Back to Journals » ClinicoEconomics and Outcomes Research » Volume 13
New Perspective to Improve Care of Patients with Infected Diabetic Foot Ulcer: Early Economic Impact of the Use of Photodynamic Therapy with RLP068 (Based) System
Authors Lorenzoni V, Chiavetta A, Curci V, Della Pepa G , Licciardello C, Pantò F, Scatena A, Turchetti G
Received 31 July 2020
Accepted for publication 12 January 2021
Published 26 February 2021 Volume 2021:13 Pages 135—144
DOI https://doi.org/10.2147/CEOR.S274897
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Samer Hamidi
Valentina Lorenzoni,1 Agatina Chiavetta,2 Vincenzo Curci,3 Giuseppe Della Pepa,4 Carmelo Licciardello,5 Felicia Pantò,6 Alessia Scatena,7 Giuseppe Turchetti1
1Institute of Management, Scuola Superiore Sant’Anna, Pisa, Italy; 2A.O. Cannizzaro, Catania, Italy; 3Centro per La Cura del Piede Diabetico, Ospedale Costantino Cantù di Abbiategrasso, Milan, Italy; 4Department of Clinical Medicine and Surgery, Federico II University, Naples, Italy; 5Unit of Metabolic and Endocrine Diseases, Centro Catanese di Medicina e Chirurgia, Catania, Italy; 6Section of Endocrinology, Biomedical Department of Internal and Specialist Medicine, University of Palermo, Palermo, Italy; 7Diabetology Unit, Cardioneurovascular Department, San Donato Hospital Arezzo Local Health Authorities South East Tuscany, Arezzo, Italy
Correspondence: Valentina Lorenzoni
Institute of Management, Scuola Superiore Sant’Anna, Piazza Martiri della Libertà 33, Pisa, 56127, Italy
Tel +39050388826
Email [email protected]
Objective: To perform an early economic evaluation of a system based on photodynamic advanced adjuvant therapy with photosensitizer RLP068/CI to facilitate the healing process of foot/leg skin lesions/ulcers with an excellent safety profile.
Design: An early short-term (10 weeks) cost-effectiveness and a budget impact analysis (over 5 years) comparing photodynamic therapy with photosensitizer RLP068/CI based (PDT-RLP068) system added to Standard of Care (SoC) vs SoC alone.
Setting: The Italian National Healthcare System perspective considering both the outpatient and the day-hospital regimen.
Participants: Hypothetical patients with diabetic foot infection (DFI) grades I/IIB.
Interventions: The PDT-RLP068 system as an add-on to Standard of Care (SoC) vs SoC alone as the first-line treatment for the management of DFIs.
Main Outcomes: Days within which the clinical target was achieved and direct health costs for patients’ management.
Results: Additional costs generated by the use of the PDT-RLP068 system progressively decreased as time to reach the target induced by the novel system decreased. In the outpatient regimen, when time to reach clinical target decreased in the range 7– 28 days, ICERs varied from about 1€ to 70€ for each additional day gained with clinical target achieved. The system was dominant when halving time to reach the target in the outpatient regimen and even for modest reduction of time in day-hospital regimen. In terms of budget impact, when considering day-hospital regimen, if the PDT-RLP068 based system allowed a shortened duration to reach the clinical target of between 7– 28 days, BI was 8,100,000€ to 700,000€, with saving less than 2,000,000€ with 50% reduction of time. Considering the inpatient setting, the use of the PDT-RLP068 system would result in saving even with the modest impact on the time needed to activate the healing process.
Conclusion: The early economic evaluation performed suggested that, if the claimed effectiveness of the technology demonstrated in case reports and in preliminary clinical studies can be confirmed in larger population studies, and allowing for shortening of the time needed to activate the healing process, the PDT-RLP068 system could offer the chance to improve care for DFI patients without compromising the sustainability of the system.
Keywords: diabetic foot ulcer, diabetic foot infection, photodynamic therapy, costs, economic, impact
Background
The worldwide burden of diabetes has increased during recent decades, with about 425 million people being affected by diabetes in 2017, and numbers are projected to increase in the near future.1 Among the sequel of consequences triggered by diabetes, diabetic foot ulcers (DFU) represent a major complication amplifying the clinical and economic burden of disease. DFUs occur in 15–34% of diabetic patients2,3 thus, according to recent Italian data suggesting that more than 3,000,000 Italians are suffering from diabetes,4 it is estimated that about 336,000 patients (9.8% of diabetic patients) developed DFUs5 and 195,000 patients had diabetic foot infections (DFIs)6 which tremendously impact both on costs and quality of life (QoL).
