Back to Journals » Open Access Emergency Medicine » Volume 11
No effect of hyperoxia on outcome following major trauma
Authors Harpsø M, Granfeldt A, Løfgren B , Deakin CD
Received 27 July 2018
Accepted for publication 12 January 2019
Published 1 April 2019 Volume 2019:11 Pages 57—63
DOI https://doi.org/10.2147/OAEM.S181629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Hans-Christoph Pape
Martin Harpsø,1,2 Asger Granfeldt,3 Bo Løfgren,1,4,5 Charles D Deakin6
1Research Center for Emergency Medicine, Aarhus University Hospital, Aarhus, Denmark; 2Department of Internal Medicine, Regional Hospital of Horsens, Horsens, Denmark; 3Department of Intensive Care, Aarhus University Hospital, Aarhus, Denmark; 4Department of Internal Medicine, Regional Hospital of Randers, Randers, Denmark; 5Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark; 6Respiratory Biomedical Research Unit, University Hospital Southampton, UK
Purpose: Oxygen supplementation has previously been considered beneficial when managing critically ill patients in order to avoid hypoxia. However, in recent years, studies have shown that hyperoxia may be harmful in critical care patients. The aim of the study was to investigate whether hyperoxia within the first 24 hours of admission following major trauma is associated with 30-day in-hospital mortality.
Patients and methods: We conducted a retrospective database study of trauma patients admitted to the general intensive care unit at University Hospital Southampton from October 2008 to October 2014. Hyperoxia was defined as one arterial blood gas with a pO2 ≥40.0 kPa during the first 24 hours of admission. Cox proportional hazards regression was used to compare 30-day in-hospital mortality between the two groups. HRs for death were calculated with 95% CIs and presented as both unadjusted and adjusted for age, sex, Acute Physiology and Chronic Health Evaluation II (APACHE II) score and number of arterial blood gases.
Results: In total, 1,462 patients had trauma as the cause for admission. Of these, 343 patients met the study inclusion criteria, of which 265 were defined as normoxic and the remaining 78 patients as hyperoxic. The cumulative in-hospital risk of death within 30 days was 7.8% (95% CI: 4.9%–12.5%) for the normoxia group and 9.7% (95% CI: 4.4 %–20.4%) for the hyperoxia group. The crude HR for 30-day in-hospital mortality was 1.15 (95% CI: 0.45–2.90) for hyperoxia compared to normoxia. Adjusting for APACHE II, age, sex and number of arterial blood gases yielded an adjusted HR of 30-day in-hospital mortality of 0.65 (95% CI: 0.24–1.73) for the hyperoxia group compared to the normoxia group.
Conclusion: In our convenience sample of 343 patients, hyperoxia within the first 24 hours following admission to intensive care with major trauma had no impact on 30-day in-hospital mortality.
Keywords: oxygen, APACHE, arterial blood gas, in-hospital mortality
Introduction
Trauma is a major cause of mortality. In the USA, unintentional injury has been the leading cause of death among persons 1–42 years.1 In critically ill patients, oxygen (O2) has traditionally been considered beneficial in order to reduce the overall risk of tissue hypoxia. Because of this, high levels of oxygen are considered standard therapy in the treatment of critically ill patients and high-flow oxygen administration is included as a part of the ABCDE approach,2 with the purpose of avoiding the detrimental effects of hypoxia.3 This long-held belief of the beneficial effects of O2 has over the last couple of years been challenged by studies suggesting that high normal levels of O2 may be detrimental through the generation of oxygen-free radicals, which cause an inflammatory response and tissue damage.4–8
Several studies have looked into the association between hyperoxia and mortality in different categories of critically ill patients,9–11 where two major patient groups have been cardiac arrest patients and patients with traumatic brain injury. A meta-analysis in patients with cardiac arrest demonstrated that hyperoxia is associated with increased mortality after adjusting for confounding variables. While not significant, the meta-analysis also showed a trend toward worse outcome if exposed to hyperoxia following traumatic brain injury.12 A newly published review and meta-analysis of 25 different randomized controlled trials comparing conservative oxygen use with liberal oxygen use found increased mortality with liberal oxygen use.13 It is worth noticing that the absolute difference in mortality in the different randomized controlled trials is small; however, combined in a meta-analysis, it results in a significant difference in in-hospital mortality.
