skillful assistance of our laboratory technicians in the University Clinic of Nephrology and Hypertension: Anne Mette Ravn, Kirsten Nygaard, and Henriette Vorup Simonsen. The authors also greatly acknowledge the skillful assistance from nurses Marianne Kirkegaard and Anja Mailund Mikkelsen in the Sleep Apnea Clinic, Department of Medicine, Holstebro Hospital. The authors thank the Department of Clinical Biochemistry, Holstebro Hospital for help in routine analyses. This research project has received research funding from The Central Denmark Region’s Research Foundation for Health Science, The Danish Heart Association, and the Axel Muusfeldt Foundation.
Disclosure
The authors alone are responsible for the content and writing of the paper. The authors report no conflicts of interest in this work.
References
Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23(1):3–16. | ||
Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20(11):2183–2189. | ||
Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007;49(6):1235–1241. | ||
Salles GF, Reboldi G, Fagard RH, et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the ambulatory blood pressure collaboration in patients with hypertension (ABC-H) meta-analysis. Hypertension. 2016;67(4):693–700. | ||
Astor BC, Hallan SI, Miller ER, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167(10):1226–1234. | ||
Meisinger C, Döring A, Löwel H, KORA Study Group. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J. 2006;27(10):1245–1250. | ||
di Angelantonio E, Chowdhury R, Sarwar N, Aspelund T, Danesh J, Gudnason V. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ. 2010;341:c4986. | ||
Fedecostante M, Spannella F, Cola G, Espinosa E, Dessì-Fulgheri P, Sarzani R. Chronic kidney disease is characterized by “double trouble” higher pulse pressure plus night-time systolic blood pressure and more severe cardiac damage. PLoS One. 2014;9(1):e86155. | ||
Farmer CK, Goldsmith DJ, Cox J, Dallyn P, Kingswood JC, Sharpstone P. An investigation of the effect of advancing uraemia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol Dial Transplant. 1997;12(11):2301–2307. | ||
Cha RH, Lee H, Lee JP, et al. Changes of blood pressure patterns and target organ damage in patients with chronic kidney disease: results of the APrODiTe-2 study. J Hypertens. 2017;35(3):593–601. | ||
Worsnop CJ, Naughton MT, Barter CE, Morgan TO, Anderson AI, Pierce RJ. The prevalence of obstructive sleep apnea in hypertensives. Am J Respir Crit Care Med. 1998;157(1):111–115. | ||
Nabe B, Lies A, Pankow W, Kohl FV, Lohmann FW. Determinants of circadian blood pressure rhythm and blood pressure variability in obstructive sleep apnoea. J Sleep Res. 1995;4(S1):97–101. | ||
Nicholl DDM, Ahmed SB, Loewen AHS, et al. Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest. 2012;141(6):1422–1430. | ||
Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50(1):197–203. | ||
Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113(5):664–670. | ||
Hoffmann-Petersen N, Lauritzen T, Bech JN, Pedersen EB. High prevalence of hypertension in a Danish population telemedical home measurement of blood pressure in citizens aged 55-64 years in Holstebro county. Am J Hypertens. 2016;29(4):439–447. | ||
Williams B, Lacy PS, Yan P, Hwee CN, Liang C, Ting CM. Development and validation of a novel method to derive central aortic systolic pressure from the radial pressure waveform using an n-point moving average method. J Am Coll Cardiol. 2011;57(8):951–961. | ||
ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31(10):1925–1938. | ||
Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. | ||
Hornstrup BG, Rosenbaek JB, Hoffmann-Petersen N, et al. Nocturnal Blood Pressure Decrease in Hypertensive Patients and Normotensives- Association with Obstructive Sleep Apnoea and Renal Function. The Open Hypertension Journal, 2018, 10: 28–40. [Electronic publication date: 17/10/2018] [Collection year: 2018]. [Publisher Id: TOHYPERJ-10-28] . Availabel from: https://benthamopen.com/TOHYPERJ/home/. | ||