DFIs are associated with long-lasting treatment and uncertain course, during which amputations and frequent relapse may occur. About 40% of patients experiencing DFI relapse within 1 year and 65% within 5 years;2,7 as a result morbidity, disability and costs increase.
Data from the European Study Group on Diabetes and the Lower Extremity (EURODIALE) suggested that overall direct costs of DFUs without infection and not requiring amputation varied between 3,771€ and 9,622€ in 2015, while for DFIs total direct costs varied between 8,113€ and 16,414€.8 Complication of diabetes including DFUs and their infection are also associated with substantial indirect costs to patients, their families and society;9,10 moreover, the ulcer healing process also affects the perception of QoL11,12 and the degree of QoL disruption is proportional to the severity of complications. In this context, management of DFIs has become crucial to contain the overall burden of disease.
Indeed, current management of DFU and DFI requires a multidisciplinary approach, and despite the availability of advanced treatment options, along with efforts at international and national levels, effective and sustainable patient management is still a challenge due to the complexity of the disease and its uncertainty.13
Current clinical practice in Italy regards topical medications as the standard of care (SoC) for the management of DFI. Although the unit cost of this medication is generally low and no clear evidence about newer and frequently expensive options exists, SoC is often not enough to guarantee wound healing; moreover the prolonged treatment coupled with the uncertain course of DFI result in significant costs for healthcare systems.
In detail, the current recommendations suggest first-line treatment with SoC (consisting of a regular assessment of the patients and including debridement, cleansing, management of exudate, and treatment of infection with antibiotic therapy) over a 4-week period to activate the healing process.14 If there is no evidence of healing process activation (reduction by 40–50% of the ulcer area) after this period, second-line options, often referred to as advanced therapies, are encouraged.14
The use of currently available options as second-line therapies is generally more expensive (compared with the standard therapy) and should be carried out by trained practitioners. These costs may be justified when they result in improved ulcer healing, reduced morbidity, fewer lower-extremity amputations and improved patient functional status.14,15
Among the advanced options, photodynamic therapy (PDT) has been shown to represent a potentially effective solution for the treatment of localized microbial infections through the in situ application of a photosensitizer followed by illumination with visible light of the photosensitizer-loaded infected area.16 PDT could strongly contribute to wound healing of infected lesions through: immediate reduction of the local bacterial load;17 effectiveness against all known classes of microorganisms; including wild type and resistant strains (also as biofilm status);18,19 no induction of resistance as shown in vitro by multiple antimicrobial PDT treatments;18 excellent safety and tolerability profile, thus safe for host tissues.20
Medical devices indicated as adjuvant therapy for the local treatment of skin lesions and ulcers through PDT are currently available and could be used to manage infection and facilitate the healing process. Unlike other advanced options, the currently available photodynamic therapy with photosensitizer RLP068/CI based (PDT-RLP068) system can be inserted as an add-on to any first-line protocol. Several observational studies in patients with DFU showed immediate significant reduction versus placebo of the total bacterial load after the initial administration17,18 and significant improvement of the ulcer healing process after repeated applications.20 Moreover, an observational study involving 36 patients with infected vascular, hard-to-heal leg ulcers treated twice over a four-day period before skin grafting surgery, has been completed.21
Accordingly, the present study aims to provide evidence about an early evaluation of the economic impact related to use of the PDT-RLP068 system, considering both a cost-effectiveness (CEA) and a budget impact analysis (BIA), accounting for the perspective of the Italian National Healthcare System (INHS), thus considering just direct health costs in both analyses, and a short-term horizon.
Methods
Target Population and Approach for the Cost-Effectiveness Analysis
Both CEA and BIA were performed considering as the target population generic patients affected by DFI (Grades I/IIB Texas scale22).
Given the paucity and the variability of data available about DFIs and on the impact of the use of the PDT-RLP068 system, taking a conservative approach, the analysis focused on resource use and effects produced within the time frame of the activation of the healing process; without considering events and costs incurred over a long-term horizon (i.e., recurrence, amputation, etc.). The analysis was limited to direct health costs related to DFI management, thus considering the INHS perspective, a system providing universal coverage to citizens and residents, with public healthcare largely free of charge.