Patients with major trauma share pathophysiological similarities to the postcardiac arrest and traumatic brain injury populations. These patients are often administered high levels of oxygen in the acute phase of their hospital treatment and are in risk of hyperoxia, with possible negative consequences. To our knowledge, the effects of hyperoxia on the outcome from major trauma have not been studied, and it represents a large group of patients in whom the effects of hyperoxia are unknown.
We hypothesized that in patients admitted due to trauma, hyperoxia during the first 24 hours of admission is associated with increased mortality.
The aim of the study was to investigate whether the presence of hyperoxia within the first 24 hours following major trauma is associated with 30-day in-hospital mortality.
Patients and methods
Study design and setting
The study was conducted as a retrospective observational study of the patients admitted at University Hospital Southampton (UHS), a designated major trauma center. A database recording patients admitted to the general intensive care unit (GICU) at the hospital was manually searched. Written informed consent was waived as the study was performed as an audit project. The National Health Service classified this study as a service evaluation and, as such, does not require ethical approval. Patients admitted from October 2008 to October 2014 were included in the study.
All patients in the GICU database who had “trauma” listed as part of either their primary or secondary reason for admission were evaluated for eligibility. The inclusion criteria were as follows: patients aged ≥18 years admitted due to trauma and the extent of injury requiring transfer to GICU within the first 24 hours after admission. The exclusion criteria were as follows: initial treatment at another hospital before being transferred to UHS, no arterial blood gas taken within the first 24 hours or the patient’s charts unable to be retrieved. Patients with burns injuries and registered hypoxia (PaO2 <8.0 kPa) during the first 24 hours were also excluded. Patients dying within the first 24 hours were excluded as they did not survive the inclusion period, and this could lead to a selection bias. In addition, patients with isolated head trauma were excluded as previous studies have demonstrated that arterial hyperoxia is associated with higher in-hospital mortality which could bias the results.11,14,15 At UHS, patients with the most severe head injuries requiring surgery or intracranial pressure monitoring would also be admitted to the neuro intensive care unit (ICU) instead of GICU. Patients were followed during hospitalization and were lost to follow-up if they died during admission.
Data collection and outcome
The following data were abstracted: patient demographics and characteristics, admission date and time, length of stay in GICU and length of total hospital stay. Data on the trauma mechanism along with affected body regions were also abstracted. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score was calculated for each patient. Hospital admission was defined as the time of arrival to the Accident and Emergency Department, and all recorded arterial blood gases during the first 24 hours of admission were included. The patients were then divided into two groups depending on the value of their highest recorded PaO2 during the first 24 hours. Similar to earlier hyperoxia studies, we defined hyperoxia as a PaO2 ≥40.0 kPa. Normoxia was defined as a PaO2 ≥8.0–<40 kPa.
Statistical analyses
Continuous data are presented as medians together with their associated quartiles (Q25% and Q75%), whereas categorical data are presented as proportions. Nonparametric data were compared using Wilcoxon rank sum test. The primary outcome was 30-day in-hospital mortality. We used Cox proportional hazards regression to investigate the impact of hyperoxia on 30-day in-hospital mortality. HRs for death were calculated with 95% CIs and presented as both unadjusted and adjusted for age, sex, number of arterial blood gases and APACHE II score. The proportional hazard assumption was assessed using log–log plots and was found to be valid. As continuous variables did not demonstrate a linear relationship, they were entered in the logistic regression analysis as categorical variables. The variables were categorized accordingly: age (<35, 35–55 and >55), APACHE II score (<10, 10–15 and >15) and number of arterial gases (<5, 5–10 and >10). Two-tailed P-values of <0.05 were considered statistically significant. Data were analyzed by using Stata version 14.0 (StatsCorp LP, College Station, TX, USA).