Pedersen EB, Eiskjaer H, Madsen B, Danielsen H, Egeblad M, Nielsen CB. Effect of captopril on renal extraction of renin, angiotensin II, atrial natriuretic peptide and vasopressin, and renal vein renin ratio in patients with arterial hypertension and unilateral renal artery disease. Nephrol Dial Transplant. 1993;8(10):1064–1070. | ||
Pedersen EB, Danielsen H, Spencer ES. Effect of indapamide on renal plasma flow, glomerular filtration rate and arginine vasopressin in plasma in essential hypertension. Eur J Clin Pharmacol. 1984;26(5):543–547. | ||
Pedersen RS, Bentzen H, Bech JN, Pedersen EB. Effect of water deprivation and hypertonic saline infusion on urinary AQP2 excretion in healthy humans. Am J Physiol Renal Physiol. 2001;280(5):F860–F867. | ||
Graffe CC, Bech JN, Pedersen EB. Effect of high and low sodium intake on urinary aquaporin-2 excretion in healthy humans. Am J Physiol Renal Physiol. 2012;302(2):F264–F275. | ||
Matthesen SK, Larsen T, Vase H, Lauridsen TG, Jensen JM, Pedersen EB. Effect of amiloride and spironolactone on renal tubular function and central blood pressure in patients with arterial hypertension during baseline conditions and after furosemide: a double-blinded, randomized, placebo-controlled crossover trial. Clin Exp Hypertens. 2013;35(5):313–324. | ||
Al Therwani S, Malmberg MES, Rosenbaek JB, Bech JN, Pedersen EB. Effect of tolvaptan on renal handling of water and sodium, GFR and central hemodynamics in autosomal dominant polycystic kidney disease during inhibition of the nitric oxide system: a randomized, placebo-controlled, double blind, crossover study. BMC Nephrol. 2017;18(1):2680686–0173. | ||
Middeke M, Schrader J. Nocturnal blood pressure in normotensive subjects and those with white coat, primary, and secondary hypertension. BMJ. 1994;308(6929):630–632. | ||
Mojón A, Ayala DE, Piñeiro L, et al. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int. 2013;30(1-2):145–158. | ||
Draman MS, Dolan E, van der Poel L, et al. The importance of night-time systolic blood pressure in diabetic patients: Dublin Outcome Study. J Hypertens. 2015;33(7):1373–1377. | ||
Pankow W, Nabe B, Lies A, et al. Influence of sleep apnea on 24-hour blood pressure. Chest. 1997;112(5):1253–1258. | ||
Herbert A, Cruickshank JK, Laurent S, Boutouyrie P, Reference Values for Arterial Measurements Collaboration. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J. 2014;35(44):3122–3133. | ||
Kohara K, Nishida W, Maguchi M, Hiwada K. Autonomic nervous function in non-dipper essential hypertensive subjects. Evaluation by power spectral analysis of heart rate variability. Hypertension. 1995;26(5):808–814. | ||
Satoh M, Hosaka M, Asayama K, et al. Aldosterone-to-renin ratio and nocturnal blood pressure decline assessed by self-measurement of blood pressure at home: the Ohasama Study. Clin Exp Hypertens. 2014;36(2):108–114. | ||
Jensen JM, Mose FH, Bech JN, Nielsen S, Pedersen EB. Effect of volume expansion with hypertonic- and isotonic saline and isotonic glucose on sodium and water transport in the principal cells in the kidney. BMC Nephrol. 2013;14:202–2369. | ||
Graffe CC, Bech JN, Pedersen EB. Effect of high and low sodium intake on urinary aquaporin-2 excretion in healthy humans. Am J Physiol Renal Physiol. 2012;302(2):F264–F275. | ||
Lauridsen TG, Vase H, Bech JN, Nielsen S, Pedersen EB. Direct effect of methylprednisolone on renal sodium and water transport via the principal cells in the kidney. Eur J Endocrinol. 2010;162(5):961–969. | ||
Pedersen RS, Bentzen H, Bech JN, Pedersen EB. Effect of water deprivation and hypertonic saline infusion on urinary AQP2 excretion in healthy humans. Am J Physiol Renal Physiol. 2001;280(5):F860–F867. |
Supplementary materials
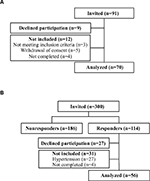  | Figure S1 Flow-chart of subjects. (A) patients. (B) healthy controls. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.