The framework for the analysis (i.e., assumptions, parameters) was developed considering current guidelines for the management of DFIs14 and inputs from a panel of experts (included as co-authors of the present work). In detail, six clinicians recognized as experts in the management of DFUs at national level and having some degree of experience in the use of the PDT-RLP068 system in clinical practice were involved in the study. The expert panel was asked to fill out a questionnaire whose general purpose was to assess facts related to an overall assessment of the PDT-RLP068 system from a Health Technology Assessment perspective, thus collecting information about current clinical practice and challenges in the management of DFIs, advantage, disadvantage and satisfaction of current SoC and the use of the PDT-RLP068 system as an add-on to SoC, related organizational aspects (see Supplementary Material for details of the questionnaire used). The questionnaire was sent to each clinician by e-mail and answers collected were then deepened into a face-to-face meeting.
Following the feedback obtained from the expert panel and on the basis of the scarce literature evidence available, the base case scenario was developed assuming as the time horizon for the analysis the length of the time frame needed for the activation of the healing process (i.e., until achievement of a meaningful clinical target associated with positive outcome). The time frame was assumed to be 10 weeks, on the basis of the inputs from the expert panel and considering treatment in an outpatient setting, the most frequent approach used in current practice according to the expert panel.
No Ethical Committee approval was needed for the conducting of the present study as it did not involve any collection of patients’ data. The clinicians participating in the expert panel, who were included as co-authors, were fully informed about the aims of the research and gave voluntary consent to participate and for the dissemination of results from the assessment they were involved in.
Interventions
Interventions considered and compared within the CEA analysis were SoC versus the use of the PDT-RLP068 system as an add-on to SoC.
Inputs about the frequency of medications, length of treatment and application of the novel technology were obtained from the expert panel.
In detail, in the base case analysis and for each treatment arm patients were assumed to perform medications twice a week. In the treatment arm considering the addition of the PDT-RLP068 system to SoC the use of that system was considered for just the first 4 weeks (8 applications)23 while in the remaining 6 weeks only management of the DFIs with conventional approaches used in SoC was assumed for both arms.
Effectiveness
Given the effectiveness and safety profile of the PDT-RLP068 system, along with preliminary data arising from clinical studies,20 the addition of the PDT-RLP068 system to SoC was assumed to provide a benefit in terms of reduction of the time needed to reach a meaningful clinical target suggestive of the activation of the healing process (i.e., 40% reduction of the ulcers’ area). Accordingly, the effectiveness was measured in term of “additional days with clinical target achieved”.
To address current uncertainty about the impact of the PDT-RLP068 system and starting from the little evidence available on the successful use of the system in patients with difficult to heal infections, which was also confirmed by the expert panel, rather than assuming a single value to model the impact of the use of the PDT-RLP068 system the base case was developed as considering multiple analyses depicting the possible impact of the novel approach over a range of possible values. In detail, assuming a general length of the time to reach the target of 10 weeks, percentage reduction of that time frame in the range from 0% (no effect of the PDT-RLP068 system) to 50% (half of the hypothesized time) were explored in the base case analysis.
Costs
Costs were valued considering tariffs associated with procedures performed within the medication from the Italian Official Gazzette24 according to the treatment regimens considered.
In detail, in the base case scenario, when the outpatient setting is considered, the list of procedures performed within a visit was derived from the expert panel, costs were then estimated valuing each procedure according to national tariffs24 except for the code referring to advanced medications that was derived from Lombardia Region; the latter was used to value additional costs related to the use of the PDT-RLP068 system to model the costs of the new technology.
Table 1 provides details of costs used to value treatment for DFI management considering the outpatient regimen.
 |
Table 1 Reimbursements Associated with the Management of DFIs |
All costs were expressed in Euro and referred to 2019.
Scenario Analysis
Despite being less frequent (according to data collected from the panel of experts) a scenario analysis considering the inpatient setting was also evaluated, assuming the management of DFI patients with day-hospital admissions, according to clinical practice in Italy.
In this scenario analysis the cost of the daily hospital admission for the management of patient with DFIs was estimated valuing the single episode using national reimbursement values set for the diagnosis related group (DRG) 294 “Adult diabetes”. The actual reimbursement for that DRG is of 236€24 and the same cost was used both for SoC and SoC plus PDT-RLP068 system as the reimbursement fully covers the cost of the novel technology.
