Back to Journals » Patient Preference and Adherence » Volume 13
New insights to improve treatment adherence in asthma and COPD
Received 22 March 2019
Accepted for publication 14 June 2019
Published 31 July 2019 Volume 2019:13 Pages 1325—1334
DOI https://doi.org/10.2147/PPA.S209532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Maureen George,1 Bruce Bender2
1School of Nursing, Columbia University, New York, NY, USA; 2Division of Pediatric Behavioral Health, National Jewish Health, Denver, CO, USA
Abstract: Chronic respiratory diseases such as asthma and COPD are typically managed by daily inhaled medication. However, the efficacy of an inhaled medication depends upon a patient’s adherence to therapy, which refers to whether the medication is actually taken as prescribed. In patients with these diseases, higher adherence has been associated with better health outcomes, such as improved disease control and a reduction in severe and potentially costly exacerbations. Adherence is a multifaceted concept that includes medication-related, intentional, and unintentional reasons that patients may or may not take their medication as directed. The purpose of this integrative review is to present the individual patient factors that contribute to suboptimal adherence to inhaled therapies and the associated effects on health outcomes, while also highlighting evidence-based strategies for health care providers to improve adherence to such therapies in patients with asthma or COPD. Working closely with patients to establish a model of shared decision-making, which takes patient beliefs and preferences into account when choosing treatment options, has the potential to improve adherence and overall patient outcomes in the management of asthma and COPD.
Keywords: chronic disease, health behavior, evidence-based medicine, inhalers
Introduction
Recent advances in the understanding of the pathophysiology of asthma and COPD have led to the development of new therapies and combinations thereof to manage these chronic respiratory diseases. Health care providers who treat these patients generally follow international and national treatment guidelines as well as strategy reports that are updated more frequently to make recommendations based on the latest research.1,2 Nevertheless, the patient is responsible for following through with taking medication as prescribed.
Suboptimal adherence is defined as the failure of patients to take their medication as directed by their clinician.3 Higher adherence has been associated with positive health outcomes, including improved disease control and a reduction in mortality.4 General medicine reviews estimate that approximately 50% of medications for chronic disease are not taken as prescribed.5 Adherence rates tend to be even lower for patients with asthma or COPD,6 with estimates ranging widely from 22% to 78%.2,3,7,8 This may be due, in part, to the additional challenges of inhaled therapy, which has a central role in asthma and COPD disease management.
The terminology used in the ABC (3-step) adherence framework includes (A) initiation, (B) implementation, and (C) persistence or discontinuation.9 Initiation refers to whether or not the patient takes the first dose of medication. Implementation is related to the alignment between the patient’s actual dosing and the prescribed regimen, which is a longitudinal measure of the patient’s dosing history. Persistence is the time between initiation and treatment cessation, which may or may not be the intended end of the prescription.
Considerable variability exists in the measurement of adherence.3 Electronic monitoring via dispensing records can determine how often prescriptions are filled, or an electronic monitoring device attached to an inhaler can be used to record the number of times the inhaler was actuated10 and the medication inhaled.11 Methods to evaluate adherence in clinical trials include patient behavioral questionnaires (eg, self-report measures like the validated Medication Adherence Report Scale for Asthma [MARS-A]12,13 and the Medication Intake Survey-Asthma [MIS-A]14), dispensing records, dose or pill counting/medication possession ratios (MPRs; number of days’ supply/number of days in study), electronic inhaler monitoring, and drug assays. Adherence in asthma and COPD is most commonly measured by self-report (37.8%) and prescription refill data (32.8%), with electronic dose monitoring (19.3%) rising in popularity.3 However, self-report measures overall are notoriously unreliable, often overestimating adherence by as much as 50%.15
This integrative review encompasses discussion of both recent studies and other reviews that were published through 2018, providing a timely update of the adherence literature for these chronic diseases. We have chosen studies published in the past 10 years that investigated the relationship between adherence and outcomes in asthma and COPD. The purpose of this manuscript is to highlight the many factors that contribute to suboptimal adherence to inhaled medication in asthma and COPD, describe the consequences for health outcomes, and present strategies supported by the literature that clinicians can employ to improve adherence to inhaled therapies and, in turn, improve patient outcomes.