Results
From October 2008 to October 2014, 1,462 patients were admitted to the GICU with “trauma” as a part of the primary or secondary reason for admission. Each patient medical record was carefully reviewed, which identified 621 patients who had been admitted because of trauma. Of the 621 patients, a total of 343 were included in the study while the remaining patients were excluded as listed in the flowchart (Figure 1). Of the 343 included patients, 265 were defined as normoxic, whereas 78 patients had at least one arterial blood gas with a pO2 ≥40.0 kPa during the first 24 hours of admission. Within the first 30 days, 6 patients from the hyperoxia group and 17 from the normoxia group died. The two groups were generally comparable with regards to age, sex and length of stay in the GICU (Table 1). Sites of injury were also similar except for head injury which was more frequent in the hyperoxia group when compared to the normoxia group. The mortality was 9.9% in patients with head injury compared to 4.3% in patients without head injury (P=0.07). Adjusting for head injury caused no change in the HR.
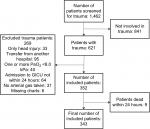  | Figure 1 Flowchart of patient inclusion and exclusion. Abbreviation: GICU, general intensive care unit. |
The APACHE II score was slightly higher in the hyperoxia group, and patients in the hyperoxia group were also transferred from the Accident and Emergency department to the GICU faster than the patients in the normoxia group (Table 1). A classification of different types of injury mechanisms can be found in Table 2.
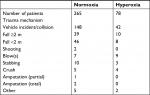  | Table 2 Trauma mechanism Note: Trauma mechanism/type that resulted in the injuries sustained to the patient. |
Characteristics of the arterial blood gases differed between the two groups, with higher oxygen levels and a higher number of arterial blood gases taken in the first 24 hours in the hyperoxia group. Data on arterial gases are listed in Table 3. Of the 78 patients in the hyperoxia group, 57 patients had only one hyperoxic blood gas, whereas 21 patients had more than one. Interestingly, PaO2 values ≥40.0 kPa were most commonly observed in the period shortly after arrival as 86% of all PaO2 values ≥40.0 kPa were observed within the first 6 hours of arrival.
The median PaCO2 was 5.2 in hyperoxia group and 5.5 in normoxia group. Looking only at the hyperoxic arterial blood gases, the corresponding median PaCO2 was 5.1 (IQR 4.7–5.6).
The in-hospital risk of death within 30 days was 7.8% (95% CI: 4.9%–12.5%) for the normoxia group and 9.7% (95% CI: 4.4%–20.4%) for the hyperoxia group. Kaplan–Meier survival curves for the two groups are shown in Figure 2. The crude HR for 30-day in-hospital mortality was 1.15 (95% CI: 0.45–2.90) for hyperoxia compared to normoxia. Adjusting for age, sex, APACHE II and number of arterial blood gases yielded an adjusted HR of 30-day in-hospital mortality of 0.65 (95% CI: 0.24–1.73) for hyperoxia compared to normoxia.
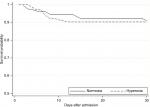  | Figure 2 Kaplan–Meier graph of patient survival in each of the two groups. |
Discussion
The current study investigated whether hyperoxia within the first 24 hours following admittance due to trauma was associated with an increase in mortality. The study did not demonstrate any association between hyperoxia and an increase in 30-day mortality. The crude estimate showed a small increase in 30-day in-hospital mortality in the hyperoxia group. After adjusting for possible confounders and baseline imbalances, this trend disappeared and showed a trend toward decreased 30-day in-hospital mortality. Both results were nonsignificant and the CIs were wide.