Results from the CEA analysis are presented using the Incremental Cost Effectiveness Ratio (ICER) expressed in terms of additional costs per additional days with clinical target achieved.
Probabilistic Sensitivity Analysis
A probabilistic sensitivity analysis (PSA) was performed to explore uncertainty assigning appropriate statistical distributions to input values used in the analysis and considering 1,000 simulations drawing samples from those distributions. Results from the PSA are represented on the cost-effectiveness plane.
Budget Impact Analysis
Considering current epidemiological data and projection about the increasing case rates in the near future1,5 a BIA was performed assuming the perspective of the INHS and considering a time horizon of 5 years (2020–2024).
The BIA aims to compare a new environment, which assumes the introduction of a PDT-RLP068 system to be used as an add-on to SoC for the management of DFIs, with the current scenario in which only SoC is considered.
Similarly to the CEA analysis, evaluation of the impact on the INHS budget was performed limiting the analysis to costs borne within the time frame needed for the activation of the healing process and considering the possibility to manage DFI patients both in outpatient and inpatient settings. The same inputs as in CEA were used for the frequency of visits, length of the time needed to activate the healing process and costs.
Table 2 reports the estimated number of patients that will be treated adding the PDT-RLP068 system to SoC, patients treated with SoC alone for each year according to the projected percentage of those treated with the PDT-RLP068 system as an add-on to SoC, or managed according to SoC alone.
Results
Cost-Effectiveness Analysis
Base Case Analyses
In the base case analyses, the PDT-RLP068 system as an add-on to SoC implied savings only when allowing 50% reduction of time to reach the clinical target.
In detail, considering treating a patient twice weekly, the cost of SoC over 10 weeks was estimated to be 1,508€ per patient; when adding the PDT-RLP068 system costs were 2,152€ per patient over the same period.
Thus, when time to reach the activation of the healing process was equal, SoC is preferable as it implied lower costs with a cost difference of about 644€ per-patient.
When the PDT-RLP068 system reduced the time needed to reach the clinical target suggesting activation of the healing process in a range of values comprised between 10% and 40% (of the overall length assumed, 10 weeks), additional costs associated with the use of the PDT-RLP068 system ranged from 493€ per patient to 40€ per patient respectively, while allowing an increase in the number of additional days with the clinical target achieved from about 7 to 28 days.
When time needed to activate the healing process is assumed to be shortened by 50% (35 days) with the use of the PDT-RLP068 system as an add-on to SoC, this strategy became dominant with a saving per patient of about 111€ and allowing to gain 35 additional days with target achieved (Table 3).
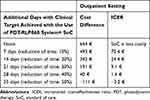 |
Table 3 Results from the Base Case Cost-Effectiveness Analysis of PDT-RLP068 System |
ICERs suggested a cost difference of about 70€ to 1€ for each additional day gained with the clinical target achieved when the PDT-RLP068 system is assumed to shorten the time to reach the target from 10% to 50% respectively (Table 3).
Scenario Analysis
When considering the management of DFUs in the inpatient setting with a weekly day-hospital admission, the use of the PDT-RLP068 system allows reducing costs even when time to reach the activation of the healing process is assumed to be shortened by 10%. In detail, savings vary between 472€ and 2,360€ when the time is shortened from 10% to 50% and ICER values suggested a cost decrease from 7€ to 26€ for each additional day gained with the clinical target achieved (Table 4).
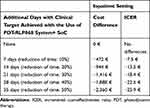 |
Table 4 Results from the Scenario Analysis |
Probabilistic Sensitivity Analysis
Figure 1 shows the results from the PSA. The sensitivity analysis shows a high degree of uncertainty of results obtained from the base case analysis.
 |
Figure 1 Results from the probabilistic sensitivity analysis. |
Budget Impact Analysis
Considering the outpatient setting the introduction of the PDT-RLP068 system to be used as an add-on to SoC implied a cumulative additional 5-year cost of about 10.6 million € when the novel system did not produce any effect on time needed to reach the clinical target suggesting the activation of the healing process.