Factors that contribute to suboptimal adherence
To understand the reasons for suboptimal adherence, health care providers should elicit patients’ beliefs and concerns about their disease and the medication(s) used to treat it.2 Factors that contribute to suboptimal adherence fall into 3 main categories (Figure 1).2 Medication factors include reasons that are directly related to the medication itself (eg, side effects, ease of inhaler use). For example, correct inhaler technique can be challenging for patients and is vital to optimal therapy delivery16 and thus a critical component in adherence.17,18 Intentional factors contributing to suboptimal adherence are the result of the patient’s choices (eg, perception that treatment is unnecessary), whereas unintentional factors are not conscious decisions made by the patient (eg, misunderstanding directions).2
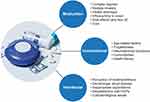 |
Figure 1 Factors contributing to suboptimal adherence in asthma and COPD. The factors that contribute to suboptimal adherence in asthma and COPD are grouped into 3 major categories.Notes: Image: iStock.com/Alessandro2802. Data from Global initiative for asthma http://www.ginasthma.org2; Makela et al3; Bryant et al7 and van Boven.18Abbreviations: COPD, chronic obstructive pulmonary disease; HCP, health care provider. |
Incorrect inhaler technique is 1 manifestation of unintentional suboptimal adherence. The use of inhalers adds to the complexity of the medication regimen. Patients may have difficulty using certain inhalers for a variety of reasons, including comorbidities such as arthritis or cognitive impairments.19 Other important aspects of the medication regimen include the use of multiple inhalers versus a single combined inhaler as well as the frequency of dose administration required each day, although available data have not shown meaningful changes in clinical outcomes with once-daily versus twice-daily dosing for inhaled therapies.20 Furthermore, it may be difficult to establish and sustain the correct technique when inhalers require different administration techniques. For example, dry-powder inhalers (DPIs) require rapid and forceful inspiration to properly deliver the drug, whereas this type of inhalation is not recommended for metered-dose inhalers (MDIs) and nebulizers.21 Furthermore, in some cases, patients may have difficulty coordinating actuation and inspiration to correctly use a pressurized MDI, whereas other patients have difficulty inhaling forcefully enough to actuate a DPI.2,22 Cognitive impairment is especially an issue in elderly patients with COPD. For patients with reduced cognitive function, DPIs have been associated with better technique than MDIs.23 In this context, it may be helpful for patients to have device types (ie, MDI vs DPI) that match for both controller and reliever therapy, avoiding complications that might arise from needing to distinguish and use more than 1 technique.24 Critical errors in inhaler technique (eg, actuation against lips, teeth, or tongue) have been shown to increase risk of hospitalization, emergency department visits, and the use of antibiotics and oral corticosteroids (OCSs). The likelihood of these errors occurring can be reduced by proper instruction and checks on inhaler technique at follow-up appointments.25
Asthma and COPD are generally accepted to be 2 distinct respiratory diseases that affect differing populations and take unique developmental courses. Each disease can present its own challenges to patient adherence; 1 such factor is the age of onset. Patients with COPD tend to be older and may have comorbidities as a result of smoking that require several medications, so it is important to consider all medications the patient is taking and any potential contraindications or potential cognitive changes that may cause difficulty with correct inhaler usage.9 In contrast, asthma can present at any age,26 and younger children with asthma may also have difficulty using inhalers correctly, especially in terms of inhalation technique.27 Concerns about adverse effects, such as growth suppression and osteoporosis,28,29 and particularly about the necessity of steroid treatment (inhaled or oral),30 may cause parents of children with asthma to be less likely to enforce adherence to prescribed therapy.31 Some clinicians may also be hesitant to prescribe inhaled corticosteroids (ICSs) to children for these same reasons.32 However, the 2018 Global Initiative for Asthma (GINA) report does recommend daily ICS therapy in children,2 and this course of therapy has not been shown to significantly impact final adult height when used at lower doses compared with higher doses.33