This is in accordance with some studies,16–18 whereas others have reported an effect of hyperoxia on mortality.11,15,19
Several reasons for this can be proposed. In our study, we excluded all isolated head trauma patients. Neurological injury following trauma is one of the most frequent causes of death,20 which is why we chose to exclude the patient group with a known high mortality. At UHS, patients with severe head injuries are admitted to the neuro ICU and as only patients from the GICU were included in the study, patients with the most severe head injuries were not included. Our study population is, therefore, different from previous studies. A factor that could influence our findings is the definition of hyperoxia. As in previous studies, we defined hyperoxia as a single value above 300 mmHg or 40 kPa.15,19,21,22 This approach can be discussed as the true significance of one reading of hyperoxia is unknown. We demonstrated that hyperoxia was most commonly observed in the period shortly after arrival and 57 (73%) patients had only one hyperoxic blood gas. This illustrates that hyperoxia was quickly corrected by the treating physicians. This could indicate that the healthcare professionals are aware of the importance of correcting hyperoxia. Even though an explorative analysis investigating the impact of a single hyperoxia blood gas vs multiple hyperoxia blood gases could be interesting, we are limited by the low number of patients.
Even though we did not find any difference in mortality between the two groups, one could speculate that patients in the hyperoxia group are more severely injured. In this study, the number of arterial blood gases taken was higher in the hyperoxia group. The higher number of arterial blood gases taken could also indicate a more severely ill patient and with more arterial blood gases taken, the greater the chance of detecting hyperoxia. This is also supported by the slightly higher APACHE II score in the hyperoxia group.
Previous studies have also demonstrated that either high or low PaCO2 is associated with increased mortality23–25 and this could impact our findings. The median PaCO2 did differ significantly between the two groups, but both groups were within the normal range of arterial PaCO2. We also looked at the median PaCO2 value for only the arterial blood gases with a PaO2 ≥40 kPa and again found that the median PaCO2 was within the normal range. This shows that the patients had neither hyper- or hypocapnia while being exposed to hyperoxia.
Limitations
The current study was a retrospective, single-center study with an inclusion period from October 2008 to October 2014. However, despite the long inclusion period, the number of included patients was relatively low and in combination with the low mortality rate, it may have limited our ability to detect a difference between groups. Due to the observational nature of the study, the results are potentially affected by confounding.
All the patients in our study were admitted to the ICU, where they received a wide variety of interventions as part of their treatment. This includes, for example, the need for surgery and different types of ventilatory therapy such as mechanical or spontaneous respiration. However, as it was a single-center study, the variability in treatment between patients should be minimal as the patients were treated by the same staff and according to the same treatment protocols.
In the current study, we used the APACHE II score to evaluate disease severity at admission to the ICU. The APACHE II score is developed as an ICU scoring system for severity of disease classification.26 The system is validated for use on all ICU patients and not just trauma patients, although studies have demonstrated that the APACHE II score is able to predict outcome in critically ill trauma patients.27–29 It could have been more appropriate to use traditional trauma scores such as the Injury Severity Score, Abbreviated Injury Scale or Revised Trauma Score which is designed for assessing specifically trauma severity. However, these scores were not available in the database.
Conclusion
In our convenience sample of 343 patients, an episode of hyperoxia within the 24 hours following admission to hospital with major trauma requiring admission to the GICU had no impact on 30-day in-hospital mortality. However, this was only a single-center study with a relatively small number of patients. Larger studies are needed to identify the effects of hyperoxia on the outcome of ICU patients admitted with major trauma.
Acknowledgment
We would like to thank the University of Aarhus and Falck Foundation for funding the study.
Disclosure
The authors report no conflicts of interest in this work.