When the use of the PDT-RLP068 system shortened time for the activation of the healing process by 10% (7 days) the cumulative budget impact (BI) was more than 8.1 million €. Additional costs progressively decreased as the time to reach the clinical target was hypothesized to be shortened because of the use of the PDT-RLP068 system. In detail, the BI was estimated to be less than 700,000 € when use of the PDT-RLP068 system decreased the time required to reach the target by 40%; finally, use of the PDT-RLP068 system produced a saving of less than 2 million € when time to reach the target with the use of the PDT-RLP068 system was reduced by 50% (Figure 2).
 |
Figure 2 Outpatient setting scenario: cumulative 5-year budget impact related to the addition of the PDT-RLP068 system to SoC. |
Similarly, when considering the inpatient regimen, introduction of the PDT-RLP068 system produced a saving for the INHS that ranged from less than 8 million € to more than 39 million € as the system is hypothesized to reduce the time to reach the target from 7 to 35 days (i.e., reduction of time from 10% to 50%) (Figure 3).
 |
Figure 3 Inpatient setting scenario: cumulative 5-year budget impact related to the addition of the PDT-RLP068 system to SoC. |
Discussion
Results from the early economic evaluation performed showed potential beneficial health-economic implications related to the use of the PDT-RLP068 system for the management of DFIs, if the successful results reported in the small amount of literature evidence available can be confirmed in larger and well-designed studies.
In detail, whilst in the case of no effect on the time to reach clinical target the PDT-RLP068 system would further increase the economic impact of DFIs – all the more so if it would lead to an increase in time, despite the fact that this option is not included in the analysis – in the case of a 10% reduction in the time to reach the target, results from the present study estimated that benefits could be achieved at the price of a limited cost increase. Indeed, although comparison with threshold values conventionally considered in Italy is not feasible since they are expressed in terms of Euro per QALY – thus not comparable with the ICER values considered in the present analysis – they varied between 25,000€ and 40,000€ per QALY.25
Despite the recent introduction of several innovative approaches (e.g., collagen dressings, skin grafts, bioengineered skin equivalents), management of DFIs is still challenging and there is no clear evidence on the potential health-economic benefits related to their use.14,15,26
Accordingly, the use of novel options is still limited as is the quality of evidence about their health-economic implications. Indeed, the available economic evaluations about strategies for the management of DFIs suggest that implementation of preventive strategies and effective first-line strategies for diabetes-related ulcerations and infections reduces the risk of amputations and the costs associated with lower-extremity amputations.27
Thus, the possibility to use the PDT-RLP068 system in first-line treatment as an add-on to SoC may result to a significant competitive advantage. Further data are needed to confirm the advantage of using the PDT-RLP068 system that would allow offsetting long-term costs because of the early and effective control of the infection.
Despite variability of diabetes costs among countries, DFUs management constitutes a significant proportion of the overall costs with DFIs with related gangrene and amputation amplifying the economic burden.3,28
According to data from the International Diabetes Federation, healthcare expenditure related to diabetes has grown from 232 billion USD$ spent by people with diabetes worldwide in 2007, to 727 billion USD$ in 2017.
Data from a recent study aiming at evaluating the cost of DFUs in Europe29 highlighted the significant economic burden of DFUs and these figures are mainly associated with the fact that there is still a lack in preventive strategy and response to treatment is variable and challenging.
In this context the role of advanced therapies is essential and evidence about their health-economic consequences serves to provide clinicians with a guide for the selection of effective and sustainable approaches. Optimal management of DFIs is essential to reduce the incidence of limb amputations and infection-related morbidities with consequent favourable impact on QoL and economic effects.10
In the actual context of uncertainty about the consequence of novel options and considering the pressure diabetes-related costs pose worldwide, the less expensive options result in the preferred treatment choice.
Regulatory barriers indeed do not facilitate or allow the widespread use of advanced options, thus in turn limiting accrual of evidence to adequately perform studies.27
As an example, in Italy one of the main barriers for the uptake of advanced therapies is represented by the lack of specific reimbursement in many regions. This prevents patients from receiving potentially effective treatment.
The main limitation of the present study is that, given the paucity of evidence available, most of the inputs used to evaluate the economic impact of the PDT-RLP068 system were drawn from a panel of experts. For the same reason the evaluation was limited to direct health costs in the time frame to reach 40% reduction of wound areas, considered as clinical target suggestive of the activation of the healing process, thus not accounting for effects on relevant outcomes such as induced co-morbidities, amputations and mortality and their related impact both in terms of direct and indirect costs.