Additional difficulties with adherence to inhalation therapy may occur as children with asthma age and become more independent. Adolescents with asthma have reported forgetfulness, lack of routine, social stigma around using inhalers, and lack of family support (eg, parents not accepting their asthma diagnosis) as reasons for suboptimal adherence.34 However, overall, increasing age has been associated with higher adherence to asthma therapy.35 Another important factor that distinguishes asthma from COPD is the course of the disease itself. The airflow obstruction that occurs in asthma is variable and can be reversed with medication, whereas COPD is a progressive and irreversible deterioration of lung function.9 Additionally, depression is a common comorbidity in asthma and COPD that undermines adherence; as a result, treatment for depression in patients with asthma or COPD is generally associated with improved adherence.36,37
Effects of suboptimal adherence on patient outcomes
Asthma
Studies have shown that higher adherence is associated with better symptom control in patients with asthma, whereas suboptimal adherence is a modifiable independent risk factor for asthma exacerbations (Table 1).2,3 Specifically, high adherence (eg, MPR ≥80%) to asthma controller therapy, such as ICS, has been associated with significantly reduced risk of exacerbation, reduced OCS use, and positive impacts on asthma-related mortality.3,8,38,39 A study in pediatric patients with moderate persistent asthma showed that medication adherence was also a strong determinant of asthma control (as defined by the 2018 GINA report).2,30
 |
Table 1 Summary of study results linking inhaled medication adherence to patient outcomes in asthma |
Given that poor asthma control can be a result of suboptimal adherence, it is important to distinguish between a patient with asthma who is not adhering to his or her current therapy and a patient with asthma who requires progression to a higher GINA treatment step to control his or her symptoms. For these reasons, it is important to assess both adherence and inhaler technique before stepping up asthma therapy so as to avoid unnecessary medications.2 The use of electronic monitoring devices has been proposed to better identify pediatric patients with severe disease who require step-up therapy despite reportedly high adherence.40
COPD
Exacerbations resulting in hospitalization are thought to account for approximately 45% of direct medical costs associated with COPD and therefore can be expensive for the health care system.41 In fact, complex admissions requiring intensive care, intubation, or both, accounted for only 5.8% of hospital-based COPD care but 20.9% of costs in 2008.42 Higher adherence has been shown to significantly reduce moderate and severe exacerbations in COPD and also to lower mortality rates (Table 2).38,43 In contrast, suboptimal adherence has been associated with increased hospitalization and mortality, reduced quality of life, and loss of productivity.38,44 For patients with severe COPD, adherence to treatment is associated with lower health care costs and a lower risk of an intensive care unit stay.45 Adherence in combination with persistence of treatment is necessary to achieve improved clinical outcomes.44
 |
Table 2 Summary of study results linking inhaled medication adherence to patient outcomes in COPD |
Improving adherence to inhaled therapies in clinical practice
According to international guidelines for both asthma and COPD, it is important for clinicians to have empathetic discussions with patients to assess adherence, along with symptom control and inhaler technique, at every office visit. Suggested topics1,2 for discussions with patients who have asthma or COPD include: 1) the number of days per week the patient takes his or her inhaled medication; 2) the patient’s beliefs about his or her medication (including the perceived necessity), the cost of medications, and how often they are refilled; and 3) the importance of adherence with daily controller medications even when symptoms are infrequent. Although forgetfulness is the most often-cited reason for suboptimal adherence,46 patients may claim that they forgot in order to avoid further discussion of the true reason(s) for not taking their medication as prescribed.