References
Mack KA, Rudd RA, Mickalide AD, Ballesteros MF. Fatal unintentional injuries in the home in the U.S., 2000–2008. Am J Prev Med. 2013;44(3):239–246. | ||
Thim T, Krarup NH, Grove EL, Rohde CV, Løfgren B. Initial assessment and treatment with the airway, breathing, circulation, disability, exposure (ABCDE) approach. Int J Gen Med. 2012;5:117–121. | ||
Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol. 2004;164(6):1875–1882. | ||
Cornet AD, Kooter AJ, Peters MJ, Smulders YM. The potential harm of oxygen therapy in medical emergencies. Crit Care. 2013;17(2):313. | ||
Farquhar H, Weatherall M, Wijesinghe M, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J. 2009;158(3):371–377. | ||
Fiskum G, Danilov CA, Mehrabian Z, et al. Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Ann N Y Acad Sci. 2008;1147:129–138. | ||
Zwemer CF, Whitesall SE, D’Alecy LG. Cardiopulmonary–cerebral resuscitation with 100% oxygen exacerbates neurological dysfunction following nine minutes of normothermic cardiac arrest in dogs. Resuscitation. 1994;27(2):159–170. | ||
Hofmann R, James SK, Jernberg T, et al. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med. 2017;377(13):1240–1249. | ||
de Jonge E, Peelen L, Keijzers PJ, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12(6): R156. | ||
Bellomo R, Bailey M, Eastwood GM, et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15(2):R90. | ||
Brenner M, Stein D, Hu P, Kufera J, Wooford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042–1046. | ||
Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, de Jonge E. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43(7):1508–1519. | ||
Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (iota): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693–1705. | ||
Davis DP, Meade W, Sise MJ, et al. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009;26(12):2217–2223. | ||
Rincon F, Kang J, Vibbert M, Urtecho J, Athar MK, Jallo J. Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. J Neurol Neurosurg Psychiatry. 2014;85(7):799–805. | ||
Ihle JF, Bernard S, Bailey MJ, Pilcher DV, Smith K, Scheinkestel CD. Hyperoxia in the intensive care unit and outcome after out-of-hospital ventricular fibrillation cardiac arrest. Crit Care Resusc. 2013;15(3):186–190. | ||
Lee BK, Jeung KW, Lee HY, et al. Association between mean arterial blood gas tension and outcome in cardiac arrest patients treated with therapeutic hypothermia. Am J Emerg Med. 2014;32(1):55–60. | ||
Raj R, Bendel S, Reinikainen M, et al. Hyperoxemia and long-term outcome after traumatic brain injury. Crit Care. 2013;17(4):R177. | ||
Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–2171. | ||
Baker CC, Oppenheimer L, Stephens B, Lewis FR, Trunkey DD. Epidemiology of trauma deaths. Am J Surg. 1980;140(1):144–150. | ||
Bellomo R, Bailey M, Eastwood GM, et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15(2):R90. | ||
Nelskylä A, Parr MJ, Skrifvars MB. Prevalence and factors correlating with hyperoxia exposure following cardiac arrest – an observational single centre study. Scand J Trauma Resusc Emerg Med. 2013;21:35–7241. | ||
Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, Abu-Hanna A, de Keizer NF, de Jonge E. Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit Care. 2015;19:348–015. | ||
Nin N, Muriel A, Peñuelas O, et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017;43(2):200–208. | ||
Ahmadi Z, Bornefalk-Hermansson A, Franklin KA, Midgren B, Ekström MP. Hypo- and hypercapnia predict mortality in oxygen-dependent chronic obstructive pulmonary disease: a population-based prospective study. Respir Res. 2014;15(1):30–992130. | ||
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. | ||
Rutledge R, Fakhry S, Rutherford E, Muakkassa F, Meyer A. Comparison of APACHE II, trauma score, and injury severity score as predictors of outcome in critically injured trauma patients. Am J Surg. 1993; 166(3):244–247. | ||
Aslar AK, Kuzu MA, Elhan AH, Tanik A, Hengirmen S. Admission lactate level and the APACHE II score are the most useful predictors of prognosis following torso trauma. Injury. 2004;35(8):746–752. | ||
Dossett LA, Redhage LA, Sawyer RG, May AK. Revisiting the validity of APACHE II in the trauma ICU: improved risk stratification in critically injured adults. Injury. 2009;40(9):993–998. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