Indeed despite results from in vivo and clinical studies17,30 suggesting the possibility for the PDT-RLP068 system to offset long-term costs due to the early and effective control of infection, the evidence was considered too weak to be included in the analysis.
Nevertheless, to overcome these limitations evidence about a wide range of possible effects induced by the use of the PDT-RLP068 system is provided. Moreover, taking a conservative approach, the economic evaluation performed assumes a similar schedule of appointments to manage patients for both options considered in the analysis. Due to uncertain course and the difficulties in patient management previously detailed, in current clinical practice it is not infrequent to manage patients considering 3 appointments per week; moreover it is assumed that the use of the PDT-RLP068 system would not impact on frequency of visits.
Feedback from the expert panel on the potential of the PDT-RLP068 system suggests a role for the addition to SoC particularly to allow better management of DFIs, reduce or even shorten the use of antibiotics but also reduce pain and smell associated with the ulcer with a clear positive impact on patients. However, as for long-term consequences, the weakness and uncertainty of the available evidence related to the impact of the use of the PDT-RLP068 system on antibiotics prevent inclusion of that parameter in the analysis.
The overall healing process, the use of antibiotics (in particular those administered within in-patient setting) and amputation have a tremendous impact on healthcare expenditure: any eventual positive impact of the PDT-RLP068 system on those outcomes could partially compensate for additional costs induced by the use of the system instead of the cheapest SoC.
To conclude, the data presented are just preliminary estimations of the potential economic impact induced by the use of the PDT-RLP068 system; these data need to be validated by a clinical study that is still ongoing.
Careful evaluation of the overall impact of advanced options for the management of DFUs and DFIs could help overcome affordability gaps and improve the effectiveness and quality of care.
Acknowledgments
VL and GT conceived the paper. VL performed the analysis and drafted the manuscript. AC, VC, GDP, CL, FP and AS participated in the panel of experts, helped to retrieve data necessary to perform the analysis and aided in interpreting results. All authors contributed to data analysis, drafting or revising the article, agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work. All authors read and approved the manuscript.
Funding
The analysis was supported by Molteni Farmaceutici S.p.A which sponsored the development of the models for the economic evaluation.
Disclosure
Dr Valentina Lorenzoni and Professor Giuseppe Turchetti report grants from Molteni Farmaceutici SpA, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Federation ID. IDF Diabetes Atlas Eighth edition 2017. International Diabetes Federation. IDF Diabetes Atlas, 8th edn. Brussels, Belgium: International Diabetes Federation; 2017. Available from: http://www.diabetesatlas.org.
2. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. doi:10.1056/NEJMra1615439
3. Yazdanpanah L, Shahbazian H, Nazari I, et al. Incidence and risk factors of diabetic foot ulcer: a population-based diabetic foot cohort (ADFC study)-two-year follow-up study. Int J Endocrinol. 2018;2018:1–9. doi:10.1155/2018/7631659
4. (No Title) [Internet]. [cited November 5, 2019]. Available from: https://www.istat.it/it/files//2019/09/La-salute-nelle-regioni-italiane.pdf.
5. Diabetic Foot Ulcer (DFU) Treatment Market Size | Industry Report 2025 [Internet]. [cited November 5, 2019]. Available from: https://www.grandviewresearch.com/industry-analysis/diabetic-foot-ulcer-dfu-treatment-market.
6. Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50:18–25. doi:10.1007/s00125-006-0491-1
7. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747–755. doi:10.1007/s00125-008-0940-0
8. Prompers L, Huijberts M, Schaper N, et al. Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia. 2008;51(10):1826–1834. doi:10.1007/s00125-008-1089-6
9. Yang W, Dall TM, Beronjia K, et al. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928.
10. Doubova SV, Borja-Aburto VH, Guerra-Y-Guerra G, Salgado-de-Snyder VN, González-Block MÁ. Loss of job-related right to healthcare is associated with reduced quality and clinical outcomes of diabetic patients in Mexico. Int J Qual Health Care. 2018;30(4):283–290. doi:10.1093/intqhc/mzy012
11. Goodridge D, Trepman E, Sloan J, et al. Quality of life of adults with unhealed and healed diabetic foot ulcers. Foot Ankle Int. 2006;27(4):274–280. doi:10.1177/107110070602700408
12. Ribu L, Wahl A. Living with diabetic foot ulcers: a life of fear, restrictions, and pain. Ostomy Wound Manage. 2004;50(2):57–67.