Good communication by health care providers helps with increased satisfaction and better adherence, in turn improving health outcomes and reducing the use of health care resources.2,47 Assessing patients’ beliefs about their preferred treatment strategies and their prescribed medications is critical.48 For example, a recent German study showed that patients with asthma or COPD who believed their specific medications were necessary were more likely to be adherent, whereas patients with asthma who were concerned about overpresciption of medication by doctors were less likely to adhere to their prescribed treatment.49
Training patients on correct inhaler technique is crucial and should be conducted routinely.25 When providing patient education about inhalers, providers should do the following: 1) know how each device works and how to optimize delivery to the lungs; 2) be able to effectively demonstrate for the patient how the device works; 3) teach the patient how to use the device with the correct technique; and 4) regularly review the patient’s technique and provide additional training as necessary.50 Educational interventions on inhaler technique are effective, and predictors of success of the intervention include low baseline performance, outpatient setting, and short follow-up time.51 A number of studies have shown that 1-on-1 educational counseling by pharmacists about correct inhaler technique, as well as the importance of adherence, smoking cessation, exercise, and follow-up, not only increases adherence in patients with COPD52 but can also reduce severe exacerbations and hospitalization rates.53 One study also showed that home visits by trained asthma educators increased adherence and decreased emergency department visits, but the improvements in outcomes were inconsistent and not long-lasting.54 In contrast, a school nurse-supervised asthma program, in which pediatric patients with persistent asthma and suboptimal adherence received nurse-supervised daily ICS therapy at school, decreased asthma-related emergency department visits, reduced asthma-related hospital admissions, and lowered asthma reliever medication refills over 1 year.55
A multidisciplinary approach to patient care supports the patient in the management of both mental and physical aspects of their disease. The inclusion of psychologists on such teams can, therefore, improve self-management by eliciting changes in behavior.58 Adherence can also be improved by implementing a shared decision-making process regarding medication and dose regimen choices between health care provider and patient.59 Shared decision-making is an approach whereby patients and health care providers make joint health care decisions based upon the best available evidence regarding possible risks and benefits associated with viable options yet still anchored to patient preferences and values.60 It is also important to consider the health literacy of the patient when developing education and treatment action plans, so these plans can be delivered at the appropriate level to support adherence to therapy.2
Electronic devices that track adherence have been available for over 3 decades,10 but new adherence-intervention smart technology includes a growing number of potential uses for asthma patients, such as feedback on inhaler technique, portable fractional exhaled nitric oxide measurement devices, and GPS devices with alerts about environmental contaminants that could worsen symptoms.61 Such technology has the potential to remotely track disease variables and remind patients to take their medication, avoid triggers, or contact their physician when symptoms worsen. A review of patient-reminder systems that are meant to combat forgetfulness in taking medications found an increase in adherence of up to 22% but no effect on outcomes or quality of life,62 whereas education tailored to patients’ illness and medication beliefs significantly improved adherence in adolescents and adults.35 A study investigating the use of a monitor attached to patients’ inhalers showed that patients with asthma who received medication reminders and feedback from such a device had significantly higher adherence than the control group who did not receive feedback or reminders.63 Another study used an electronic monitoring device in patients with COPD to track the quality and timing of inhaler use to improve adherence in distinct clusters of patients (regular use, frequent technique errors; irregular use, good technique; and irregular use, frequent technique errors).64 One recently completed trial (NCT02864342) showed that electronic medication reminders improved adherence to inhaled medication in patients with COPD. The majority of patients indicated that they found the device with accompanying smartphone application easy to use and the reminders helped to ensure their medication was taken as prescribed.65 Additional studies in progress are investigating electronic means of increasing adherence to inhalation therapies, but electronic interventions also raise a number of potential ethical questions related to patient privacy.66 Furthermore, the real-world feasibility of shared decision-making, in which providers deliver a brief, 7-minute intervention during 1 regular office visit, is currently being explored. An assessment of the effects of this decision process on patient adherence to ICS therapy is one of the secondary outcomes being measured in this study.67
In COPD, multifaceted interventions have been shown to have the greatest impact on improved medication adherence, although not all studies have shown a positive effect.7 For example, pulmonary rehabilitation is a comprehensive intervention to promote long-term adherence to health-enhancing behaviors in patients with COPD, but the specific effects on medication adherence are not well studied.1,68 However, individually tailored care plans, educational sessions about the illness and inhaler technique, health care provider visits, and/or weekly phone calls do improve adherence.7,53,69 Figure 2 illustrates how these different types of interventions can fit together in a patient’s overall care plan to improve medication adherence.