13. Martínez-Gómez DA, Moreno-Carrillo MA, Campillo-Soto A, Carrillo-García A, Aguayo-Albasini JL. Reduction in diabetic amputations over 15 years in a defined Spain population. Benefits of a critical pathway approach and multidisciplinary team work. Rev Esp Quimioter. 2014;27(3):170–179.
14. Position document Local management of diabetic foot ulcers - Wounds International [Internet]. [cited November 5, 2019]. Available from: https://www.woundsinternational.com/resources/details/position-document-local-management-diabetic-foot-ulcers.
15. IWGDF Guidelines on the prevention and management of diabetic foot disease IWGDF Guidelines [Internet]. [cited November 5, 2019]. Available from: www.iwgdfguidelines.org.
16. Wainwright M, Maisch T, Nonell S, et al. Photoantimicrobials—are we afraid of the light? Lancet Infect Dis. 2017;17(2):e49–e55. doi:10.1016/S1473-3099(16)30268-7
17. Mannucci E, Genovese S, Monami M, et al. Photodynamic topical antimicrobial therapy for infected foot ulcers in patients with diabetes: a randomized, double-blind, placebo-controlled study - The D.A.N.T.E (Diabetic ulcer Antimicrobial New Topical treatment Evaluation) study. Acta Diabetol. 2014;51(3):435–440. doi:10.1007/s00592-013-0533-3
18. Giuliani F, Martinelli M, Cocchi A, Arbia D, Fantetti L, Roncucci G. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob Agents Chemother. 2010;54(2):637–642. doi:10.1128/AAC.00603-09
19. Vassena C, Fenu S, Giuliani F, et al. Photodynamic antibacterial and antibiofilm activity of RLP068/Cl against Staphylococcus aureus and Pseudomonas aeruginosa forming biofilms on prosthetic material. Int J Antimicrob Agents. 2014;44(1):47–55. doi:10.1016/j.ijantimicag.2014.03.012
20. Monami M, Scatena A, Schlecht M, et al. Antimicrobial photodynamic therapy in infected diabetic foot ulcers: a multicenter preliminary experience. J Am Podiatr Med Assoc. 2020;110. doi:10.7547/18-069
21. Mosti G, Picerni P, Licau M, Mattaliano V. Photodynamic therapy in infected venous and mixed leg ulcers: a pilot experience. J Wound Care. 2018;27(12):816–821. doi:10.12968/jowc.2018.27.12.816
22. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855–859. doi:10.2337/diacare.21.5.855
23. Pantò F, Adamo L, Giordano C, Licciardello C. Efficacy and safety of photodynamic therapy with RLP068 for diabetic foot ulcers: a review of the literature and clinical experience. Drugs Context. 2020;9. doi:10.7573/dic.2019-10-3
24. Italian Ministry of Health. Remunerazione prestazioni di assistenza ospedaliera per acuti, assistenza ospedaliera di riabilitazione e di lungodegenza post acuzie e di assistenza specialistica ambulatoriale. Gazzetta Ufficiale - Serie Generale; 2013.
25. Fattore G. Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. PharmacoEconomics. 2009.
26. Piaggesi A, Låuchli S, Bassetto F, et al. Advanced therapies in wound management: cell and tissue based therapies, physical and bio-physical therapies smart and IT based technologies. J Wound Care. 2018;27(Sup6a):S1–137.
27. Chow I, Lemos EV, Einarson TR. Management and prevention of diabetic foot ulcers and infections: a health economic review. PharmacoEconomics. 2008;26(12):1019–1035. doi:10.2165/0019053-200826120-00005
28. Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabet Med. 2014;31(12):1498–1504. doi:10.1111/dme.12545
29. Tchero H, Kangambega P, Lin L, et al. Cost of diabetic foot in France, Spain, Italy, Germany and United Kingdom: a systematic review. Ann Endocrinol (Paris). 2018;79(2):67–74. doi:10.1016/j.ando.2017.11.005
30. Martinelli N, Curci V, Quarantiello A, Saldalamacchia G. The benefits of antimicrobial photodynamic therapy with RLP068 in the management of diabetic foot ulcers. Drugs Context. 2019;8:1–8. doi:10.7573/dic.212610
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