 |
Figure 2 Strategies for improving adherence to inhaled medications for asthma and COPD. A multifaceted care plan tailored to an individual patient can improve adherence and, ultimately, health outcomes.Notes: Upper left image: iStock.com/Vesnaandjic. Upper right image reprinted from The Lancet, Vol. 3, No. 3, Chan AH, Stewart AW, Harrison J, Camargo CA, Black PN, Mitchell EA, The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial, p210-219, Copyright 2015, with permission from Elsevier.70 Bottom left image: iStock.com/Steve Debenport. Bottom right image: iStock.com/DragonImages. Data from Tommelein et al53 Wilson et al59and Chan et al.70Abbreviations: COPD, chronic obstructive pulmonary disease; HCP, health care provider. |
Conclusions
Although inhaled maintenance therapy is vital for many patients with asthma or COPD, suboptimal adherence is frequently an issue, and the underlying causes of it are multifactorial and complex. Suboptimal adherence is associated with negative consequences related to disease control, mortality, and health care resource use; it is, therefore, important to optimize each patient’s therapy to improve adherence and maximize the therapeutic effects of prescribed medications. Therapy optimizations may include routine inhaler technique training or changing to a different type of inhaler that is easier for the patient to use correctly. Shared decision-making allows for collaboration between patient and physician to develop a treatment plan that addresses barriers to adherence and fosters an environment that is favorable to patient education, communication, and counseling.
Abbreviation list
COPD, chronic obstructive pulmonary disease; GINA, Global Initiative for Asthma; GPS, global positioning system; ICS, inhaled corticosteroid; MARS-A, Medication Adherence Report Scale for Asthma; MIS-A, Medication Intake Survey-Asthma; MPR, medication possession ratio; OCS, oral corticosteroid.
Acknowledgments
Medical writing support was provided by Shane Walton, PhD, of MedErgy (Yardley, PA, USA), under the direction of the authors, in accordance with Good Publication Practice (GPP3) guidelines, and was funded by AstraZeneca (Wilmington, DE, USA).
Author contributions
Both authors were involved in the analysis and interpretation of the literature search results. Both authors participated in the development and critical review of the manuscript, provided final approval to submit for publication, and agree to be accountable for all aspects of the work.
Disclosure
MG has served as a consultant for AstraZeneca and Teva. MG also reports personal fees from Teva and AstraZeneca, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2018Report); 2018. Available from: http://goldcopd.org.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; 2018. Available from: http://www.ginasthma.org. Accessed February 8, 2019.
3. Makela MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490. doi:10.1016/j.rmed.2013.04.005
4. Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi:10.1136/bmj.38875.675486.55
5. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. doi:10.7326/0003-4819-157-11-201212040-00538
6. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209.
7. Bryant J, McDonald VM, Boyes A, Sanson-Fisher R, Paul C, Melville J. Improving medication adherence in chronic obstructive pulmonary disease: a systematic review. Respir Res. 2013;14:109. doi:10.1186/1465-9921-14-19
8. Barnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care. 2015;60(3):455–468. doi:10.4187/respcare.03200
9. Vrijens B, Dima AL, Van Ganse E, et al. What we mean when we talk about adherence in respiratory medicine. J Allergy Clin Immunol Pract. 2016;4(5):802–812. doi:10.1016/j.jaip.2016.05.019
10. Kikidis D, Konstantinos V, Tzovaras D, Usmani OS. The digital asthma patient: the history and future of inhaler based health monitoring devices. J Aerosol Med Pulm Drug Deliv. 2016;29(3):219–232. doi:10.1089/jamp.2015.1267
11. PROAIR® DIGIHALERTM (Albuterol Sulfate) [Packet Insert]. Frazer, PA: Teva Respiratory, LLC; December 2018.
12. Clatworthy J, Price D, Ryan D, Haughney J, Horne R. The value of self-report assessment of adherence, rhinitis and smoking in relation to asthma control. Prim Care Respir J. 2009;18(4):300–305. doi:10.4104/pcrj.2009.00037
13. Cohen JL, Mann DM, Wisnivesky JP, et al. Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: the Medication Adherence Report Scale for Asthma. Ann Allergy Asthma Immunol. 2009;103(4):325–331.
14. Dima AL, van Ganse E, Laforest L, Texier N, de Bruin M, The Astro-Lab Group. Measuring medication adherence in asthma: development of a novel self-report tool. Psychol Health. 2017;32(10):1288–1307. doi:10.1080/08870446.2017.1290248
15. Bender BG. Nonadherence to asthma treatment: getting unstuck. J Allergy Clin Immunol Pract. 2016;4(5):849–851. doi:10.1016/j.jaip.2016.07.007
16. DePietro M, Gilbert I, Millette LA, Riebe M. Inhalation device options for the management of chronic obstructive pulmonary disease. Postgrad Med. 2018;130(1):83–97. doi:10.1080/00325481.2018.1399042
17. Mokoka MC, Lombard L, MacHale EM, et al. In patients with severe uncontrolled asthma, does knowledge of adherence and inhaler technique using electronic monitoring improve clinical decision making? A protocol for a randomised controlled trial. BMJ Open. 2017;7(6):e015367. doi:10.1136/bmjopen-2016-015367
18. van Boven JF, Trappenburg JC, van der Molen T, Chavannes NH. Towards tailored and targeted adherence assessment to optimise asthma management. NPJ Prim Care Respir Med. 2015;25:15046. doi:10.1038/npjpcrm.2015.46
19. Sanduzzi A, Balbo P, Candoli P, et al. COPD: adherence to therapy. Multidiscip Respir Med. 2014;9(1):60. doi:10.1186/2049-6958-9-60
20. Perez de Llano L, Sanmartin AP, Gonzalez-Barcala FJ, et al. Assessing adherence to inhaled medication in asthma: impact of once-daily versus twice-daily dosing frequency. The ATAUD study. J Asthma. 2018;55(9):933–938. doi:10.1080/02770903.2018.1426769
21. Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl). 2015;8:131–139. doi:10.2147/MDER.S48888
22. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19(1):10. doi:10.1186/s12931-017-0710-y
23. Baird C, Lovell J, Johnson M, Shiell K, Ibrahim JE. The impact of cognitive impairment on self-management in chronic obstructive pulmonary disease: a systematic review. Respir Med. 2017;129:130–139. doi:10.1016/j.rmed.2017.06.006
24. Kaplan A, Price D. Matching inhaler devices with patients: the role of the primary care physician. Can Respir J. 2018;2018:9473051. doi:10.1155/2018/9473051
25. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–938. doi:10.1016/j.rmed.2011.01.005
26. Sears MR. Predicting asthma outcomes. J Allergy Clin Immunol. 2015;136(4):829–836; quiz 837. doi:10.1016/j.jaci.2015.04.048
27. The Inhaler Error Steering Committee, Price D, Bosnic-Anticevich S, et al. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med. 2013;107(1):37–46. doi:10.1016/j.rmed.2012.09.017
28. Philip J. The effects of inhaled corticosteroids on growth in children. Open Respir Med J. 2014;8:66–73. doi:10.2174/1874306401408010066
29. Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354(19):1985–1997. doi:10.1056/NEJMoa051378
30. Jentzsch NS, Silva GCG, Mendes GMS, Brand PLP, Camargos P. Treatment adherence and level of control in moderate persistent asthma in children and adolescents treated with fluticasone and salmeterol. J Pediatr (Rio J). 2017;95(1):69–75. doi:10.1016/j.jped.2017.10.008
31. Bender BG, Bender SE. Patient-identified barriers to asthma treatment adherence: responses to interviews, focus groups, and questionnaires. Immunol Allergy Clin North Am. 2005;25(1):107–130. doi:10.1016/j.iac.2004.09.005
32. Saini K, Griffiths P. Fluticasone and beclometasone: what are their effects on children’s growth? Br J Community Nurs. 2003;8(5):221–225. doi:10.12968/bjcn.2003.8.5.11201
33. Loke YK, Blanco P, Thavarajah M, Wilson AM. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS One. 2015;10(7):e0133428. doi:10.1371/journal.pone.0133428
34. De Simoni A, Horne R, Fleming L, Bush A, Griffiths C. What do adolescents with asthma really think about adherence to inhalers? Insights from a qualitative analysis of a UK online forum. BMJ Open. 2017;7(6):e015245. doi:10.1136/bmjopen-2016-015245
35. Petrie KJ, Perry K, Broadbent E, Weinman J. A text message programme designed to modify patients’ illness and treatment beliefs improves self-reported adherence to asthma preventer medication. Br J Health Psychol. 2012;17(1):74–84. doi:10.1111/j.2044-8287.2011.02033.x
36. Wei YJ, Simoni-Wastila L, Albrecht JS, et al. The association of antidepressant treatment with COPD maintenance medication use and adherence in a comorbid Medicare population: a longitudinal cohort study. Int J Geriatr Psychiatry. 2018;33(2):e212–e220. doi:10.1002/gps.4772
37. Thomas M, Bruton A, Moffat M, Cleland J. Asthma and psychological dysfunction. Prim Care Respir J. 2011;20(3):250–256. doi:10.4104/pcrj.2011.00058
38. Ismaila A, Corriveau D, Vaillancourt J, et al. Impact of adherence to treatment with fluticasone propionate/salmeterol in asthma patients. Curr Med Res Opin. 2014;30(7):1417–1425. doi:10.1185/03007995.2014.908827
39. Makhinova T, Barner JC, Richards KM, Rascati KL. Asthma controller medication adherence, risk of exacerbation, and use of rescue agents among texas medicaid patients with persistent asthma. J Manag Care Spec Pharm. 2015;21(12):1124–1132. doi:10.18553/jmcp.2015.21.12.1124
40. Jochmann A, Artusio L, Jamalzadeh A, et al. Electronic monitoring of adherence to inhaled corticosteroids: an essential tool in identifying severe asthma in children. Eur Respir J. 2017;50:6. doi:10.1183/13993003.00711-2017
41. Mapel D, Roberts MH, Blanchette CM, Petersen H, Ramachandran S. Effectiveness of inhaled combined corticosteroid/long-acting bronchodilator treatment in reducing COPD exacerbations and short-acting bronchodilator use. JCOM. 2013;20(2):60–68.
42. Dalal AA, Shah M, D’Souza AO, Rane P. Costs of COPD exacerbations in the emergency department and inpatient setting. Respir Med. 2011;105(3):454–460. doi:10.1016/j.rmed.2010.09.003
43. Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–943. doi:10.1136/thx.2009.113662
44. van Boven JF, Chavannes NH, van der Molen T, Rutten-van Molken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103–113. doi:10.1016/j.rmed.2013.08.044
45. Kim JA, Lim MK, Kim K, Park J, Rhee CK. Adherence to inhaled medications and its effect on healthcare utilization and costs among high-grade chronic obstructive pulmonary disease patients. Clin Drug Investig. 2018;38(4):333–340. doi:10.1007/s40261-017-0612-2
46. Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35–44. doi:10.2147/RMHP.S19801
47. Clark NM, Cabana MD, Nan B, et al. The clinician-patient partnership paradigm: outcomes associated with physician communication behavior. Clin Pediatr (Phila). 2008;47(1):49–57. doi:10.1177/0009922807305650
48. George M. Health beliefs, treatment preferences and complementary and alternative medicine for asthma, smoking and lung cancer self-management in diverse Black communities. Patient Educ Couns. 2012;89(3):489–500. doi:10.1016/j.pec.2012.05.003
49. Brandstetter S, Finger T, Fischer W, et al. Differences in medication adherence are associated with beliefs about medicines in asthma and COPD. Clin Transl Allergy. 2017;7:39. doi:10.1186/s13601-017-0175-6
50. Scullion J. The nurse practitioners’ perspective on inhaler education in asthma and chronic obstructive pulmonary disease. Can Respir J. 2018;2018:2525319. doi:10.1155/2018/2525319
51. Klijn SL, Hiligsmann M, Evers S, Roman-Rodriguez M, van der Molen T, van Boven JFM. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. NPJ Prim Care Respir Med. 2017;27(1):24. doi:10.1038/s41533-017-0022-1
52. Abdulsalim S, Unnikrishnan MK, Manu MK, Alrasheedy AA, Godman B, Morisky DE. Structured pharmacist-led intervention programme to improve medication adherence in COPD patients: a randomized controlled study. Res Social Adm Pharm. 2018;14(10):909–914. doi:10.1016/j.sapharm.2017.10.008
53. Tommelein E, Mehuys E, Van Hees T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): a randomized controlled trial. Br J Clin Pharmacol. 2014;77(5):756–766. doi:10.1111/bcp.12242
54. Otsuki M, Eakin MN, Rand CS, et al. Adherence feedback to improve asthma outcomes among inner-city children: a randomized trial. Pediatrics. 2009;124(6):1513–1521. doi:10.1542/peds.2008-2961
55. Trivedi M, Patel J, Lessard D, et al. School nurse asthma program reduces healthcare utilization in children with persistent asthma. J Asthma. 2018;55(10):1131–1137. doi:10.1080/02770903.2017.1396473
56. Bender BG, Apter A, Bogen DK, et al. Test of an interactive voice response intervention to improve adherence to controller medications in adults with asthma. J Am Board Fam Med. 2010;23(2):159–165. doi:10.3122/jabfm.2010.02.090112
57. Vollmer WM, Feldstein A, Smith DH, et al. Use of health information technology to improve medication adherence. Am J Manag Care. 2011;17(12Spec No.):SP79–S87.
58. Lunn S, Restrick L, Stern M. Managing respiratory disease: the role of a psychologist within the multidisciplinary team. Chron Respir Dis. 2017;14(1):45–53. doi:10.1177/1479972316688914
59. Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566–577. doi:10.1164/rccm.200906-0907OC
60. Blaiss MS, Steven GC, Bender B, Bukstein DA, Meltzer EO, Winders T. Shared decision making for the allergist. Ann Allergy Asthma Immunol. 2019;122(5):463–470. doi:10.1016/j.anai.2018.08.019
61. Bender BG. Technology interventions for nonadherence: new approaches to an old problem. J Allergy Clin Immunol Pract. 2018;6(3):794–800. doi:10.1016/j.jaip.2017.10.029
62. Tran N, Coffman JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. J Asthma. 2014;51(5):536–543. doi:10.3109/02770903.2014.888572
63. Foster JM, Usherwood T, Smith L, et al. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. J Allergy Clin Immunol. 2014;134(6):1260–1268 e3. doi:10.1016/j.jaci.2014.05.041
64. van Boven JFM, Cushen B, Sulaiman I, et al. Personalising adherence-enhancing interventions using a smart inhaler in patients with COPD: an exploratory cost-effectiveness analysis. NPJ Prim Care Respir Med. 2018;28(1):24. doi:10.1038/s41533-018-0092-8
65. Criner GJ, Cole T, Hahn K, Kastango K, Eudicone J, Gilbert I. Late breaking abstract - a randomized clinical study to assess the impact of budesonide/formoterol (BUD/FM) pMDI medication reminders on adherence in COPD patients. Eur Respir J. 2018;52(Suppl 62):PA1988. doi:10.1183/13993003.01675-2018
66. Campbell JI, Eyal N, Musiimenta A, Haberer JE. Ethical questions in medical electronic adherence monitoring. J Gen Intern Med. 2016;31(3):338–342. doi:10.1007/s11606-015-3502-4
67. George M, Pantalon MV, Sommers MLS, et al. Shared decision-making in the BREATHE asthma intervention trial: a research protocol. J Adv Nurs. 2019;75(4):876–887. doi:10.1111/jan.13916
68. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi:10.1164/rccm.201309-1634ST
69. Leiva-Fernandez J, Leiva-Fernandez F, Garcia-Ruiz A, Prados-Torres D, Barnestein-Fonseca P. Efficacy of a multifactorial intervention on therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled trial. BMC Pulm Med. 2014;14:70. doi:10.1186/1471-2466-14-70
70. Chan AH, Stewart AW, Harrison J, Camargo CA, Black PN, Mitchell EA. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respir Med. 2015;3(3):210–219. doi:10.1016/S2213-2600(15)00008-9
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
